 |
 |
| Plant Pathol J > Volume 33(1); 2017 > Article |
Abstract
Seed dehiscence of ginseng (Panax ginseng C. A. Mayer) is affected by moisture, temperature, storage conditions and microbes. Several microbes were isolated from completely dehisced seed coat of ginseng cultivars, Chunpoong and Younpoong at Gumsan, Korea. We investigated the potential of five Talaromyces flavus isolates from the dehiscence of ginseng seed in four traditional stratification facilities. The isolates showed antagonistic activities against fungal plant pathogens, such as Cylindrocarpon destructans, Fusarium oxysporum, Rhizoctonia solani, Sclerotinia nivalis, Botrytis cinerea, and Phytophthora capsici. The dehiscence ratios of ginseng seed increased more than 33% by treatment of T. flavus GG01, GG02, GG04, GG12, and GG23 in comparison to control (28%). Among the treatments, the reformulating treatment of T. flavus isolates GG01 and GG04 showed the highest of stratification ratio of ginseng seed. After 16 weeks, the reformulating treatment of T. flavus isolates GG01 and GG04 significantly enhanced dehiscence of ginseng seed by about 81% compared to the untreated control. The candidate’s treatment of T. flavus GG01 and GG04 showed the highest decreasing rate of 93% in seed coat hardness for 112 days in dehiscence period. The results suggested that the pre-inoculation of T. flavus GG01 and GG04 found to be very effective applications in improving dehiscence and germination of ginseng seed.
The genus Panax is geographically distributed in many regions of the world, including North America and northeast Asia for its reputed medicinal and herbal properties (Hu et al., 1977; Proctor and Bailey, 1987). Ginseng (Panax ginseng Meyer) is an important medicinal plant that has been used for medicinal purposes for centuries in Korea (Hu, 1977; Liu and Xiao, 1992; Yun, 2001). The worldwide demand for ginseng and its products has increased significantly.
The ginseng plant are propagated sexually from stratified seed, which are normally planted during the fall season (September to October) to give rise to one year seedlings in the following spring (April to May). Ginseng seeds that have an underdeveloped embryo at the time of dispersal, distinguished by a very thick and hard seed coat require specific a cold temperature conditions for embryo development and radical and cotyledon emergence have morphophysiological dormancy (Baskin and Baskin, 2004; Lee et al., 2008; Proctor and Louttit, 1995).
Numerous studies have been conducted to improve seed germination and dormancy of ginseng seed using exogenous plant hormones (Kim et al., 2014; Lee et al., 2008), optimal chilling conditions (Kwon et al., 2001), dehiscent materials (Yang et al., 1982), embryo growth and temperature (Proctor and Louttit, 1995; Proctor and Stechyshyn-Nagasawa, 2008), and microorganisms (Yang et al., 1982). However, limited studies were carried to develop methods hastening and improving dormancy and stimulating germination of the seeds, as an alternative to other laborious methods.
In order to enhance the germination rate of ginseng seeds, the ripening treatment method such as hierarchical storage using sand was used to stretch embryo and cleavage the seed coat under low temperature and high humidity conditions (Li et al., 2000; Won and Jo, 1988). Seed coat dehiscence and survival ratio of ginseng seedlings were not affected by seed size (Lee et al., 2008). Stratified ginseng seed with full-size embryos did not germinate at room temperature and under the required chilling treatment for 75 days in outdoors and 90 days in cold chamber (Kwon et al., 2001). Kwon et al. (1998) reported that cytokinins are closely related with the germination of ginseng seed and dihydrozeatin, and that possibly trans-zeatin is the most important cytokinins involved in the germination process. Also, Kim et al. (2014) showed that the embryo growth ratio of ginseng seed during the seed collection period revealed a similar trend in which the total gibberellic acid and abscisic acid contents were inversely related.
Talaromyces flavus (anamorph, Penicillium dangeardii sometimes reported as Penicillium vermiculatum) was a biocontrol agent and shown to control several soil borne plant pathogens including Sclerotinia sclerotiorum (McLaren et al., 1982), Rhizoctonia solani (McLaren et al., 1982), and Verticillium dahliae (Fravel et al., 1987; Marois et al., 1982; Naraghi et al., 2010). Marois et al. (1984) also reported that this fungus grows on the rhizosphere of greenhouse cucumber, cotton, and egg-plant, and inhibit the germination of the microsclerotia of V. dahliae. Forty percent of non-volatile extracts (Talaron) of T. flavus are thought to affect production of hydrogen peroxide due to the glucose oxidase enzyme giving antibacterial and antifungal activities (Kim et al., 1990). The action of T. flavus in the mycoparasitism of plant pathogenic fungi has also been reported to be able to secrete fungal hydrolytic enzymes, chitinase (Duo-Chuan et al., 2005; Madi et al., 1997) and proteinase (Haggag et al., 2006).
Therefore, this study aims to enhance the dehiscence of Panax ginseng seeds treated with antifungal fungus, T. flavus GG01 and GG04 obtained from the traditional stratification facilities for the dehisced ginseng seed coat in Korea.
Fresh-harvested ginseng seeds of a 4-year-old P. ginseng plant (var. ‘Chunpoong’) were collected from the eco-friendly agricultural ginseng farm at Gumsan in late July, 2014. The ginseng berries were mechanically harvested by hand de-pulped, and the seeds were staked in the sand for one week. The seeds were washed discarding the floating seeds and then dried in the shade. Semi-dried ginseng seeds were kept at 4°C for use in this study.
We selected five isolates of T. flavus GG01, GG02, GG04, GG12, and GG23 from the Korean traditional ginseng seed stratification facilities. The ascospores were produced by growing five T. flavus isolates in the dark for three weeks on potato dextrose agar (PDA; Difco, Detroit, MI, USA). For morphological study of five isolates of T. flavus, we recommended using Czapek yeast extract agar (CYA; Czapek concentrate 10 ml, sucrose 30 g, yeast extract 5 g, K2HPO4 1 g, CuSO4·5H2O 0.005 g, ZnSO4·7H2O 0.01 g, agar 20 g) and malt extract agar (MEA; malt extract 20 g, dextrose 20 g, peptone 6 g, agar 15 g). The conidia were prepared separately for every isolates. Twenty milliliters sterile distilled water was poured into 50 cm-wide × 80-cm-tall sterile plastic bags containing 250 g of rice polish media mixed with 10 ml D-lactose monohydrate (20 g/l). The plastic bags were incubated at 30°C for 30 days. The content of each plastic bag was removed after the rice polish media was completely covered with T. flavus hyphae. The number of ascospores of five isolates T. flavus in each gram of rice polish media was then determined using a haemocytometer (Chet and Baker, 1981).
All of the plant pathogens, Cylindrocarpon destructans (KACC 40123), Fusarium oxysporum (KACC 44452), R. solani AG-2-1(KACC 40123), Sclerotinia nivalis (KACC 45152), and Botrytis cinerea (KACC 43521) were isolated from the ginseng plant except for Phytophthora capsici (KACC 40181). Fungal isolates were maintained on PDA slants respectively, at room temperature for further study.
The assay for antagonism was performed on PDA on petri dishes using the dual culture method (Hwang et al., 2006). The mycelial plugs (5-mm diameter) of pathogens and fungal antagonists were placed on the same dish in a gap to 6 cm from each other. Isolates of C. destructans, F. oxysporum, R. solani AG-2-1, S. nivalis, B. cinerea, and P. capsici were cultured on PDA at 25°C for seven days. To test the five isolates of T. flavus GG01, GG02, GG04, GG12, and GG23, a 5 mm of mycelia agar disc from the pathogen cultures was placed on one side of a petri dish containing PDA medium. The dishes were incubated at 25°C for one week. Paired cultures were incubated at 25°C. Dishes inoculated only with test pathogens served as controls. The experiment was repeated twice with three replications of each treatment. After one week, the diameter of colonies of six species of plant pathogens was recorded and the percent of mycelial growth inhibition (PMGI) was calculated using the formula:
where radicle of control (RC) represents the distance (measured in mm) from the point of inoculation to the colony margin on the control dishes and inhibition radicle (IR) the distance of fungal growth from the point of inoculation to the colony margin on the treated dishes in the direction of the antagonists.
The moisture content of 100 g of rice polish media adjusted to 30% (v/w) with distilled water in 500 ml flask and autoclaved at 121°C for 20 min. The sterile rice polish media inoculated with T. flavus conidia was suspended in 30 ml sterile distilled water and incubated at 30°C in BOD-incubator under dark conditions. After 30 days of incubation, the number of ascospores in 1 ml of this suspension was counted using a haematocytometer. Five isolates of T. flavus inoculum was added to the ginseng seed in the pots at 107 ascospores per 1 g of seeds and incubated at 25°C for seven days before the thorough stratification procedure.
After the inoculation with T. flavus based on weight of 0.01%, 0.10%, 1.00%, and 5.00% of the concentration of the ginseng seed, the hardness of the seed coat, somatic embryo growth ratio and the dehiscence rate of ginseng seeds, were investigated after 12 weeks and 16 weeks. The ratio of ginseng seed dehiscence was determined by visual inspection of seed coat cleavage. A simple dehiscence unit for small quantities of fresh dried ginseng seed is a pouch made of lightweight screen in plastic pot (width × height, 15 × 25 cm). The container was filled with alternating layers of ginseng seed and at least twice the volume of moistened river sand, to break the dormancy by dehiscence under low temperature. It was also stacked for more than three months, watering the sample two to three times daily. The sample were kept at minimum temperature of 13°C or higher during the night time, below 25°C in phytotron during the day time (Kim et al., 2008). The containers were opened and checked for the dehiscence rate and hardness of seeds. Ginseng seed coat impermeability was evaluated in 50 seeds which have monochromatic brown seeds and no mimetic displays.
Seed hardness was evaluated as a defense against accelerated force. A TA-XT plus texture analyser (Stable Micro Systems Ltd., Surrey, UK) was used for the textural analyses of ginseng seed coat hardness. The analysis employed was the return-to-start method, measuring force under compression with a 2 mm cylindrical probe (P2), and recording the peak of maximum force. P2 is the probe indicated for assessing ginseng seed coat hardness because its small area affects the tegument and could help to differentiate similar samples, even when they present soft cotyledon but with hard tegument (Revilla and Vivar-Quintana, 2008). Whole ginseng seed coats were axially compressed to 90% of its original height. Force-time curves were recorded at a speed of 1 mm/s and the corresponding results were average values expressed in Newtons (N).
Data analysis. Basic statistic parameters were calculated and the obtained information was presented in tables and figures. All of the comparison of means of inhibition of mycelial growth and inhibition of plant pathogens were subjected to a two factorial ANOVA. The significant differences between the treatments were determined by the t-test (P = 0.05). All statistical analyses were performed using SAS v.9.1 software (SAS Institute, Cary, NC, USA).
We obtained the five isolates, T. flavus CG01, CG02, CG04, CG12, and CG23 from the completely dehisced seed coat of two ginseng cultivars, Chunpoong and Younpoong at three locations of traditional ginseng seed stratification facilities, Nami, Burim, and Jewon in Gumsan. The dominant ginseng cultivar was Chungpoong ginseng in Gumsan. Also, the fungus T. flavus was normally established on the traditional ginseng seed stratification facilities. The characteristics of five isolates obtained from completed dehiscence ginseng seed coats were studied. The mycelium color was white and embedded in the mycelium on the PDA (Fig. 1A, B). The exudate and soluble pigment of colony was absent on PDA. On the other hand, the exudate and soluble pigment of colony of CG01 and CG04 were absent and pale orange on the CYA, respectively (Fig. 1C, D). These isolates could grow in either dark or light, but lighting was beneficial for vegetative growth and sporulation. The results showed that the PDA media were most favorable to its colony growth and conidial production. The optimal temperature ranged from 28-34°C for its colony growth and the optimal initial pH of culture media was pH 6 (data not shown).
Peterson (1959) reported that the fungi associated with the ginseng seed took little part in the colonization of roots in the soil. Species of Aspergillus, Penicillium, and Alternaria, abundant on the seed, were rarely obtained from root samples. The genera of Phoma, Collectotrichum, and Fusarium were the most common fungal en- dophytes found in ginseng roots cultivated in Korea (Park et al., 2012). Plant-microbe interactions like endophytic microbes are noted for natural methods for sustainable agriculture and environmental conservation. Most of the endophytes have antibiotic and plant growth promoting (PGP) activities (Doty, 2011).
However, in spite of the infinite potential, there are only a few reports on the endophytes present in ginseng seed (Li et al., 2012; Um et al., 2014).
The five fungal isolates of T. flavus, GG01, GG02, GG04, GG12, and GG23 were cultured on PDA and tested for the potential as biocontrol agents against six plant pathogens isolated from ginseng plant. The test of antimicrobial spectrum of the isolates successfully inhibited the mycelial growth of six fungal plant pathogens with 26.5-59.5% in vitro (Fig. 2). Among the isolates, T. flavus GG04 was the most effective antagonistic fungus.
Marois et al. (1984) found that significantly higher populations of T. flavus were found in rhizosphere soils than non-rhizosphere soils when the antifungal agent was applied as an ascospore drench to soil planted to potato, cotton or eggplant. Nagtzaam et al. (1998) found that the eggplant seeds were simultaneously coated with T. flavus and talcum powder. The treatment could reduce colonization of roots and infection of eggplants by V. dahliae. A similar result was found in this study. Duo-Chuan et al. (2005) reported that the two chitinases, 41 kDa (CHIT41) and 32 kDa (CHIT32) chitinase had activity against cell wall of V. dahliae, S. sclerotiorum and R. solani, and inhibited spore germination and germ tube elongation of Alternaria alternata, Fusarium moniliforme, and Magnaporthe grisea. Also, the fungus T. flavus is reported to be antagonistic to various plant pathogens, either through hyperparasitism such as in infections with the fungus S. sclerotiorum (McLaren et al., 1986) or by production of glucose oxidase which is released, in presence of glucose and hydrogen peroxide, which in turn prevents the growth and germination of the plant pathogen V. dahlia (Kim et al., 1988; Stosz et al., 1996).
In the results of the study, five fungal isolates of T. flavus showed a broad spectrum of antifungal potential against six fungal plant pathogens originated from ginseng plants.
The dehiscence rate of ginseng seed treated with the five fungal isolates of T. flavus increased by more than 39.7% when compared to the untreated ginseng seeds for 112 days after treatment. In the single treatment GG01 and the combined treatment GG01 and GG04, the percentage of seed with opened seed coat had already reached to 23.9% and 27.0%, respectively at 56 days. Among five isolates, GG01 and GG04 have the higher dehiscence activity with 63.2% and 65.3%, respectively at 112 days of stratification. Notably, the combined treatment of two isolates, T. flavus GG01 and GG04 showed the highest dehiscence ratio of ginseng seed coat than each single treatments and untreated control (Table 1).
Son and Reuther (1977) reported that the slow germination of P. ginseng seeds seemed to be mainly due to the dormancy of endosperm or seed coat rather than of the embryo. Ginseng seed is the characteristics of triple dormancy. At the time harvest goes through the process of dehiscence, the rigid endocarp surrounding the seed and growth doubled after-ripening process to go through immature embryo state. It also has properties that must be overcome until germination to physiological dormancy by cold treatment (Kwon et al., 2001). Madi et al. (1997) showed that the enzymatic activity of T. flavus varied considerably between isolates, with the greatest differences in chitinase (25-fold), followed by glucanase (16-fold), cellulose (11-fold), and glucose oxidase (7-fold).
T. flavus produces extracellular pectin esterase and polygalacturonase after 24 h in submerged culture supplemented with 0.5-0.8% citrus pectin, preceded by a pre-culture for 24 h in 2% (w/v) sucrose or in solid substrate culture on passion fruit peel, lemon or orange pulp pellets after three to six days of incubation (Crotti et al., 1999). T. flavus was described as a good producer of viscosity diminishing activity when cultured in solid substrate culture on orange pulp pellets (Siésser and Said, 1989). Pectin substances, generically known as pectin, are present in primary cell walls and in middle lamellae of plants and constituted by polymers of galacturonic acid.
Our results showed that the treatments of five fungal isolates having antifungal activities were effective for breaking dormancy and triggering the embryo development of ginseng seed.
The ginseng seeds inoculated with five isolates of T. flavus showed decreased seed coat hardness at the rate of more than 70% compared to the untreated control for 112 days after treatment. The hardness of ginseng seed coat significantly decreased at the rate of at least 44% compared to the initial hardness of ginseng seeds at 56 days, except for the untreated control. Among the seven treatments, the combined treatment of T. flavus GG01 and GG04 showed the highest seed coat hardness during 112 days of stratificaqtion period (Fig. 3). Also, significant morphological and physiological changes in ginseng seeds were observed after dehiscence.
Ginseng seeds belong to the morphophysiological dormancy class, which describes a combination of morphological and physiological dormancy. Many factors affect the morphophysiological dormancy of ginseng seed germination, including the stratification period, seeding time and depth, temperature, and spacing (Baskin and Baskin, 2004; Lee et al., 2008; Proctor and Louttit, 1995).
Ginseng seeds started to become dormant as the treatments of T. flavus isolates and the seed coat color changed light brown to typical brown-black discoloration. This demonstrated the continuity of physiological changes in the seeds even after their maturity, when the physiological connection with the break of ginseng seed dormancy had ceased.
To improve the embryo growth of dehisced ginseng seeds were treated with five antifungal isolates of T. flavus before stratification of ginseng seeds. All of the antifungal isolates had a good embryo development and swelling. Also these antifungal isolates enhanced the embryo length by at least 73.3% than the untreated control at 112 days under cold chamber. The highest growth ratio of ginseng embryo was shown in the combined treatments of GG01 and GG04 (Table 2).
A similar result on the germination rate of the embryos grown after dehiscence was found in this study. Through embryos continue growing after dehiscence to a morphologically mature stage, at which there is a less than 85% embryo to endosperm ratio, they cannot germinate due to physiological dormancy, which can be removed by cold stratification at 4-5°C for about three months (Kwon et al., 2001). Due to the ginseng seeds requiring both morphological and physiological after-ripening of the embryo prior to germination (Liu et al., 2011), the embryo of ginseng is too immature to be noticed with the naked eye observations during harvest time. The embryo grew to half the size of the endosperm where seeds were dehisced after three months.
In this study, these results suggested that relatively low percentage of seed with seed dehiscence may be affect to the problem of seed rot during more than three months of stratification. However, applying the candidate antifungal isolates once to the ginseng seed may improve the stratification and germination more effectively in farm conditions.
To shorten the time and increase the rate of dehiscence, ginseng seed treated with the alternative reformation of T. flavus GG01 and GG04 isolates significantly increased the rate of dehiscence compared to the untreated seeds for 112 days (Fig. 4).
It is suggested that the antagonistic microbes, 1% (w/w) T. flavus GG01 and GG04, be established on the endocarp surface of depulped seeds of P. ginseng. This will enable the facilitation of the softening of the endocarp, thereby enhancing the supply of oxygen and water necessary for the embryo development, and accelerate the embryo growth and cause the dehiscence at the beginning. Therefore, antifungal microbes should aim at establishing high densities of the antagonist on the surface of ginseng seed coat at the start of the dehisced and germination time, particularly near the rooting stage of the ginseng seedlings.
There are some discussions about the presence of antifungal agents in the seed coats of persistence species and their possible role in protecting seed against plant pathogens (Warr et al., 1992). The priming effect of PGP rhizobacteria (PGPR) describes the potentiated activation of cellular defense responses on pathogen attack, which includes oxidative burst, cell wall reinforcement, activation of defense-related enzymes, and accumulation of secondary metabolites (Conrath et al., 2006).
However, ginseng is most commonly propagated by seed. Fresh, non-stratified ginseng seeds may be planted immediately after harvest of the berries. It will stratify naturally in the seedbed over the next year and a half, although loss to rodents and disease may be quite high. The seeds require special handling because, to germinate, they must first be subjected to a long period of storage in a moist medium with a warm/cold treatment; a process known as dehiscence. Because of this requirement, ginseng seed normally does not germinate until the second spring following harvest of berries in the fall. Commercial seed suppliers store seed for a year and then market it in the fall as stratified seed. Fall planted, stratified ginseng seed will usually emerge the following April to June.
In this study, each of the isolates of G01 and G04 individually showed the difference results of antagonistic spectra and efficacy of dehiscence rate and embryo growth. The best application was to combine the isolates on the pre inoculation on the ginseng seed coat for improving stratification ratio of ginseng seed.
According to early studies, ginseng seeds ripen in the fall, but generally do not germinate until the following fall without dehiscence. Complete dehiscence also requires extensive labor, time and cost. Thus, it is useful to find out and develop an alternative method to hasten the dormancy breaking and stimulate ginseng seed germination.
Acknowledgments
This study was carried out with the support of the “Co-operative Research Program for Agricultural Science & Technology Development (Project No. PJ00999701), Rural Development Administration, Republic of Korea.
Fig. 1
Overview of colony color of GG01 (A) and GG04 (B) isolates of Talaromyces flavus cultured on potato dextrose agar at 7 days at 25°C. The exudate and soluble pigment of colony of CG01 (C) and CG04 (D) on the Czapek yeast extract agar (CYA) at 7 days at 25°C. The conidiophores (E) and conidia (F) formed on the CYA.
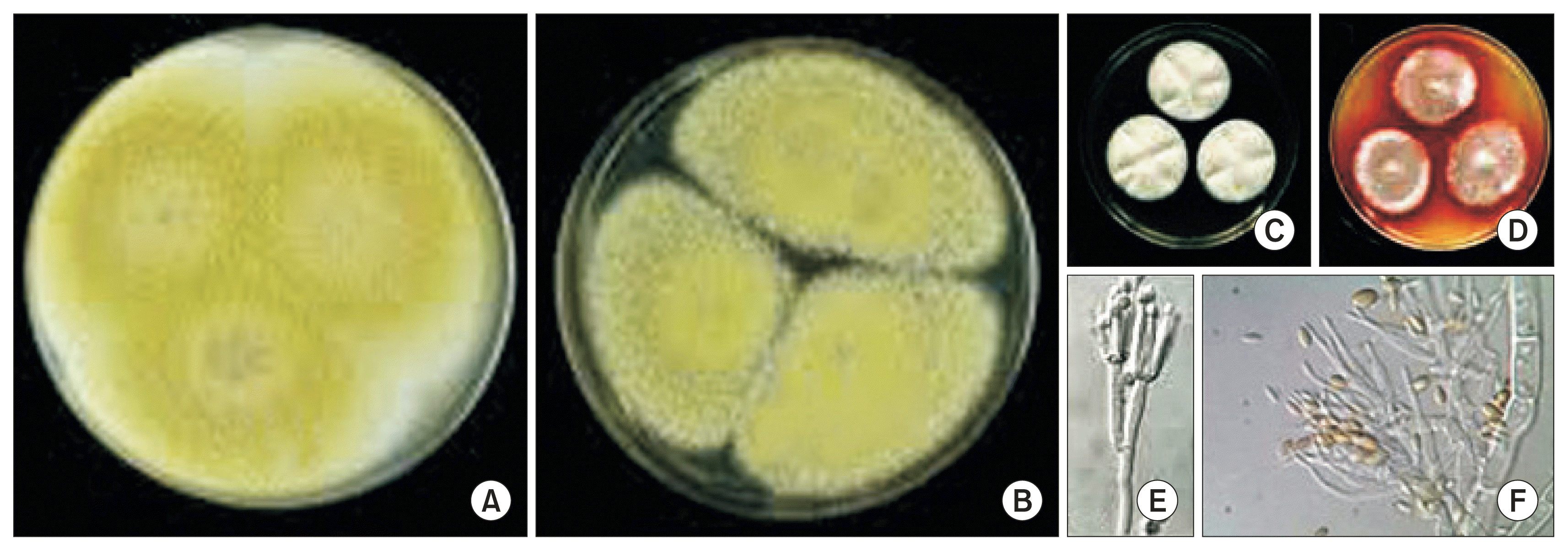
Fig. 2
Antifungal activities of five isolates of Talaromyces flavus on potato dextrose agar at 25°C for seven days in dark condition. CD, Cylindrocarpon destructans (KACC 40123); FO, Fusarium oxysporum (KACC 44452); RS, Rhizoctonia solani AG2-1 (KACC 40123); PC, Phythopthora capsici (KACC 40181); SN, Sclerotinia nivalis (KACC 45152); BC, Botrytis cinerea (KACC 43521). Means followed by the same letters are not significantly different at P ≤ 0.05 according to Fisher’s protected least significant difference test.
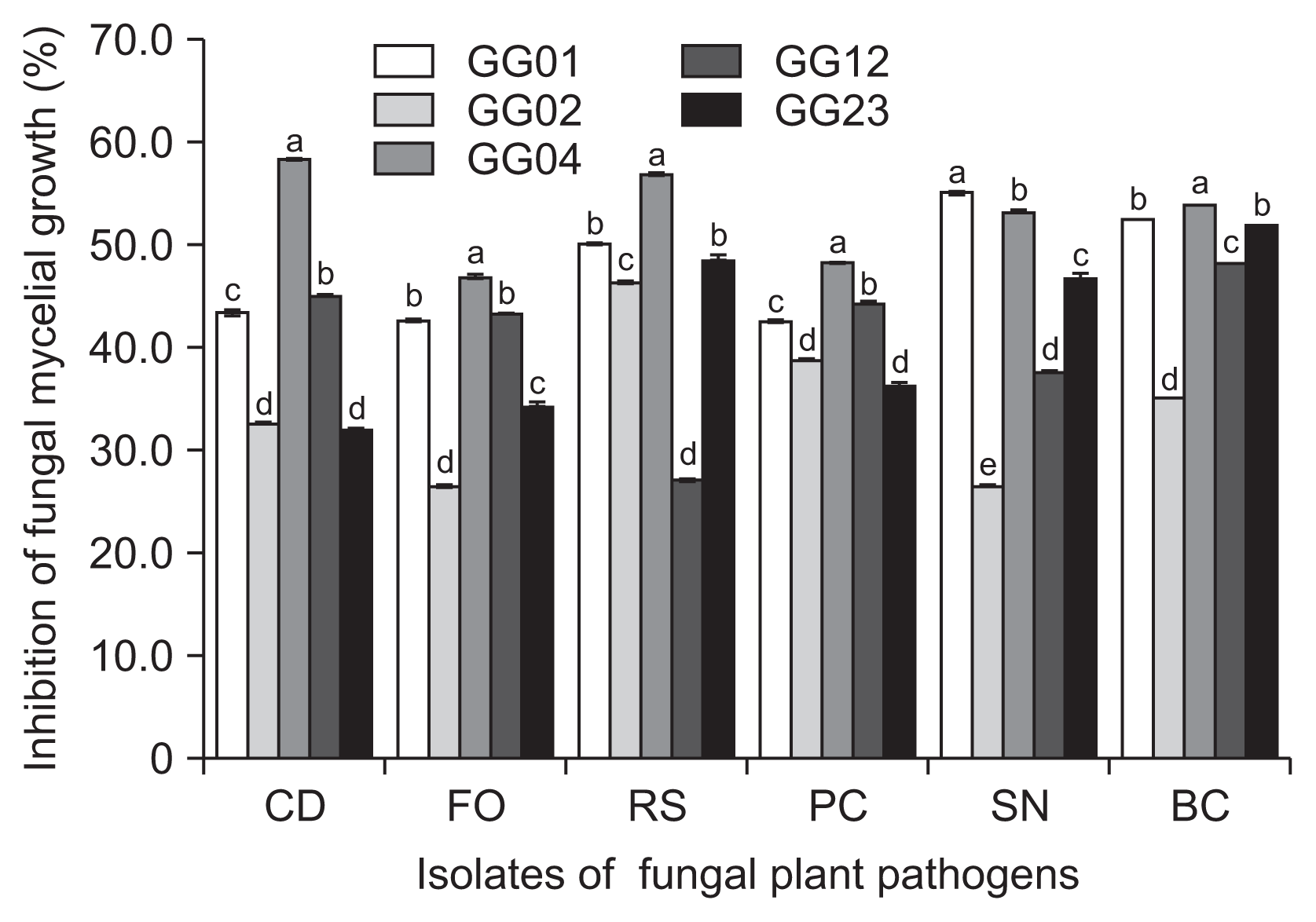
Fig. 3
Evaluation of seed coat hardness treated with 1.0% (w/w) Talaromyces flavus treatments for the enhancing of ginseng seed coat dehiscence for 112 days. Means followed by the same letters are not significantly different at P ≤ 0.05 according to Fisher’s protected least significant difference test.
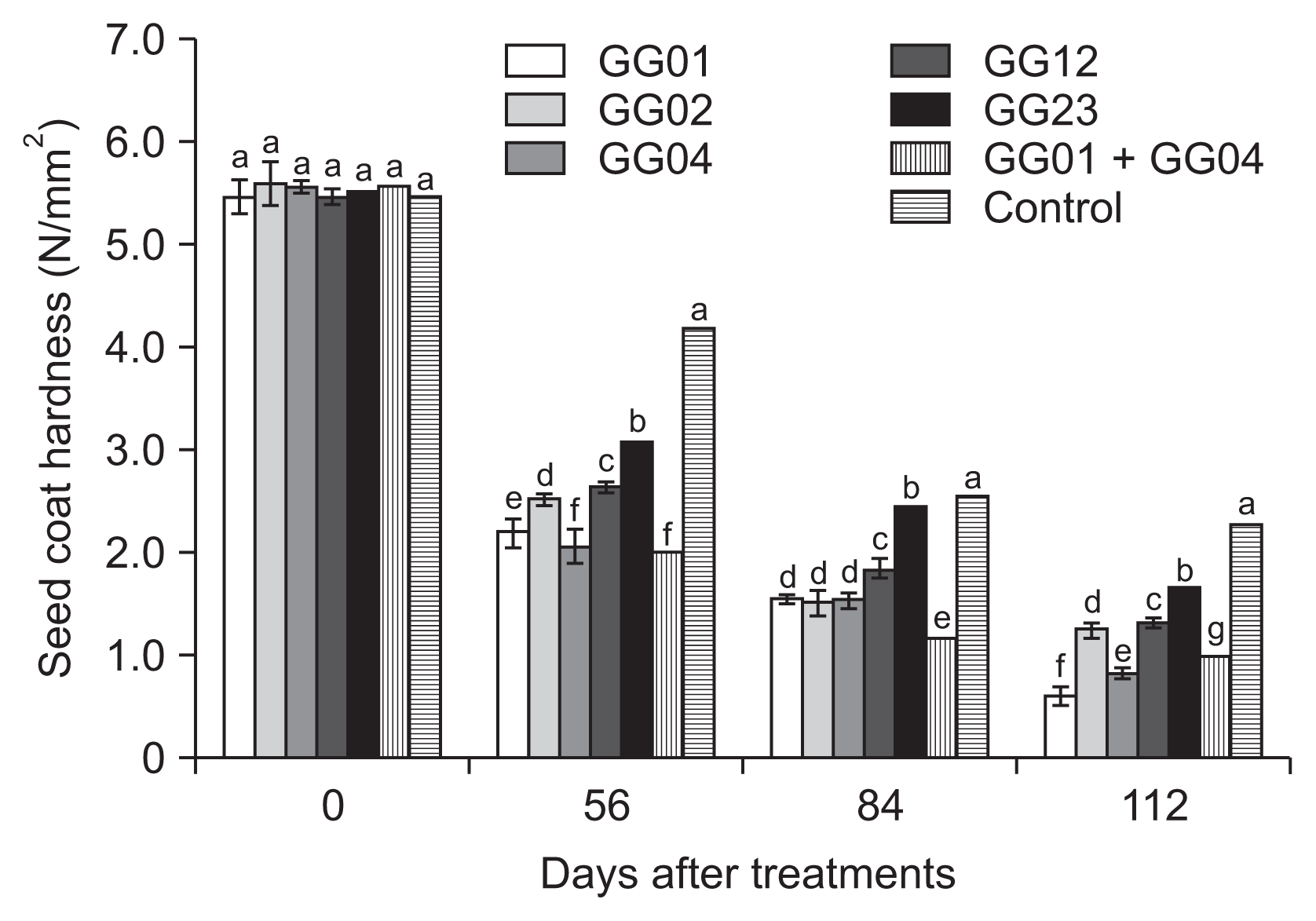
Fig. 4
Estimation of inoculation contents of the reformulating treatment with Talaromyces flavus GG01 and GG04 (1:1, v/v) for enhancement of ginseng seed dehiscence for 112 days. Means followed by the same letters are not significantly different at P ≤ 0.05 according to Fisher’s protected least significant difference test. Control, untreated control.
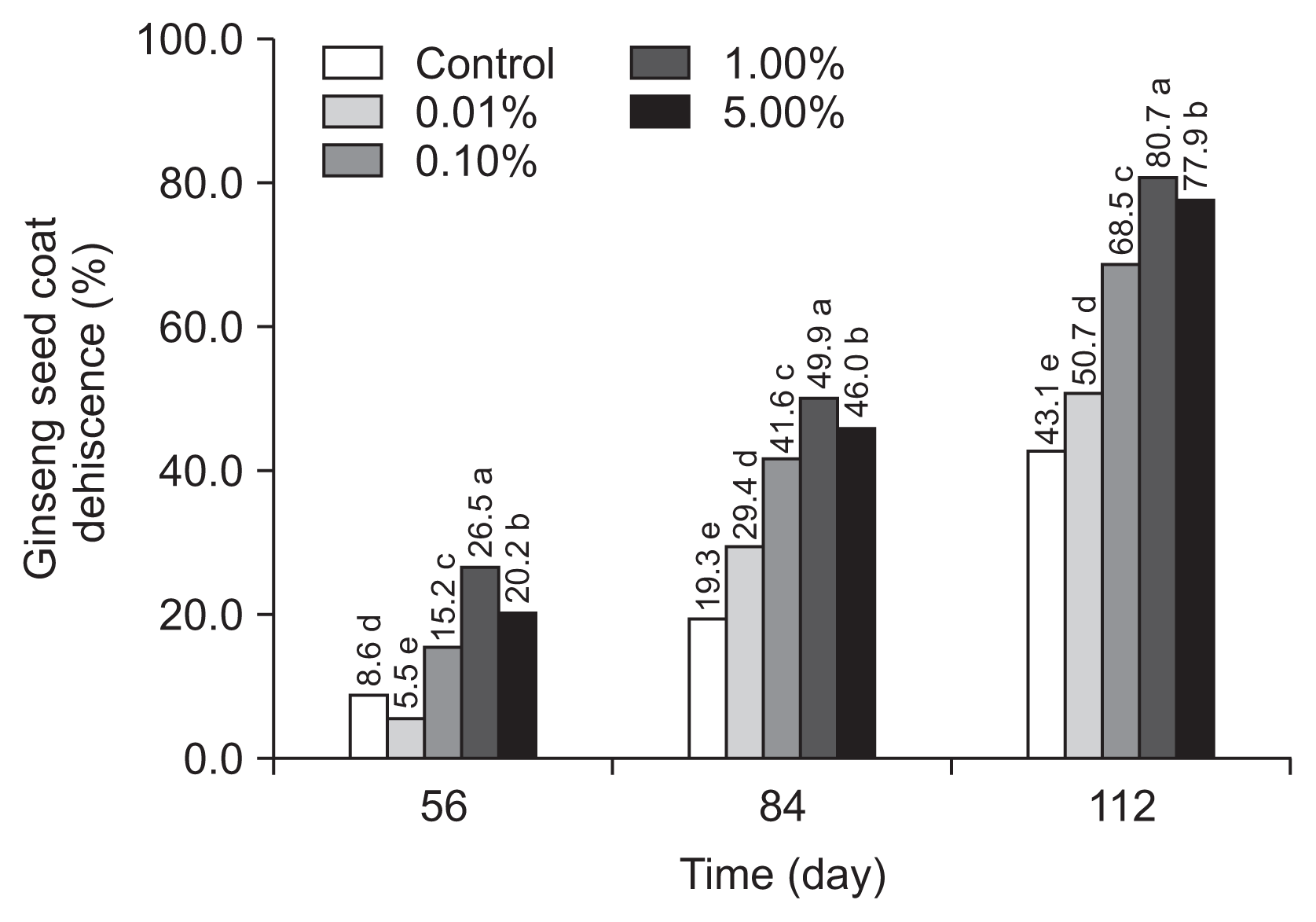
Table 1
Effect of five isolates of Talaromyces flavus treatment on ginseng seed dehiscence
Table 2
Effect of five isolates of Talaromyces flavus treatment on ginseng seed embryo growth
References
Chet, I and Baker, R 1981. Isolation and biocontrol potential of Thrichoderma hamatum from soil naturally suppressive to Rhizoctonia solani. Phytopathology. 71:286-290.

Conrath, U, Beckers, GJ, Flors, V, García-Agustín, P, Jakab, G, Mauch, F, Newman, MA, Pieterse, CM, Poinssot, B, Pozo, MJ, Pugin, A, Schaffrath, U, Ton, J, Wendehenne, D, Zimmerli, L and Mauch-Mani, B Prime-A-Plant Group. 2006. Priming: getting ready for battle. Mol Plant-Microbe Interact. 19:1062-1071.


Crotti, LB, Jabor, VA, Chellegatti, MA, Fonseca, MJ and Said, S 1999. Studies of pectic enzymes produced by Talaromyces flavus in submerged and solid substrate cultures. J Basic Microbiol. 39:227-235.


Duo-Chuan, LI, Chen, S and Jing, LU 2005. Purification and partial characterization of two chitinases from the mycoparasitic fungus Talaromyces flavus. Mycopathologia. 159:223-229.



Fravel, DR, Kim, KK and Papavizas, GC 1987. Viability of microsclerotia of Verticillium dahliae reduced by a metabolite produced by Talaromyces flavus. Phytopathology. 77:616-619.

Haggag, WM, Kansoh, AL and Aly, AM 2006. Proteases from Talaromyces flavus and Trichoderma harzianum: purification characterization and antifungal activity against brown spot disease on faba bean. Plant Pathol Bull. 15:231-239.
Hwang, JY, Shim, CK, Ryu, KY, Choi, DH and Jee, HJ 2006 Selection of Brevibacillus brevis B23 and Bacillus stearothermophilus B42 as biological control agents against sclerotinia rot of lettuce. Res Plant Dis. 12:254-259 (in Korean).

Kim, HH, Lee, JH, Shin, DJ, Ko, HC, Hwang, HS, Kim, T, Cho, EG and Engelmann, F 2008. Desiccation sensitivity and cryopreservation of Korean ginseng seeds. Cryo Letters. 29:419-426.

Kim, KK, Fravel, DR and Papavuzas, GC 1988. Identification of a metabolite preduced by Talaromyces flavus as glucose oxidase and its role in the biocontrol of Verticillium dahlie. Phytopatholology. 78:488-492.
Kim, KK, Fravel, DR and Papavizas, GC 1990. Glucose oxidase as the antifungal principle of talaron from Talaromyces flavus. Can J Microbiol. 36:760-764.


Kim, YH, Ahn, IO, Khan, AL, Kamran, M, Waqas, M, Lee, JS, Kim, DH, Jang, SW and Lee, IJ 2014. Regulation of endogenous gibberellins and abscisic acid levels during different seed collection periods in Panax ginseng. Hortic Environ Biotechnol. 55:166-174.


Kwon, WS, Lee, JH and Lee, MG 2001. Optimum chilling terms for germination of the dehisced ginseng (Panax ginseng C. A. Meyer) seed. J Ginseng Res. 25:167-170.
Kwon, WS, Min, BH and Lee, JM 1998 Separation of cytokinins in ginseng seeds during after-ripening by HPLC. J Korean Soc Hortic Sci. 38:371-375 (in Korean).
Lee, JS, Lee, SS, Lee, JH and Ahn, IO 2008 Effect of seed size and cultivars on the ratio of seed coat dehiscence and seedling performance in Panax ginseng. J Ginseng Res. 32:257-263 (in Korean).

Li, TSC, Bedford, KE and Sholberg, PL 2000. Improved germination of American ginseng seeds under controlled environments. HortTechnology. 10:131-135.

Li, Y, Ying, Y, Zhao, D and Ding, W 2012. Microbial community diversity analysis of Panax ginseng rhizosphere and non-rhizosphere soil using randomly amplified polymorphic DNA method. Open J Genet. 2:95-102.
Liu, CX and Xiao, PG 1992. Recent advances on ginseng research in China. J Ethnopharmacol. 36:27-38.


Liu, SQ, Ren, YY and Xu, HB 2011. Effect of thinning flower buds on fruit and seed traits and root yield of Panax ginseng C. A. Meyer. Ind Crops Prod. 33:559-565.

Madi, L, Katan, T, Katan, J and Henis, Y 1997. Biological control of Sclerotium rolfsii and Verticillium dahliae by Talaromyces flavus is mediated by different mechanisms. Phytopathology. 87:1054-1060.


Marois, JJ, Fravel, DR and Papavizas, GC 1984. Ability of Talaromyces flavus to occupy the rhizosphere and its interaction with Verticillium dahliae. Soil Biol Biochem. 16:387-390.

Marois, JJ, Johnston, SA, Dunn, MT and Papavizas, GC 1982. Biological control of Verticillium wilt of eggplant in the field. Plant Dis. 66:1166-1168.

McLaren, DL, Huang, HC and Rimmer, SR 1982. Hyphal interactions occurring between Sclerotinia sclerotiorum and Penicillium vermiculatum. Can J Plant Pathol. 4:308.
McLaren, DL, Huang, HC and Rimmer, SR 1986. Hyper-parasitism of Sclerotinia sclerotiorum by Talaromyces flavus. Can J Plant Pathol. 8:43-48.

Nagtzaam, MPM, Bollen, GJ and Termorshuizen, AJ 1998. Efficacy of Talaromyces flavus alone or in combination with other antagonists in controlling Verticillium dahliae in growth chamber experiments. J Phytopathol. 146:165-173.

Naraghi, L, Heydari, A, Rezaee, S, Razavi, M and Afshari-Azad, H 2010. Biological control of Verticillium wilt of greenhouse cucumber by Talaromyces flavus. Phytopathol Mediterr. 49:321-329.
Park, YH, Kim, YC, Park, SU, Lim, HS, Kim, JB, Cho, BK and Bae, H 2012. Age-dependent distribution of fungal endophytes in Panax ginseng roots cultivated in Korea. J Ginseng Res. 36:327-333.



Peterson, EA 1959. Seed-borne fungi in relation to colonization of roots. Can J Microbiol. 5:579-582.


Proctor, JTA and Louttit, D 1995. Stratification of American ginseng seed: embryo growth and temperature. Korean J Ginseng Sci. 19:171-174.
Proctor, JTA and Stechyshyn-Nagasawa, A 2008. Extended stratification of the North American ginseng seed. J Ginseng Res. 32:155-160.
Revilla, I and Vivar-Quintana, AM 2008. Effect of canning process on texture of Faba beans (Vicia Faba). Food Chem. 106:310-314.

Siéssere, V and Said, S 1989. Pectic enzymes production in solid state fermentation using citrus pulp pellets by Talaromyces flavus, Tubercularia vulgaris and Penicillium charlesi. Biotechnol Lett. 11:343-344.


Son, ER and Reuther, G 1977. Preliminary studies on breaking of dormancy and germination of Panax ginseng seeds. Korean J Crop Sci. 22:45-51.
Stosz, SK, Fravel, DR and Roberts, DP 1996. In vitro analysis of the role of glucose oxidase from Talaromyces flavus in biocontrol of the plant pathogen Verticillium dahliae. Appl Environ Microbiol. 62:3183-3186.




Um, Y, Kim, BR, Jeong, JJ, Chung, CM and Lee, Y 2014 Identification of endophytic bacteria in Panax ginseng seeds and their potential for plant growth promotion. Korean J Med Crop Sci. 22:306-312 (in Korean).

Warr, SJ, Thompson, K and Kent, M 1992. Antifungal activity in seed coat extracts of woodland plants. Oecologia. 92:296-298.



Won, JY and Jo, JS 1988. Studies on the germination characters of Korean ginseng (Panax ginseng C. A. Meyer) seed. Res Rep Agric Sci Technol. 15:47-68.
Yang, DC, Cheon, SK, Lee, SS, Yang, DC and Kim, HJ 1982. The effects of various dehiscence materials, growth regulators and fungicides on the of ginseng seed (Panax ginseng C A. Meyer). Korean J Ginseng Sci. 6:55-66.
- TOOLS
-
METRICS

- Related articles
-
Seed Transmission of
Tomato yellow leaf curl virus in White Soybean (Glycine max )2017 ;33(4)



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



