Most postharvest losses caused by fungal pathogens occur as a result of mechanical damage during storage or transportation. Postharvest diseases caused by fungi are widespread diseases of ornamental crops, flowers, and vegetables. It is estimated that about 25% of the fruits and vegetables are rotten by postharvest diseases (Singh and Sharma, 2007). Control of postharvest diseases has applied synthetic chemical fungicides either as pre-harvest sprays in the greenhouse or as postharvest dips of products (Gachanggo et al., 2012). However, continued use of chemical fungicides is prohibited because of increased global demand for chemical-free fresh produce, development of fungal resistance, and increased costs of synthetic fungicides (De Costa and Gunawardhana, 2012; Korsten, 2006).
Ionizing radiation is a viable alternative and an effective nonchemical treatment for control of postharvest disease (Hallman, 2011). Ionizing radiation is radiation with sufficient energy that, during an interaction with an atom, causes atoms and molecules to become ionized or excited, thereby producing free radicals that break chemical bond, and damaged molecules involved in the cell process without leave any residue, which gives rise to chemical and metabolic or physiological changes in fungal pathogens. Currently, only three sources of ionizing radiation, i.e., gamma-rays driven by the isotopes cobalt-60 or cesium-137; X-rays; and electron-beams (e-beams) generated by a machine source, are commercially used for the control of pests. Although gamma-rays, e-beams, or X-rays are produced from different sources, they have the same mode of action. The unit of irradiation dose is the gray (Gy), which is the energy absorbed in J kg-1 of material. Gamma irradiation has been shown to successfully inactivate fungi from different materials, such as stored seeds, paper, wood, and soil (De Silva et al., 2006). It has been reported in many studies that the sensitivity of storage pathogenic fungi was evaluated by gamma irradiation (Geweely and Nawar, 2006; Maity et al., 2011). However, very limited in vitro studies have been conducted to determine the dose of irradiation to reduce the fungal survival and germination to gamma-rays, X-rays, and e-beams. This study aimed at evaluating the dose-range of irradiations required to achieve D10 values, i.e., the dose required for reducing the given population by 90% of its initial value, on postharvest fungal pathogens, spore germination, and the development of fungal growth, and for providing information on the response of some physiological properties of fungi to irradiation.
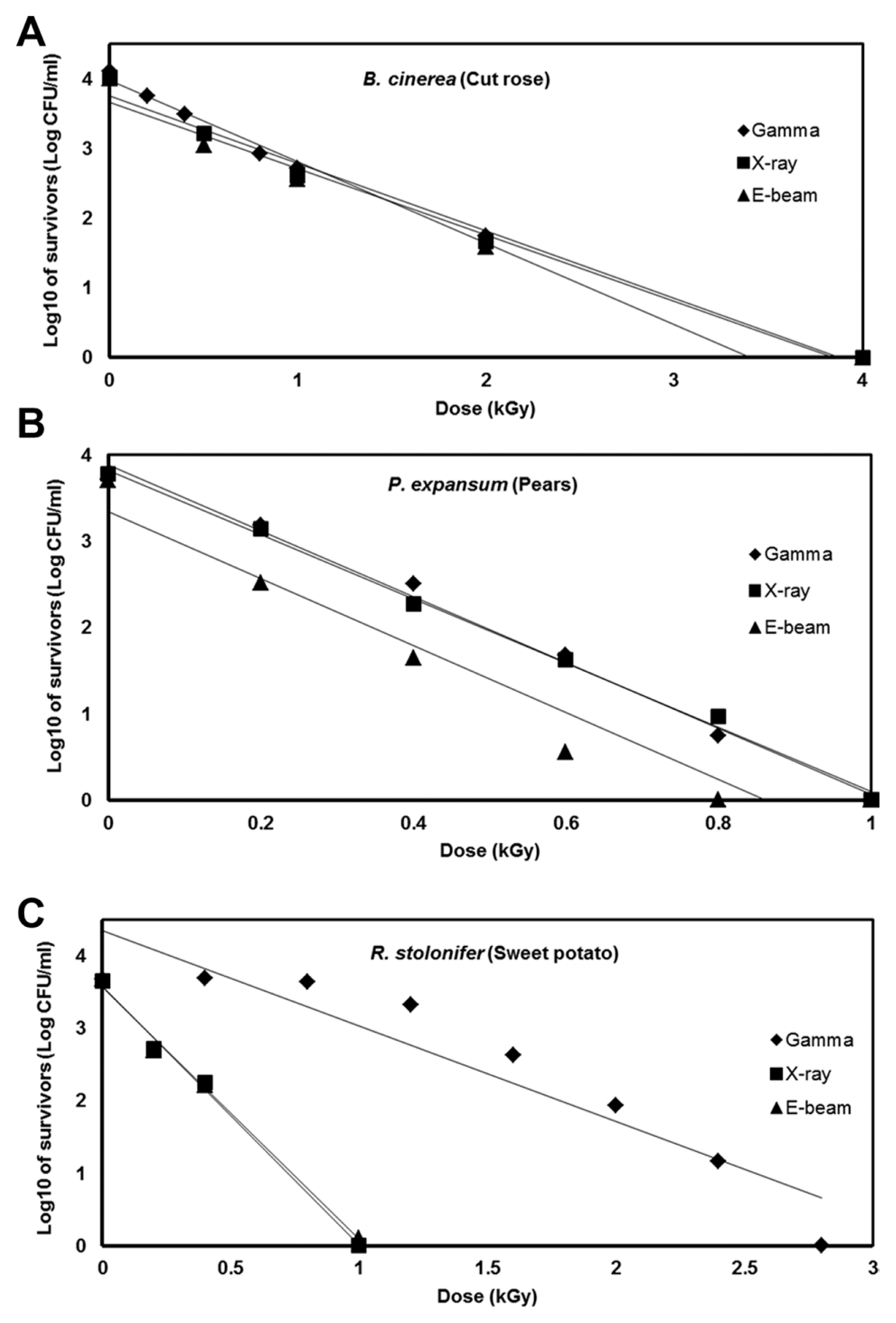
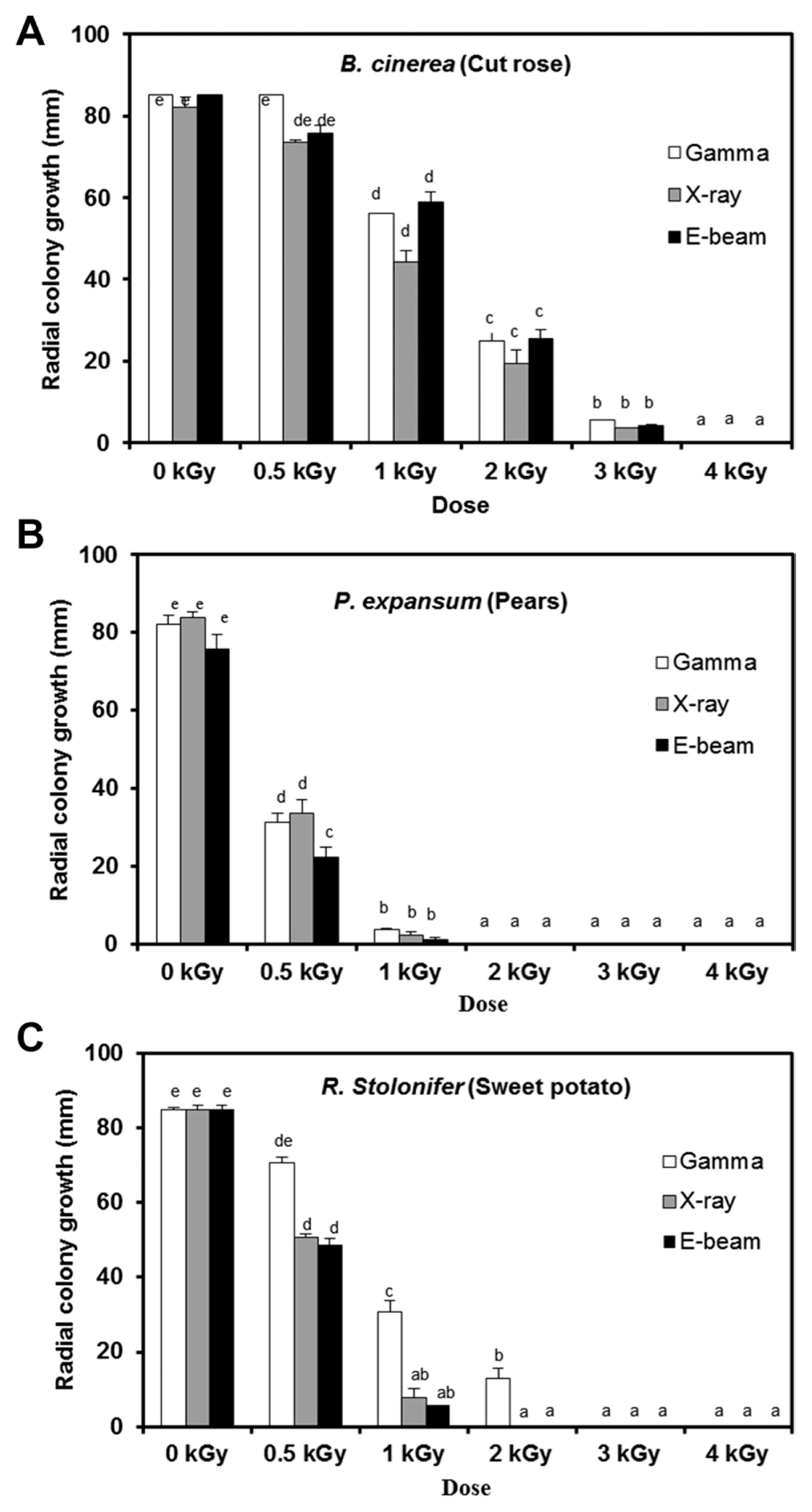
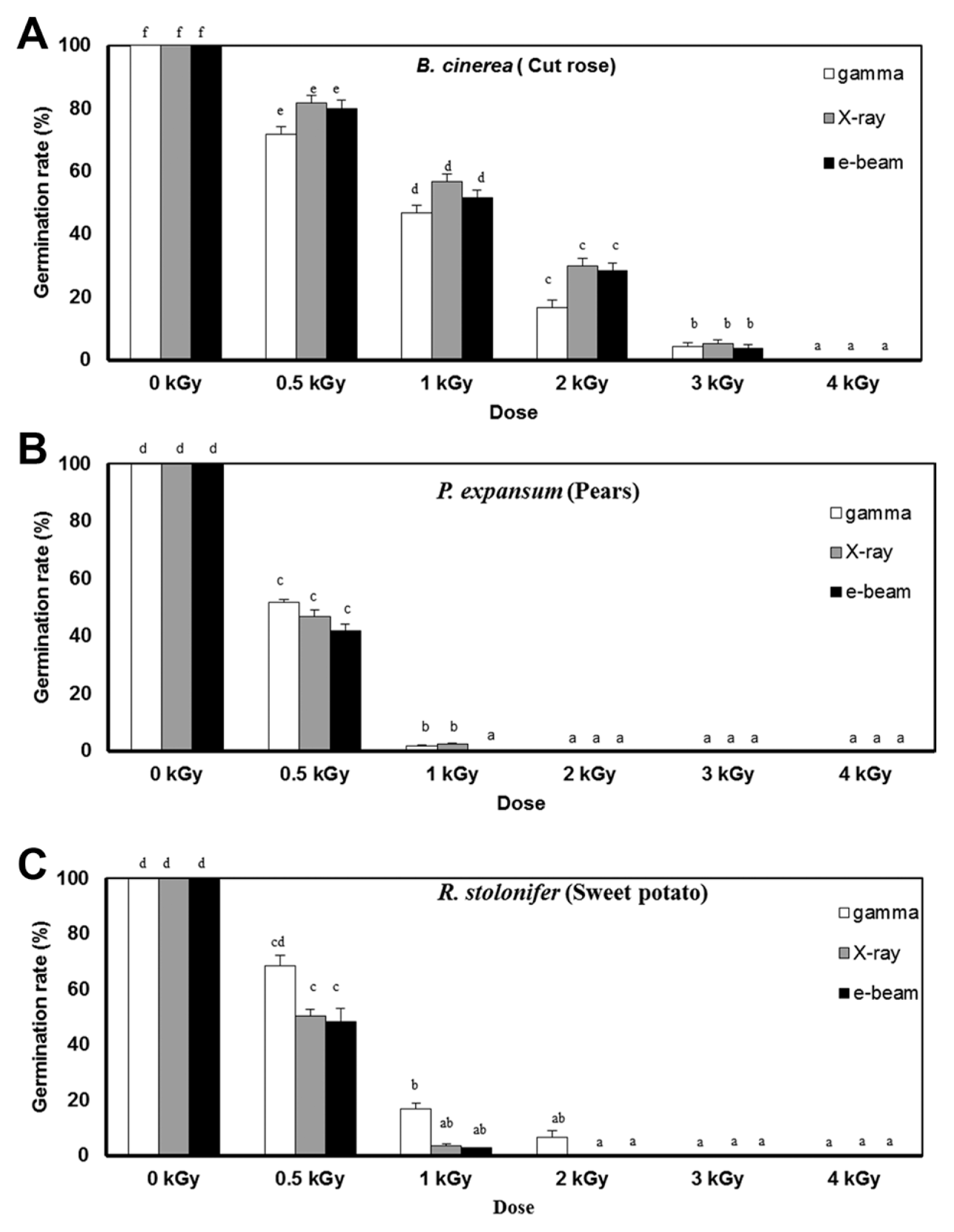
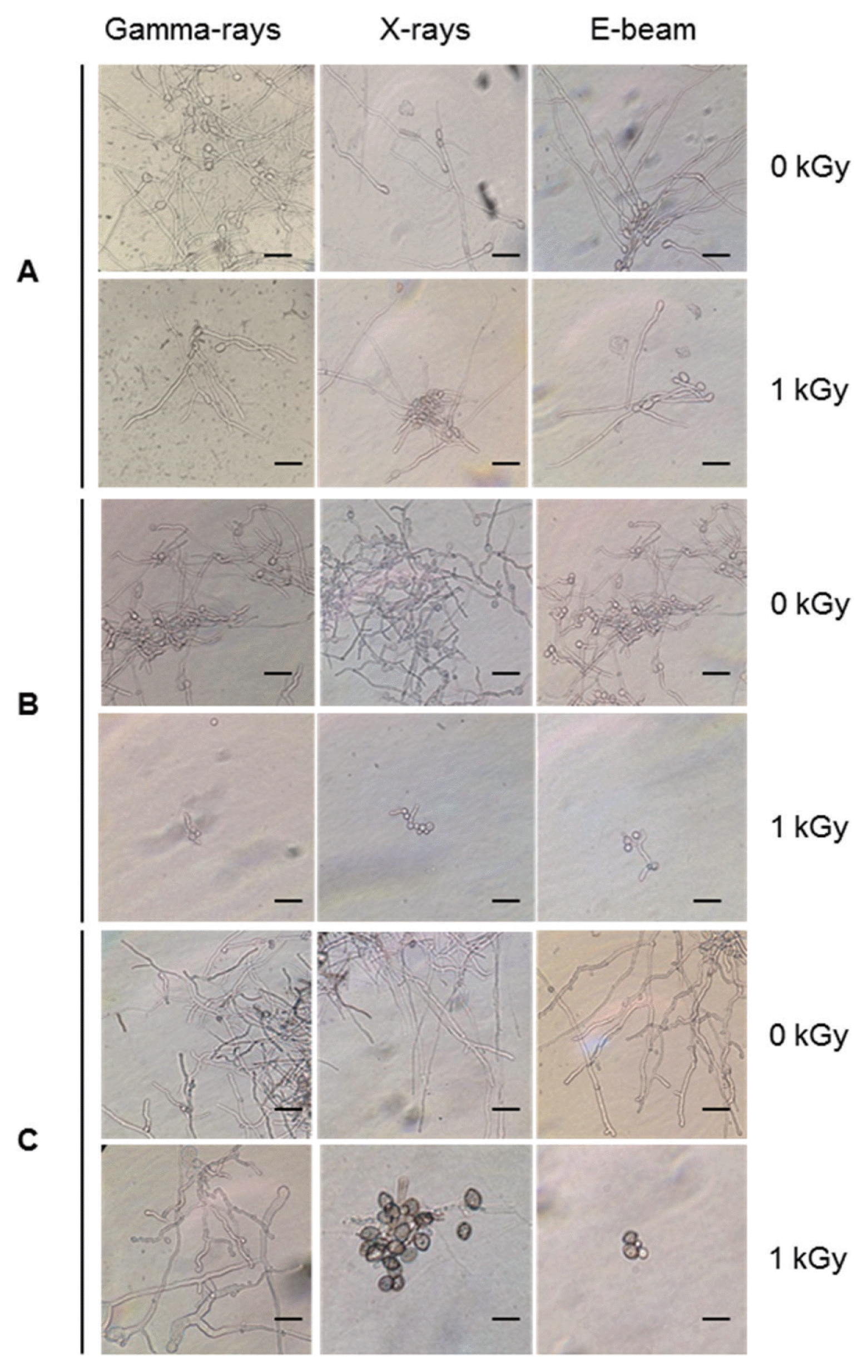
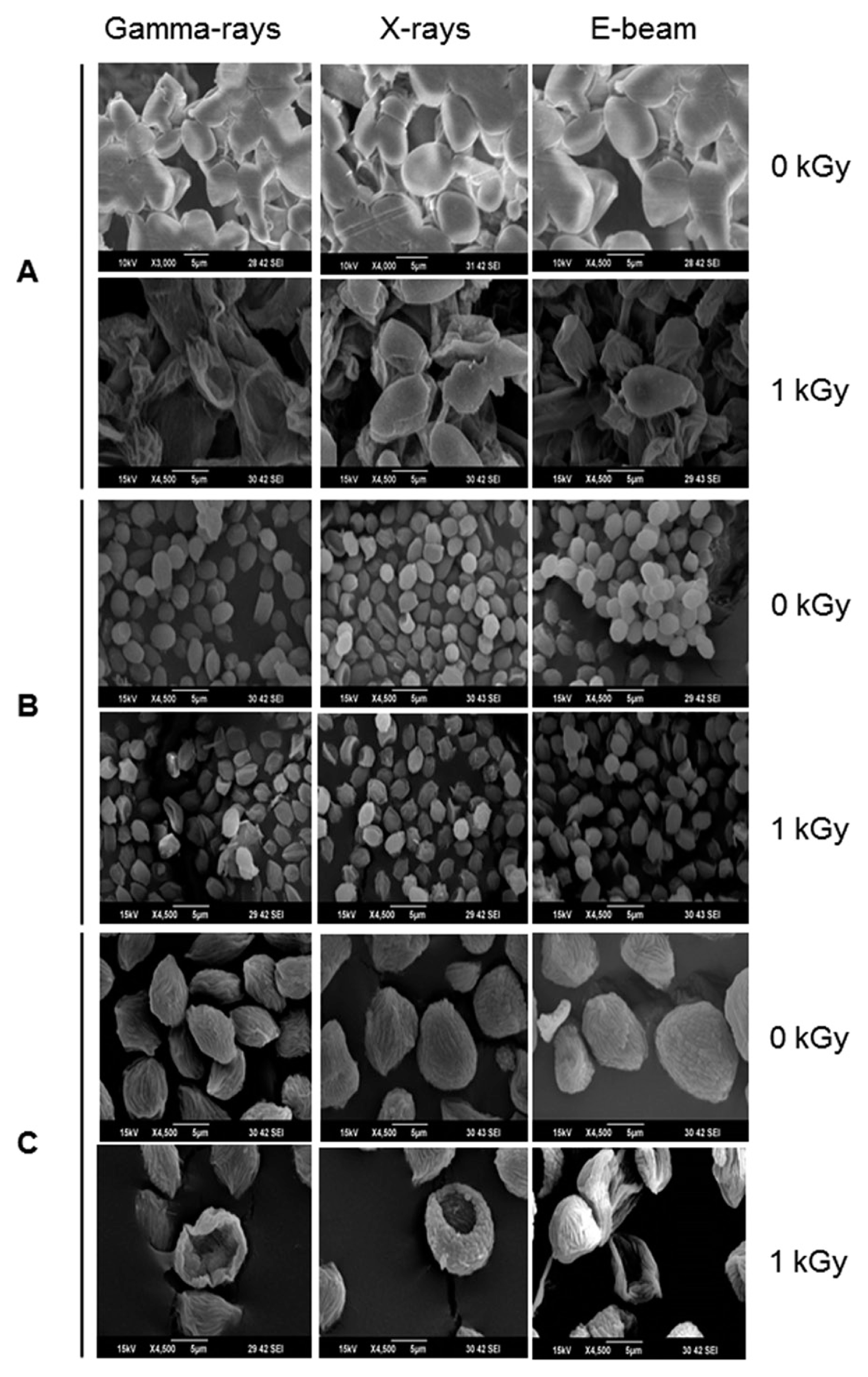
Postharvest fungal pathogens, Botrytis cinerea, Penicillium expansum, and Rhizopus stolonifer, were obtained from Korean Agricultural Culture Collection (KACC). For a pure culture, isolated individual conidia were transferred to potato dextrose agar (PDA, Difo Laboratories, Detroit, MI). Prior to each experiment, all cultures were grown on PDA at 25┬░C for 14 days. For fungal identification, the genomic DNA was isolated from the fungus using a DNA extraction kit (Bioneer, Korea) according to the manufacturerŌĆÖs manual. The extracted DNA was used as a template to amplify 18s rRNA genes using the primers ITS1 and ITS4 (White et al., 1990). PCR products were sequenced, and a blast search was requested from a commercial analysis service (Solgent Inc., Daejeon, Korea). The accession numbers were listed in Table 1. To examine the effect of irradiations on fungal viability, fungal spores were exposed to gamma-rays, X-rays, and e-beams. A cobalt-60 gamma irradiator at the Korea Atomic Energy Research Institute, Jeongeup, Korea (150 TBq capacity; ACEL, MDS Nordion, Canada) was used for the irradiation. The viability of irradiated and non-irradiated spores was determined by a dilution plate method in three subsets (up to 10ŌłÆ4 or 10ŌłÆ5 dilution with sterile distilled water) for 72 h in PDA media (Lacey et al., 1980). Survival curves were constructed by plotting the survivor CFU/ml versus the actual radiation dose. The curves were fitted through a linear regression. Radiation sensitivity was measured using the D10 value determined from the reciprocal of the slope for the straight-line portion of the survival. Data were subjected to analyses of variance (ANOVA) at p<0.05 using SPSS 13.0 software to evaluate significant differences among irradiation doses analyzed (SPSS Inc., USA), and DuncanŌĆÖs multiple range tests were used to compare the differences among the mean values. The sensitivity for B. cinerea, P. expansum, and R. stolonifer to different types of irradiation are shown in Fig. 1. The viability results for fungi after irradiation treatments revealed that each fungi show different viability to irradiation. In the case of B. cinerea, it shows similar sensitivity to gamma-rays, X-rays, and e-beams (p<0.05). However, P. expansum exhibited significantly (p<0.05) higher sensitivity to e-beams than to gamma-rays and X-rays. The D10 values for the fungal pathogens were determined from the slopes of the survivor curves, and are summarized in Table 1. For the B. cinerea and P. expansum, the D10 values were similar to the irradiations, but in the case of R. stolonifer, the D10 values to gamma rays were about 2-times greater than the X-rays and e-beam. In our study, the D10 values of the selected fungi were in the order of B. cinerea > R. stolonifer > P. expansum. The higher the D10 value, the more resistant the fungus is to radiation. The D10 values varied from 0.257 kGy to 1.049 kGy, indicating the existence of a spectrum of radioresistance in the plant fungal pathogens. For example, Alternaria alternate showed high D10 value (about 1.049 kGy), whereas Aspergillus flavus showed low D10 value (about 0.25 kGy). The radioresistance is occurred because of chromosome number, thickness of cell wall, and melanin pigment. In addition, there is no any difference in the same species (Saleh et al., 1988). In this way, several studies have shown that plant pathogenic fungi show different radiosensitivity to radiations (Saleh et al., 1988; Tiryaki, 1990). To examine major changes in fungal morphology including the diameter of colonies and germination rate when exposed to different irradiations, agar discs (5 mm in diameter) were isolated from a solid culture of irradiated B. cinerea, P. expansum, and R. stolonifer, and each agar disc was placed on a PDA medium. Agar discs of irradiated fungal pathogens were inoculated in a petridish (90 mm in diameter). After incubation at 25┬░C in the dark for three days, the mycelial growth of fungal pathogens was measured as the average colony radius (Fig. 2). The colony diameter of B. cinerea and R. stolonifer manifested a significant (p<0.05) decrease at 1 kGy and 2 kGy absorbed dose in irradiation after three days. In the case of P. expansum, the colony diameter was significantly decreased at irradiation levels of 0 kGy and 0.5 kGy. The growth parameter gradually decreased to reach a minimum at 3 kGy, and 4 kGy was the lethal dose to three irradiation levels in B. cinerea. On the other hand, 2 kGy was the lethal dose of irradiation to P. expansum and R. stolonifer. The obtained lethal dose values were in accordance with the D10 values. There was no specific trend in the radioresistance of tested fungi toward different types of irradiation. The difference in the response of the tested fungal species to irradiation may be associated with certain factors such as the presence of mycelial water content as a natural radioprotector (EL-Sherbeny, 1982), the ability to repair DNA breaks (El-Bazza et al., 2001), the induction of certain enzymes associated with the recovery of radiation damage, and chemical substances suppressing the process of growth (Nahed, 1999). For further study on the inhibition of growth to irradiation, spore germination was undertaken in tested fungi to gamma-rays, X-rays, and e-beams. Spores were treated with irradiation and then incubated for the indicated times at 25┬░C. Under a microscope (40├Ś), for every zone selected, germinated and non-germinated spores were counted (Eclipse Ni, Nikon, Japan). Ten representative zones were selected at random. The sum of the germinated spores over the total spores present determined the percentage of germination for B. cinera, P. expansum, and R. stolonifer at the time of the sampling. Sampling was carried out in triplicate. The percentages of spore germination of the fungal pathogens decreased with a further increase in the radiation dose, and were completely inhibited at 4 kGy for B. cinerea (p<0.05) and at 2 kGy for P. expansum (p<0.05). In the case of R. stolonifer (p<0.05), spore germination to X-rays and e-beams was completely inhibited at 2 kGy, while completely inhibited at 3 kGy to gamma-rays (Figs. 3 and 4). The pattern of fungal responses to irradiation shows a different spore germination enhancement. It is possible that the difference in sensitivity to different types of irradiation may be linked to the pigmentation and cell wall. B. cinerea with a lighter pigmentation and thin cell walls are more sensitive to gamma-rays than the dark walled Alternaria tenuissima (Geweely and Nawar, 2006). In addition, gamma irradiation affected the membrane integrity and cellular leakage of conidia in a dose-dependent manner. Furthermore, the leakage of protein and sugar from mycelia increased with the dose. The mechanisms by which gamma irradiation showed antifungal activity of some fungal pathogens can be directly associated with the disruption of cell membrane of the fungal pathogen, resulting in a loss of cytoplasmic materials from the hyphae (Unpublished data). Morphological changes of fungal spores after irradiation were examined by analyzing the scanning electron microscope (SEM) images. For an SEM analysis, dehydrated spores were dried by incubating in hexamethyldisilazane (HMDS) for 15 min twice, mounted on carbon tape, coated with platinum, and then examined under a scanning electron microscope (SEM) (JEOL, Tokyo, Japan). SEM was employed with spores with irradiation at dose of 1.0 kGy, lethal dose of P. expansum and R. stolonifer, to compare morphological changes after irradiation. When spores were irradiated at 1.0 kGy, structural changes were much different compared to spores irradiated at 0 kGy. The treated spores were crushed and exhibited a high degree of hollowness on the spore surface in P. expansum and R. stolonifer, whereas in case of the B. cinerea, the spores were not much crushed because of its radioresistance (Fig. 5). These results suggest that the radiosensitivity of the fungi was in the order of B. cinerea < R. stolonifer < P. expansum, and at the P<0.05 level, the means are significantly different.
Taken together, the in vitro studies suggest that the lethal dose of the three tested fungal pathogens to irradiation was within 2-4 kGy. These doses greatly affected the essential metabolic process. The radiosensitivity of spores to different types of irradiation varies according to the species, which indicate that differences in fungi radiation response reflect species-specific radiosensitivity, which might be associated with their adaptive strategies under radiation stress. Our results suggest that ionizing radiation is promising approach for the utilization of synthetic fungicides for control of pathogens. However, further research should be undertaken interactions between the irradiation and pathogens infected the fruit tissues at the biochemical and molecular levels in in vivo assay. In addition, ionizing radiation has potential use in the control of other pathogens such as bacteria and viruses.



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print






