|
 |
| Plant Pathol J > Volume 30(4); 2014 > Article |
Abstract
A fungus Pyrenophora tritici-repentis induces tan spot of wheat which is a foliar disease that causes yield loss to wheat crops worldwide. In this study, a new, simple and non-costly technique was performed to produce the sexual stage of this fungus in culture, within 9 weeks using wheat straw. This protocol will be helpful to researchers studying the biology of sexual stage development, disease epidemiology and genetics of this fungus.
Tan spot, caused by Pyrenophora tritici-repentis (Died.) Drechsler (Ascomycete; anamorph: Drechslera tritici-repentis (Died.) Shoemaker), is a damaging foliar disease of both bread wheat (Triticum aestivum L.) and durum wheat (T. turgidum L. var. durum) (Fig. 1). The life cycle of this pathogen involves two forms, ascospores produced during the sexual stage and conidia produced during the asexual stage (Schilder and Bergstrom, 1992). The ascospores (primary inoculum) are released from pseudothecia, which develop on crop residues, while conidia are produced on the infected plants during the growing season (Hosford, 1971). Researchers need a simple and efficient method to produce ascospores in the laboratory, because genetic variability in the fungal pathogens occurs generally through their sexual stage, and understanding the genetic mechanism of the pathogen is required to undertake any management strategies for disease. The in vitro production of ascospores on green or senescent maize leaves, described by Friesen et al. (2003), is an efficient technique, but it can be limiting for researchers because the use of maize leaves in the method could add much more labor to grow corn in the greenhouse; mainly where it is not available in the field. To address this disadvantage we were especially interested in producing pesudothecia and ascospores in vitro on wheat straw.
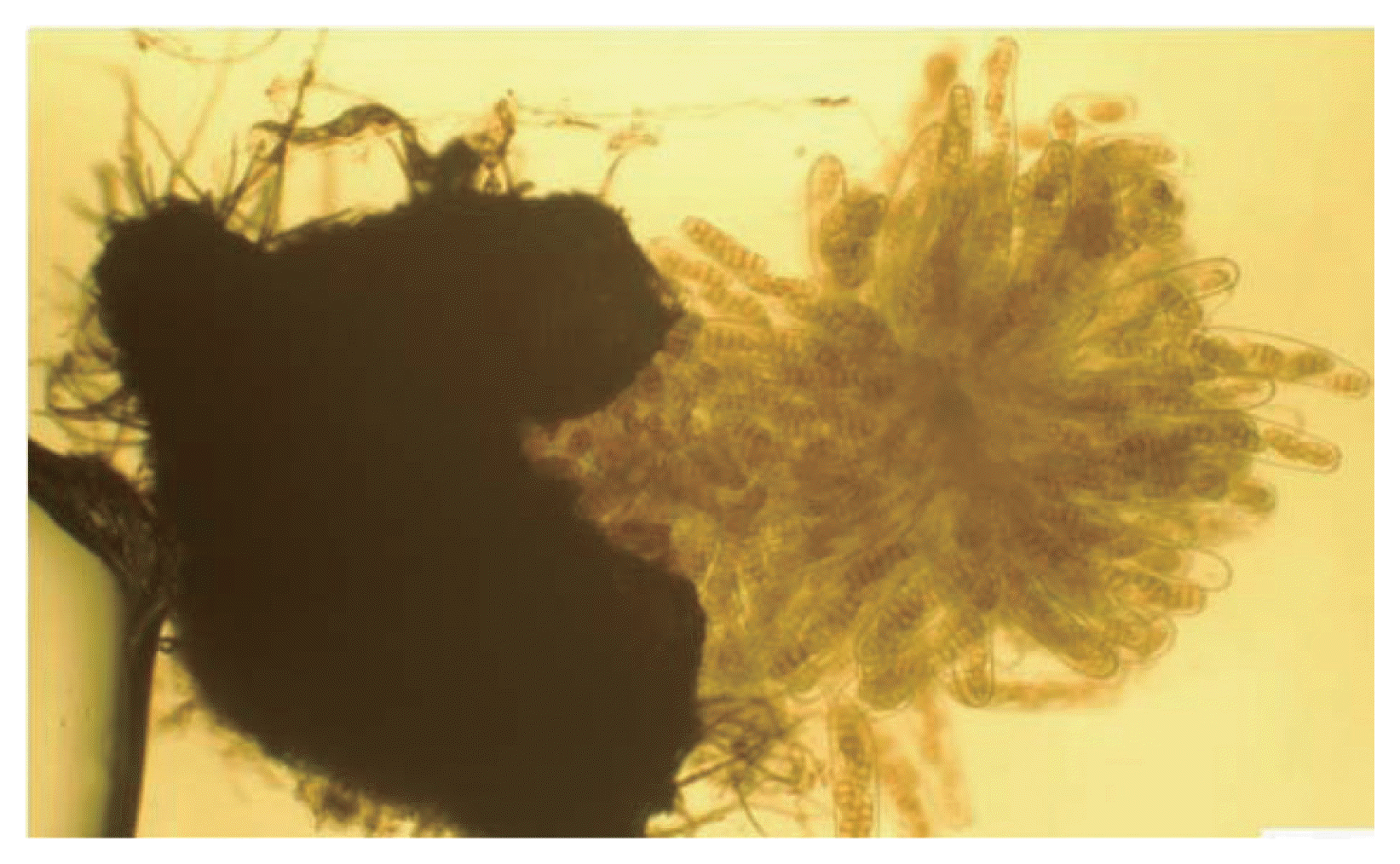
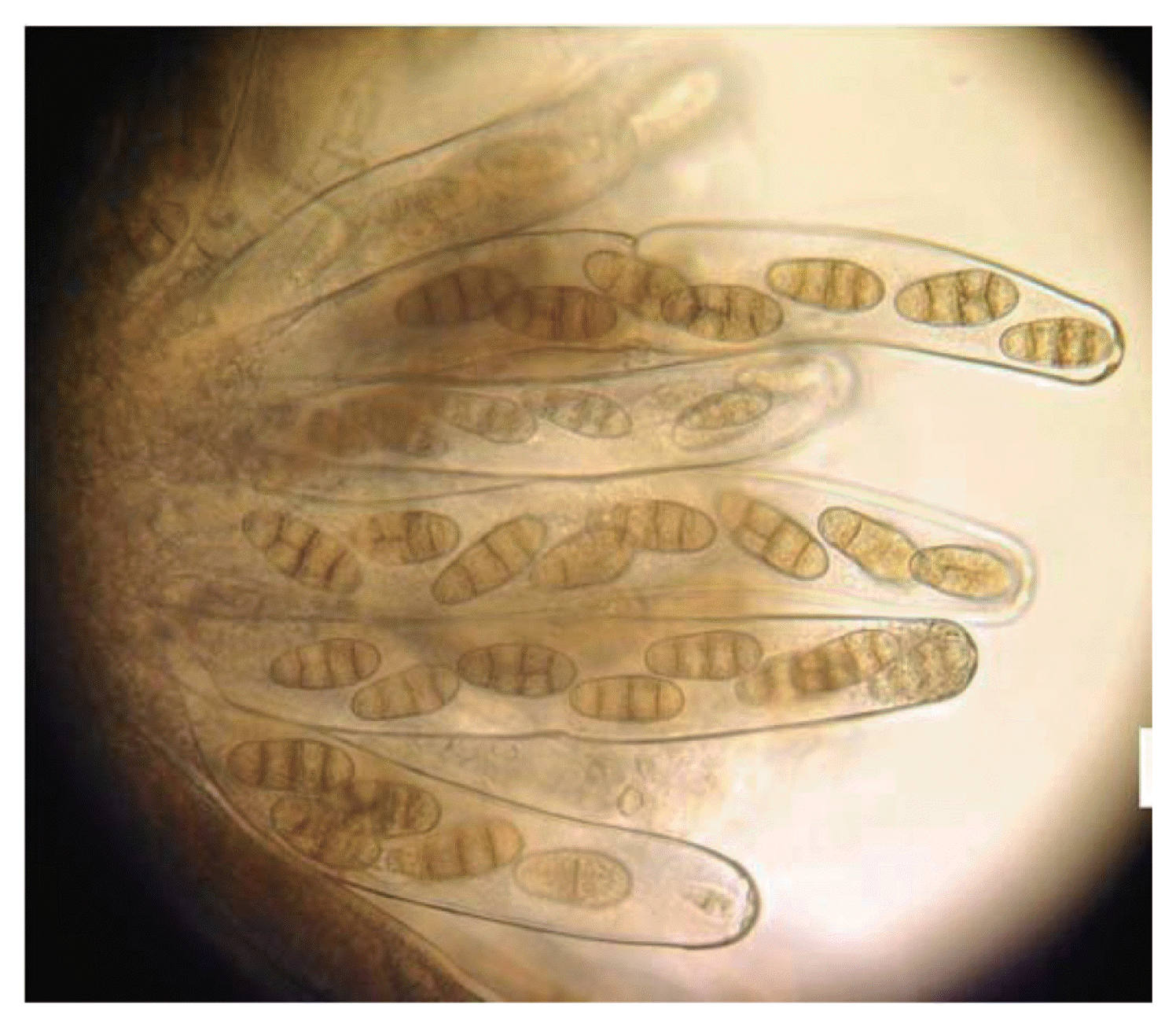
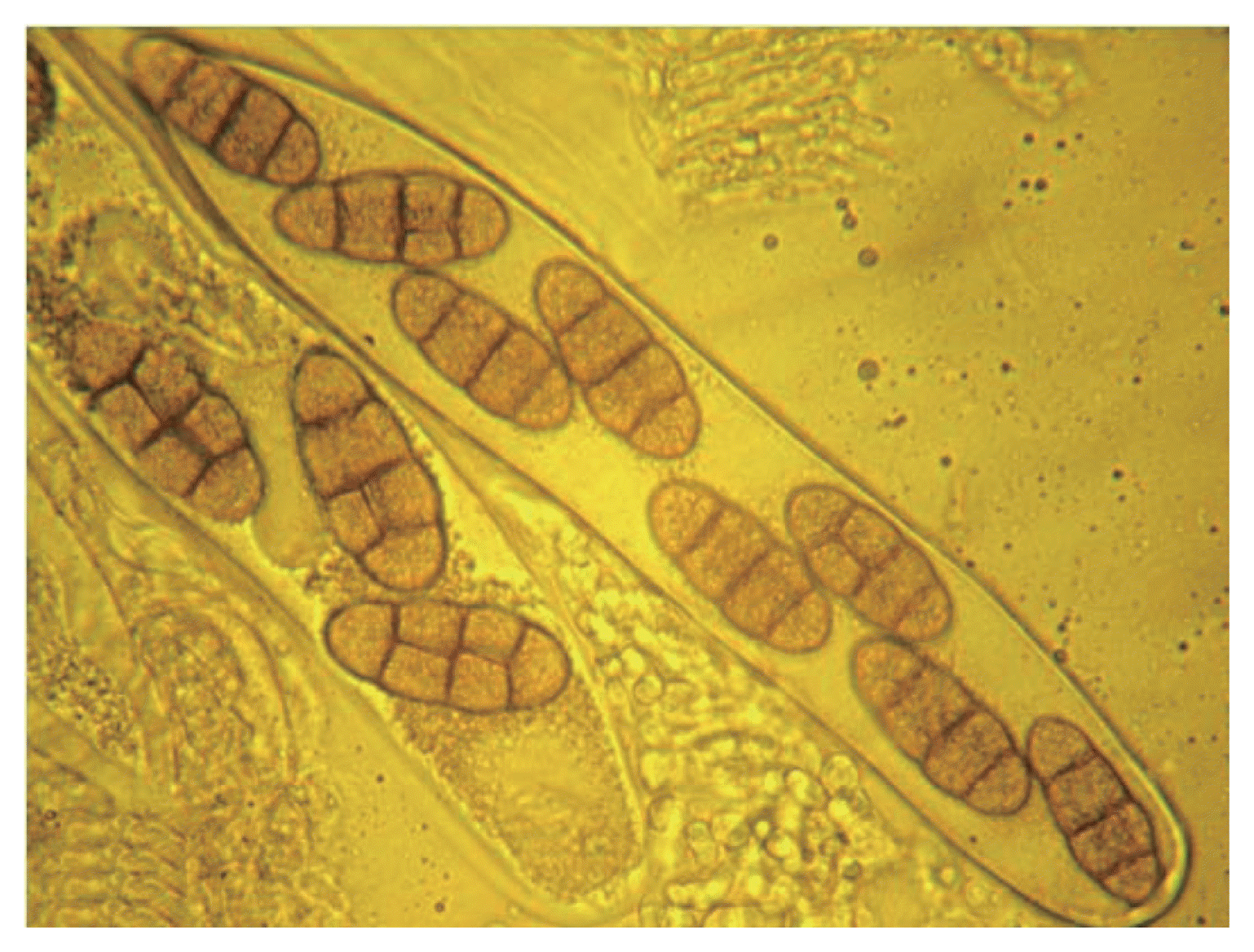
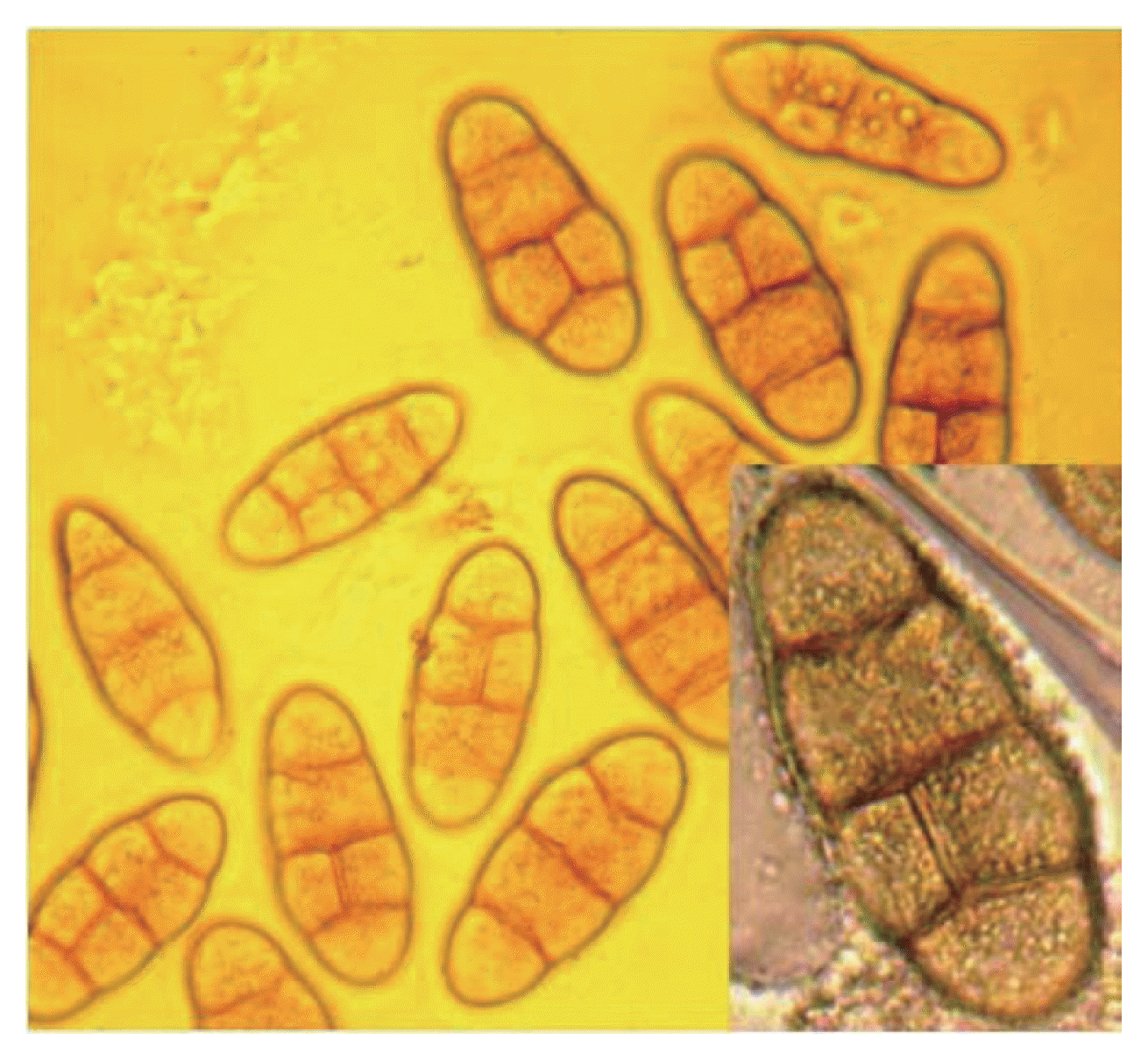
Sexual stage of P. tritici-repentis was produced in laboratory using two isolates; Ptr65 and Ptr68. Straw segments (4 cm) were sterilized, immersed in a spore suspension (1,350 spores/ml) and placed in water agar plates (2%). Dishes were incubated at 18°C in the darkness for 2 weeks, and then at 4°C in continuous light for seven weeks. The sub-epidermal pseudothecia were observed on the straw fragments to the naked eye and under microscope. They were black, with a spherical shape and with short spines surrounding short beaks (Fig. 2). Asci were bitunicate and narrow at the base, each ascus contained eight ascospores (Figs. 3, 4, 5). They were transversely three septate with one or two longitudinal septa in one of the central cell (Fig. 6).
To confirm the identity of P. tritci-repentis telomorph, the diameter of 50 pseudothecia, the length of 50 asci and the length, width, and septa numbers of 50 ascospores were measured. Our measurements compared to those of other authors, are summarized in Table 1; they show close coherence with the description given by Wiese (1987) and Zillinski (1983).
The rational for this method is based on three considerations: in nature, pseudothecia mostly occur on wheat straw (Rees and Platz, 1980), availability of wheat straw in all tan spot areas and water agar is a simple and inexpensive medium. In this method water Agar is used as a medium to provide enough moisture needed for Pseudothecia development. Summerell and Burgess (1988) indicated that initiation and maturation of Pseudothecia in the field would require a period of moist conditions for optimal production of ascospores. A Petri dish with water agar serves as an excellent moist chamber to the plated straw segments. The agar does not dry out as quickly as filter paper used in previously described protocol and provides even moisture. According to previous studies, initiation and maturation of Pseudothecia in the field would require a moderate temperature (15°C-20°C). Frisen et al. (2003) observed higher pseudothecial densities in the dark; they supposed that pseudothecial development may be inhibited by light. Therefore, the first part of our experiment was conducted with incubations at 18°C under darkness and the second part under continuous light to induce ascus neck formation, because phototropism gives ascospores a better chance of being discharged into open air as reported by Ingold (1971). Positive phototropism during ascus development has also been reported in other fungi including Aleuria vesiculosa, Ascobolus magnificus, Podospora curvula, and Sordaria spp. (Buller, 1934; Ingold, 1971).
This easy and rapid protocol of P. tritici-repentis telomorph development would help researchers in carrying a biochemical and molecular genetic analysis of teleomorph developmental biology, production of ascospores inoculums, and other aspects of tan spot-wheat system.
Acknowledgments
This research has been supported by the Phytopathology Laboratory (Department of plant sciences) of the University of Manitoba, Canada, and the Algerian Ministry of High Education and Scientific Research. We thank Dr. S. Ali (South Dakala State University, USA) for his comments and retical reading of the paper.
Table 1
Pseudothecia, asci and ascospores characteristics of the two isolates of Pyrenophora tritici-repentis, as compared with sizes of P. tritici-repentis reported in the literature
| Character | Results from this paper (μm) | Wiese, 1987 Max-Min (μm) | Zillinsky, 1983 | |||
|---|---|---|---|---|---|---|
|
|
||||||
| Ptr65 | Ptr68 | |||||
|
|
|
|||||
| Max-Min | Avg | Max-Min | Avg | |||
| Pseudothecia | 238-365 | 340 | 210-344 | 310 | 200-350 | n.r. |
| Ascus | ||||||
| Length | 195-220 | 212 | 200-219 | 202 | n.r; | n.r. |
| Ascospore | ||||||
| Length | 48-58.5 | 53.79 | 42-54.6 | 49.16 | 40-70 | 40-60 |
| Width | 18-24 | 21.12 | 18-22.5 | 19.68 | 18-25 | 18-25 |
References
Buller, AHR 1909-1950. Researches on fungi. 6: Longman, Green and Co, London.
Friesen, TL, Ali, S, Stack, RW, Francl, LJ and Rasmussen, JB 2003. Rapid and efficient production of the Pyrenophora tritici-repentis teleomorph. Can J Bot. 81:890-895.

Hosford, RM Jr 1971. A form of Pyrenophora trichostoma pathogenic to wheat and other grasses. Phytopathology. 61:28-32.

Ingold, CT 1971. Fungal spores. Oxford University Press, London.
Rees, RG and Platz, GJ 1980. The epidemiology of yellow spot of wheat in southern Queensland. Aust J Agr Res. 31:259-267.

Schilder, AM and Bergstrom, GC 1992. In: The dispersal of conidia and ascospores of Pyrenophora tritici-repentis, eds. by In : LJ Francl, JM Krupinsky and MP McMullen, In: Proceedings of 2nd Tan Spot Workshop; 96-99. Fargo.
Summerell, BA and Burgess, LW 1988. Factors influencing production of pseudothecia by Pyrenophora tritici-repentis. Trans. Br. Mycol. Soc. 90:557-562.

Wiese, MV 1987. Compendium of Wheat Disease. 2nd Ed. APS Press, The American Phytopathological Society, St. Paul, MN, USA.
Zillinsky, FJ 1983. Common Diseases of Small Grain Cereals: A Guide to Identification. Centro International de Mejoramiento de Maiz y Trigo (CIMMYT), Mexico, D.F., Mexico.
- TOOLS
-
METRICS

-
- 1 Crossref
- 0 Scopus
- 2,842 View
- 26 Download
- Related articles





 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print