 |
 |
| Plant Pathol J > Volume 29(2); 2013 > Article |
Abstract
Acknowledgments
Fig. 1

Fig. 2
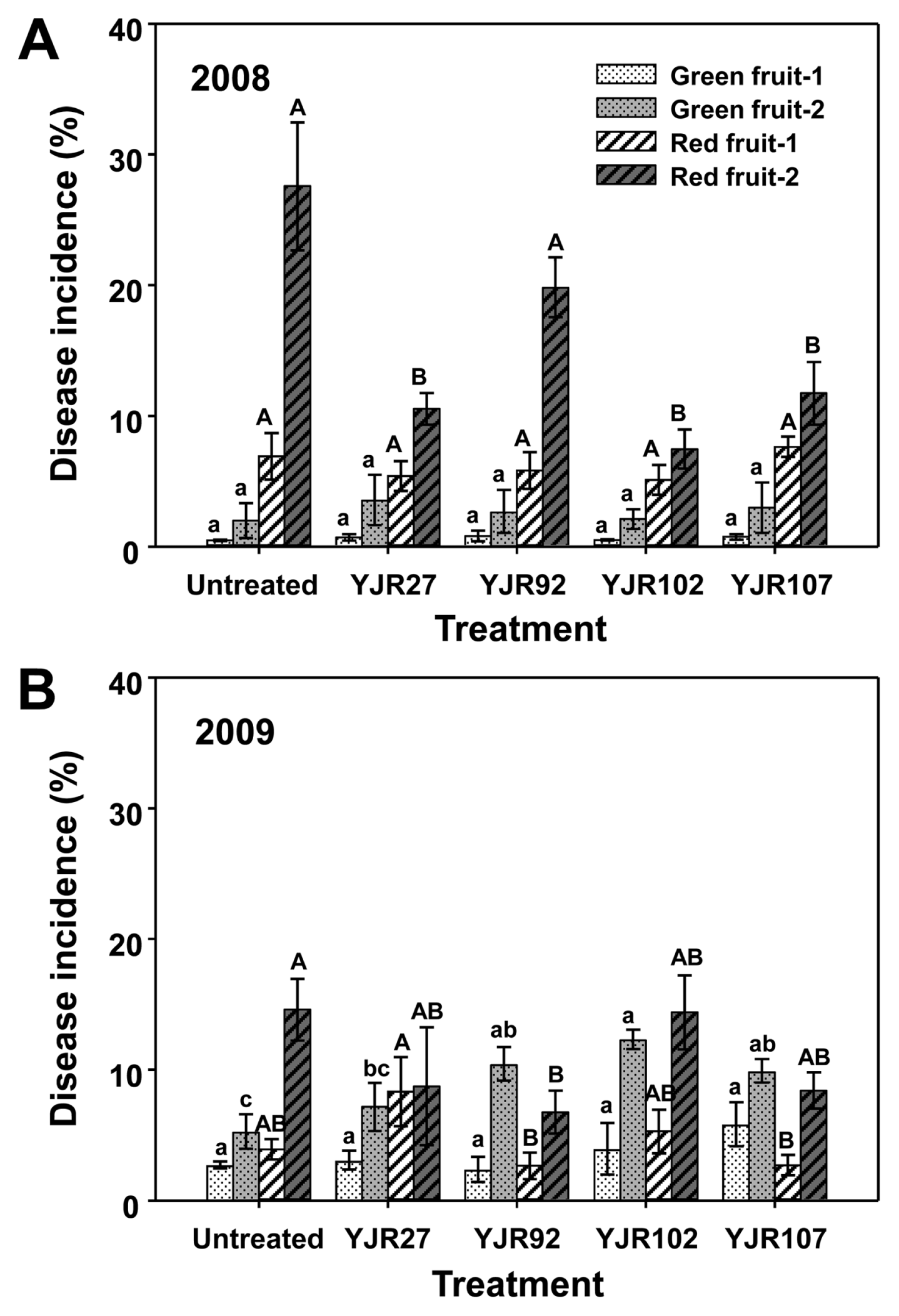
Fig. 3
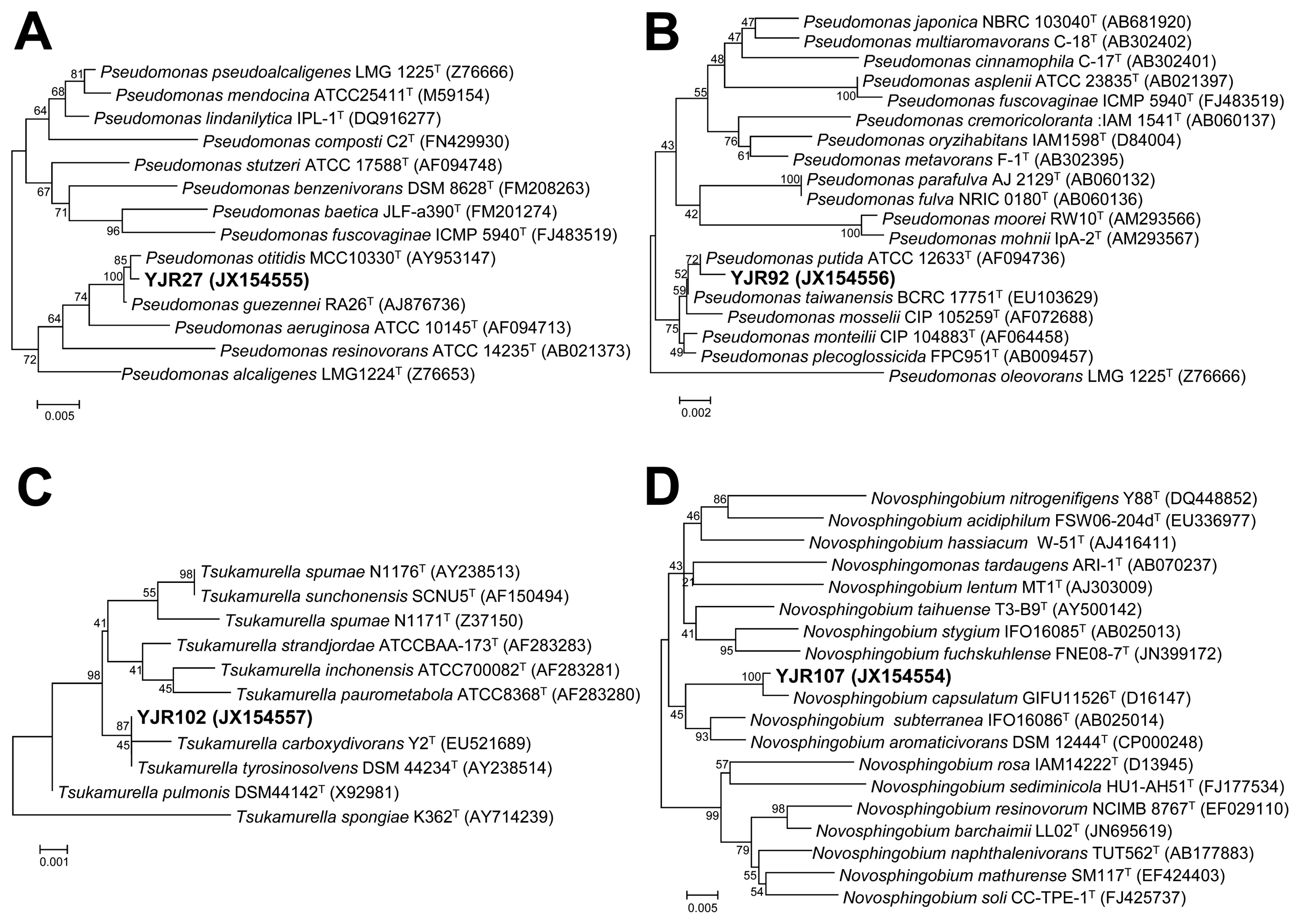
Table 1
| Location | Year | Source | Host | Strain numbers isolated |
|---|---|---|---|---|
| Andong | 2006 | RI, RS, Sa | Cucumber | 61 |
| RI, RS, S | Tomato | 41 | ||
| Youngjongdo | 2004 | RI, RS | Pepper | 137 |
|
|
||||
| Total | 239 | |||
Table 2
| Treatment | Radicle assaya (disease incidence) | Seedling assayb (disease incidence) | In planta trialc (disease severity) |
|---|---|---|---|
| Untreated | 96.67 ± 2.11 ad | 87.50 ± 4.56 a | 3.20 ± 0.20 a |
| YJR03 | 65.83 ± 2.71 b | 58.93 ± 3.39 bc | 2.30 ± 0.19 b |
| YJR07 | 68.33 ± 3.07 b | 50.89 ± 5.25 bc | 2.60 ± 0.20 ab |
| YJR13 | 54.17 ± 3.27 c-e | 43.06 ± 6.43 bc | 2.50 ± 0.24 b |
| YJR27 | 50.00 ± 5.77 e | 40.18 ± 5.70 c | 0.93 ± 0.20 cd |
| YJR59 | 65.00 ± 2.24 bc | 51.19 ± 7.31 bc | 2.47 ± 0.18 b |
| YJR89 | 66.67 ± 2.11 b | 61.01 ± 7.67 b | 2.63 ± 0.22 ab |
| YJR92 | 53.33 ± 4.22 de | 53.87 ± 5.44 bc | 1.00 ± 0.15 cd |
| YJR96 | 63.33 ± 2.11 b-d | 41.96 ± 5.92 bc | 2.43 ± 0.22 b |
| YJR100 | 63.33 ± 3.33 b-d | 52.68 ± 10.74 bc | 2.37 ± 0.19 b |
| YJR102 | 51.67 ± 6.54 e | 58.63 ± 6.77 bc | 0.70 ± 0.16 d |
| YJR107 | 66.67 ± 2.11 b | 50.00 ± 8.54 bc | 1.30 ± 0.23 c |
a In the radicle assay, germinated seeds treated with 10-mM MgSO4 solution (untreated control) or bacterial strains were placed in the margins of P. capsici cultures on water agar (WA) containing 0.02% glucose. Disease incidence (%) of infected radicles was determined 2-3 days after placing the seeds on WA. Values are means of six replicates with 10 seeds each from two experiments.
b Two-week-old pepper seedlings treated with the bacterial strains were inoculated with 2 × 103 zoospores of P. capsici per g dry wt. potting mix. Disease incidence (%) of the seedlings was determined 4 days after inoculation. Values are means of six replicates of eight seedlings each from two experiments.
c Five-week-old pepper plans were inoculated with 25 zoospores per g dry wt. soil. Disease severity was evaluated on a scale of 0 (symptomless) to 5 (plants dead) 17 and 18 days after inoculation in two experiments. Values are means of 30 replicates from two experiments.
d Means ± standard errors within a column followed by the same letter are not significantly different according to the LSD test at P < 0.05. Arcsine square root-transformed data for disease incidence (%) on radicle and seedling assays, and the nonparametric rank test for disease severity in in planta trial were conducted for statistic analysis; however, untransformed data are presented.
Table 3
| Treatmenta | Infected root (%)b | ||
|---|---|---|---|
|
|
|||
| 2007 | 2008 | 2009 | |
| Untreated | 78.17 ± 3.14 ac | 47.33 ± 1.01 a | 68.31 ± 4.70 a |
| Metalaxyl | 33.13 ± 3.94 b | 27.50 ± 10.36 a | -d |
| YJR27 | 31.57 ± 0.95 b | 36.75 ± 5.10 a | 38.93 ± 4.10 b |
| YJR92 | 34.07 ± 3.73 b | 36.17 ± 5.22 a | 27.10 ± 3.81 b |
| YJR102 | 29.53 ± 2.70 b | 34.50 ± 7.82 a | 27.05 ± 3.15 b |
| YJR107 | 28.00 ± 5.05 b | 29.67 ± 7.60 a | 25.67 ± 5.97 b |
a Plant roots were dip-treated with bacterial strains, 10-mM MgSO4 solution (untreated control), or metalaxyl (fungicide control) on 1 June 2007, 16 June 2008, and 14 May 2009. Plant roots were collected on 17 September 2007, 10 September 2008, and 2 September 2009.
b The percentage of infected plant roots was determined on PARPH medium selective for Phytophthora spp.
c Means ± standard errors within a column followed by the same letter are not significantly different according to the LSD test at P < 0.05. Arcsine square root-transformed data were used for statistical analysis; however, untransformed data are presented. These experiments were conducted with three replicates of three plants each in 2007 and four replicates of three plants each in 2008 and 2009.
Table 4
| Treatment a | Total bacteria (log CFU/g dry wt. soil) b | Total fungi (log CFU/g dry wt. soil) | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| 2007 | 2008 | 2009 | 2007 | 2008 | 2009 | |
| Untreated | 6.39 ± 0.02 ac | 7.70 ± 0.11 a | 7.99 ± 0.16 a | 3.11 ± 0.04 a | 4.15 ± 0.04 a | 4.64 ± 0.06 a |
| Metalaxyl | 6.38 ± 0.04 a | 7.72 ± 0.16 a | 7.77 ± 0.12 a | 3.18 ± 0.04 a | 4.18 ± 0.08 a | 4.71 ± 0.17 a |
| YJR27 | 6.32 ± 0.16 a | 7.83 ± 0.06 a | 7.89 ± 0.08 a | 3.22 ± 0.06 a | 4.23 ± 0.05 a | 4.47 ± 0.06 a |
| YJR92 | 6.37 ± 0.10 a | 7.80 ± 0.01 a | 7.98 ± 0.19 a | 3.24 ± 0.03 a | 4.22 ± 0.03 a | 4.42 ± 0.15 a |
| YJR102 | 6.43 ± 0.09 a | 7.67 ± 0.07 a | 7.31 ± 0.50 a | 3.26 ± 0.10 a | 4.18 ± 0.06 a | 4.00 ± 0.14 b |
| YJR107 | 6.29 ± 0.06 a | 7.68 ± 0.06 a | 7.61 ± 0.31 a | 3.14 ± 0.05 a | 4.10 ± 0.07 a | 4.41 ± 0.10 a |
a Plant roots were dip-treated with bacterial strains, 10-mM MgSO4 solution (untreated control), or metalaxyl (fungicide control) on 1 June 2007, 16 June 2008, and 14 May 2009. Plant roots were collected on 17 September 2007, 10 September 2008 and 2 September 2009.
Table 5
| Treatmenta | Number of pepper fruitb | Fresh weight of pepper fruit (kg) | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Pre-inoculation | Post-inoculation | Pre-inoculation | Post-inoculation | |||||
|
|
|
|
|
|||||
| Harvest-1 | Harvest-2 | Harvest-3 | Harvest-4 | Harvest-1 | Harvest-2 | Harvest-3 | Harvest-4 | |
| Untreated | 144 ± 21 ac | 56 ± 6 ab | 57 ± 13 b | 75 ± 8 c | 1.91 ± 0.25 a | 0.41 ± 0.04 ab | 0.93 ± 0.27a-c | 0.53 ± 0.06 b |
| Metalaxyl | 104 ± 12 ab | 86 ± 17 a | 149 ± 9 a | 169 ± 28 a | 1.40 ± 0.19 ab | 0.69 ± 0.15 a | 1.28 ± 0.06 ab | 1.14 ± 0.17 a |
| YJR27 | 93 ± 29 ab | 55 ± 11 ab | 101 ± 3 ab | 164 ± 0 a | 1.37 ± 0.48 ab | 0.42 ± 0.09 ab | 0.75 ± 0.02 bc | 1.15 ± 0.12 a |
| YJR92 | 84 ± 16 ab | 36 ± 12 b | 98 ± 6 ab | 137 ± 20 ab | 1.07 ± 0.24 ab | 0.23 ± 0.07 b | 0.75 ± 0.05 bc | 0.98 ± 0.07 a |
| YJR102 | 92 ± 20 ab | 46 ± 12 b | 154 ± 42 a | 134 ± 2 ab | 1.28 ± 0.36 ab | 0.36 ± 0.11 b | 1.38 ± 0.37 a | 1.13 ± 0.17 a |
| YJR107 | 55 ± 14 b | 30 ± 7 b | 61 ± 17 b | 106 ± 6 bc | 0.71 ± 0.19 b | 0.22 ± 0.07 b | 0.48 ± 0.13 c | 0.76 ± 0.11 ab |
a Plant roots were treated with bacterial strains, 10-mM MgSO4 solution (untreated control), or metalaxyl (fungicide control) on 1 June 2007.
b Numbers and fresh weights (kg) of marketable pepper fruits (> 8 cm long), both red (ripe) and green (unripe), were evaluated on August 6 and 13 as well as September 8 and 22 in 2007. After the second harvest, 1 × 105 zoospores of P. capsici were inoculated to the soil around each plant on 13 August 2007.
Table 6
| Treatmenta | Numbers of pepper fruitb | Fresh weight of pepper fruit (kg) | ||
|---|---|---|---|---|
|
|
|
|||
| Harvest-1 | Harvest-2 | Harvest-1 | Harvest-2 | |
| Untreated | 229 ± 31 ac | 215 ± 24 a | 3.10 ± 0.46 ab | 1.74 ± 0.25 a |
| Metalaxyl | 177 ± 29 a | 201 ± 52 a | 2.32 ± 0.36 ab | 1.57 ± 0.39 a |
| YJR27 | 171 ± 29 a | 163 ± 15 a | 2.32 ± 0.47 ab | 1.19 ± 0.13 a |
| YJR92 | 211 ± 27 a | 180 ± 29 a | 2.93 ± 0.42 ab | 1.40 ± 0.23 a |
| YJR102 | 164 ± 28 a | 231 ± 32 a | 2.18 ± 0.41 b | 1.59 ± 0.27 a |
| YJR107 | 242 ± 18 a | 203 ± 22 a | 3.48 ± 0.22 a | 1.61 ± 0.18 a |
a Plant roots were dip-treated with bacterial strains, 10-mM MgSO4 solution (untreated control), or metalaxyl (fungicide control) on 16 June 2008.
Table 7
| Year, treatment a | Number of pepper fruitb | Fresh weight of red pepper fruit (kg)c | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Green fruit | Red fruit | |||||
|
|
|
|
||||
| Harvest-1 | Harvest-2 | Harvest-1 | Harvest-2 | Harvest-1 | Harvest-2 | |
| 2008 | ||||||
| Untreated | 195 ± 19 a d | 245 ± 10 a | 243 ± 15 a | 201 ± 16 b | 4.08 ± 0.20 b | 3.96 ± 0.20 a |
| YJR27 | 206 ± 11 a | 239 ± 24 a | 213 ± 27 a | 230 ± 11 ab | 4.92 ± 0.30 a | 3.90 ± 0.46 a |
| YJR92 | 213 ± 20 a | 274 ± 17 a | 237 ± 26 a | 226 ± 18 ab | 5.24 ± 0.27 a | 3.70 ± 0.34 a |
| YJR102 | 201 ± 22 a | 264 ± 25 a | 221 ± 22 a | 261 ± 17 a | 4.62 ± 0.33 ab | 4.02 ± 0.42 a |
| YJR107 | 191 ± 10 a | 287 ± 24 a | 239 ± 17 a | 231 ± 22 ab | 4.48 ± 0.17 ab | 3.52 ± 0.37 a |
| 2009 | ||||||
| Untreated | 204 ± 21 a | 86 ± 10 a | 260 ± 20 a | 160 ± 19 a | 2.70 ± 0.06 b | 2.10 ± 0.42 a |
| YJR27 | 214 ± 31 a | 122 ± 31 a | 252 ± 11 a | 208 ± 44 a | 4.00 ± 0.37 a | 3.50 ± 0.39 a |
| YJR92 | 243 ± 63 a | 125 ± 23 a | 250 ± 15 a | 264 ± 61 a | 4.03 ± 0.53 a | 3.48 ± 0.66 a |
| YJR102 | 225 ± 28 a | 149 ± 30 a | 261 ± 12 a | 219 ± 33 a | 4.38 ± 0.45 a | 3.38 ± 0.09 a |
| YJR107 | 207 ± 44 a | 119 ± 18 a | 220 ± 33 a | 188 ± 49 a | 3.90 ± 0.34 ab | 2.90 ± 0.50 a |
a Plant seedlings were treated with bacterial strains, 10 mM MgSO4 solution (untreated control), or metalaxyl (fungicide control) on 19 April 2008 and 18 April 2009. These seedlings were transplanted into the beds on 22 April 2008 and 20 April 2009.
b The numbers of marketable green and red fruit (> 8 cm long) were evaluated on August 2 and 25 in 2008 and on July 29 and August 19 in 2009.
References
- TOOLS
-
METRICS

-
- 29 Crossref
- 0 Scopus
- 4,515 View
- 44 Download
- Related articles



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



