From the Photosynthesis to Hormone Biosynthesis in Plants
Article information
Abstract
Land plants produce glucose (C6H12O6) through photosynthesis by utilizing carbon dioxide (CO2), water (H2O), and light energy. Glucose can be stored in various polysaccharide forms for later use (e.g., sucrose in fruit, amylose in plastids), used to create cellulose, the primary structural component of cell walls, and immediately metabolized to generate cellular energy, adenosine triphosphate, through a series of respiratory pathways including glycolysis, the tricarboxylic acid cycle, and oxidative phosphorylation. Additionally, plants must metabolize glucose into amino acids, nucleotides, and various plant hormones, which are crucial for regulating many aspects of plant physiology. This review will summarize the biosynthesis of different plant hormones, such as auxin, salicylic acid, gibberellins, cytokinins, ethylene, and abscisic acid, in relation to glucose metabolism.
Plant hormone biosynthesis is intricately controlled by both internal and external cues, playing a crucial role in regulating various aspects of plant physiology, including growth, development, and defense responses. Plants, being autotrophs, produce their own food, glucose (GLU, C6H12O6), through photosynthesis. This process involves transforming carbon dioxide (CO2), water (H2O), and light energy into GLU and oxygen (O2). GLU can be catabolized to produce cellular energy in the form of adenosine triphosphate (ATP) through a series of respiratory pathways, consist of glycolysis, the tricarboxylic acid (TCA) cycle, and oxidative phosphorylation. Alternatively, GLU can be anabolized into storage forms like sucrose and amylose, and structural forms such as cellulose. Significantly, GLU is also metabolized into various phytohormones, including auxin, salicylic acid (SA), gibberellins (GAs), cytokinins (CKs), ethylene (ET), and abscisic acid (ABA) (Fig. 1). Therefore, understanding the complete biosynthetic pathways of different plant hormones from GLU metabolism is essential to provide insights into various aspects of plant physiology.
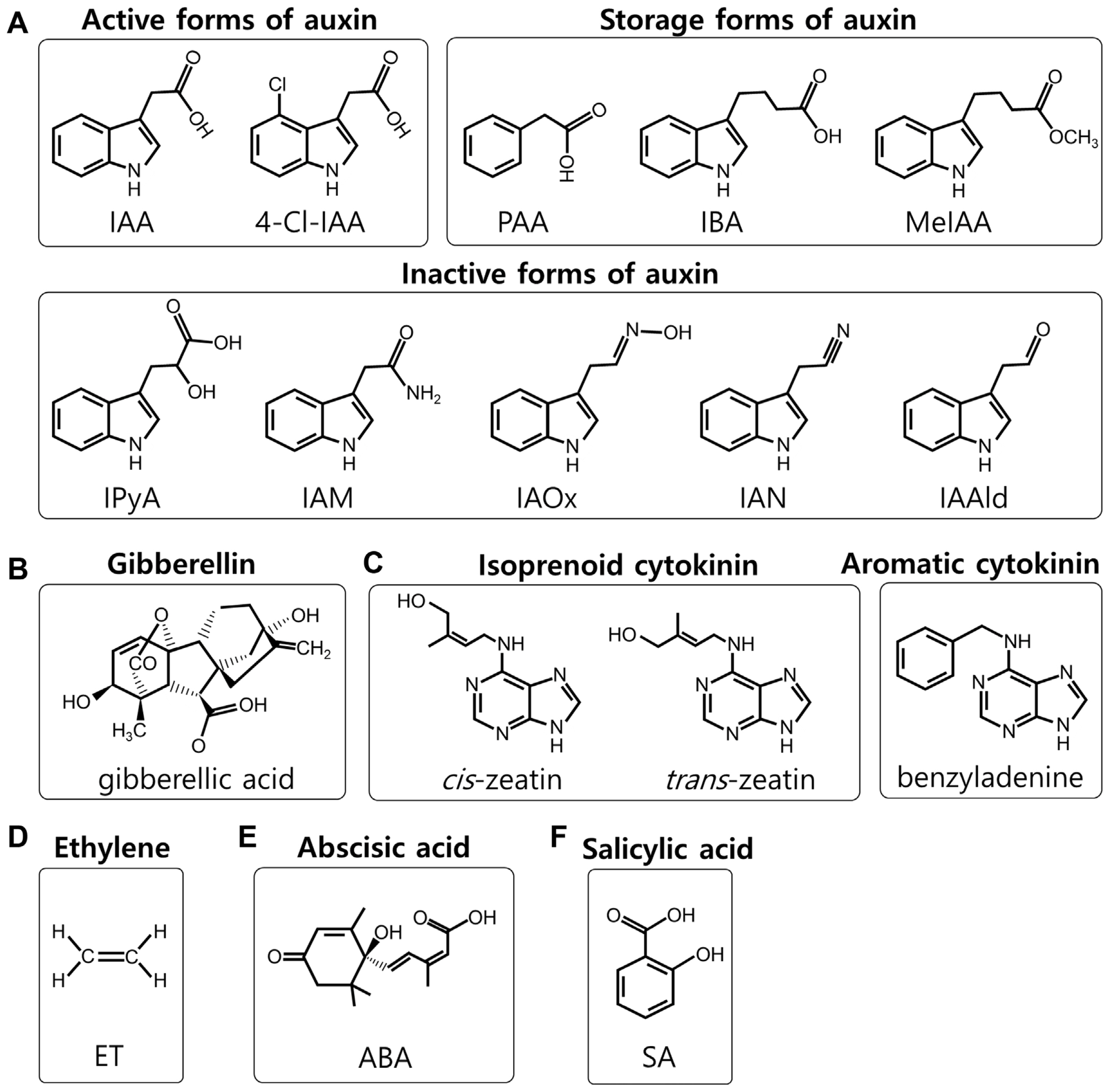
Chemical structures of plant hormones. (A) Active, storage and inactive forms of auxins. IAA, indole-3-acetic acid; 4-Cl-IAA, 4-chloroindole-3-acetic acid; PAA, phenylacetic acid; IBA, indole-3-butyric acid; MeIAA, methyl-IAA; IPyA, indole-3-pyruvic acid; IAM, indole-3-acetaldoxime; IAOx, indole-3-acetaldoxime; IAN, indole-3-acetonitrile; IAAld, indole-3-acetaldehyde. (B) Gibberellic acid. (C) Isoprenoid and aromatic cytokinins. (D) Ethylene (ET). (E) Abscisic acid (ABA). (F) Salicylic acid (SA).
Auxin, one of the first major plant hormones discovered, plays a pivotal role not only in regulating plant growth and development but also in responding to various biotic and abiotic stimuli (Korasick et al., 2013). In nature, auxins exist in different forms: (1) active forms such as indole-3-acetic acid (IAA), 4-chloroindole-3-acetic acid, and phenylacetic acid; (2) inactive forms including indole-3-pyruvic acid, indoleacetamine, indole-3-acetaldoxime, indole-3-acetonitrile, and indole-3-acetaldehyde; and (3) storage forms like indole-3-butyric acid, methyl-IAA, and auxins conjugated to amino acids or sugars (Fig. 1A) (Zhao, 2014). IAA is the most significant natural auxin in plants. Its biosynthesis can occur via tryptophan (Trp)-dependent or independent pathways (Wang et al., 2015). In the Trp-dependent pathway, the Trp aminotransferase of Arabidopsis (TAA) family of aminotransferases removes the amino group (NH2) from the amino acid Trp to produce indole-3-propionic acid, which is then converted into IAA through oxidative decarboxylation by the YUCCA (YUC) family of flavin-containing monooxygenases. On the other hands, indole synthase plays a key role in the Trp-independent auxin biosynthesis.
SA is a crucial plant hormone associated with defense, but it also influences various aspects of plant growth and development, such as seed germination, vegetative growth, root development, flowering, fruit yield, senescence, stomatal closure, and thermogenesis (heat production) (Klessig et al., 2018). Genetic studies in Arabidopsis have identified two primary pathways for SA biosynthesis: the phenylalanine ammonia lyase (PAL)-dependent pathway and the isochorismate synthase (ICS)-dependent pathway. In the PAL-dependent pathway, PAL converts the amino acid phenylalanine (Phe) to trans-cinnamic acid, which is then metabolized into SA. Upon various pathogen challenge, enhanced PAL expression has been observed in various plants, including Arabidopsis, pepper, soybean, and tobacco. However, it should be noted that Arabidopsis mutants lacking all four PAL genes displayed a 90% reduction in basal PAL activity but only a 50% decrease in pathogen-induced SA accumulation (Huang et al., 2010). Regarding the ICS-dependent pathway, Arabidopsis has two ICS genes, ICS1 and ICS2. ICS1 expression is induced by pathogen infection, whereas ICS2, primarily expressed in veins and hydathodes, is not pathogen-responsive (Hunter et al., 2013; Ellis et al., 2007; Wildermuth et al., 2001). Mutants lacking the ICS1 gene showed a drastic reduction in pathogen-induced SA accumulation. This suggests that the ICS1-dependent pathway plays a more critical role in pathogen-induced SA accumulation and defense response compared to the PAL- and ICS2-dependent pathways.
GAs, a vast group of diterpenoid carboxylic acids, are classified based on their structure (Hedden, 2020). Remarkably, over 136 GAs have been isolated from bacteria, fungi, and plants (Khan et al., 2008). GAs regulate a variety of developmental processes throughout a plant’s life cycle, including stem growth, germination, flowering, sexual expression, dormancy, enzyme induction, and leaf and fruit aging. Among these, gibberellic acid is one of the first structurally characterized GAs, commonly referred to as GA3. In plants, the precursor to GAs, trans-geranylgeranyl diphosphate, is biosynthesized via the methylerythritol 4-phosphate (MEP) and mevalonate (MVA) pathways.
CKs a group of naturally occurring adenine derivatives that regulate cell division, growth, and other physiological, such as response to environmental cues (Sakakibara, 2021; Zürcher and Müller, 2016). CKs influence shoot and root growth, leaf expansion, chloroplast development, and delay of senescence. Structurally, CKs are adenine derivatives and can be classified into two main groups: (1) isoprenoid CKs, which include zeatin and its derivatives, and (2) aromatic CKs, like benzyladenine (Fig. 1C). CKs are typically biosynthesized in the roots and is then transported to other plant parts. The isoprenoid CKs are primarily synthesized through the MEP pathway. In this pathway, dimethylallyl pyrophosphate (DMAPP) combines with ATP or adenosine diphosphate (ADP) to form isopentenyladenosine-5′-monophosphate, catalyzed by the enzyme isopentenyl transferase. Subsequent hydroxylation processes lead to the formation of various forms of zeatin. They also interact with other hormones, like auxins and GAs, to fine-tune growth and developmental processes.
ET is a small hydrocarbon gas that acts as a crucial phytohormone in plants, playing a significant role in various aspects of plant growth, development, and response to environmental stress (Abeles et al., 1992). It is involved in processes such as fruit ripening, flower wilting, leaf abscission, and response to mechanical stress. ET is unique among plant hormones due to its gaseous state at ambient temperatures. The biosynthesis of ET in plants follows a simple pathway. It starts with the amino acid methionine (Met), which is converted to S-adenosyl-L-methionine (SAM) by SAM synthetase. SAM then undergoes a series of reactions involving the enzyme 1-aminocyclopropane-1-carboxylic acid (ACC) synthase, which results in the production of ACC. Finally, ACC is converted to ET gas by the action of ACC oxidase. This pathway is tightly regulated and can be rapidly activated in response to various internal and external stimuli, such as physical damage or infection (Kende, 1993; Wang et al., 2002). Its role in fruit ripening is particularly well-studied. It acts as a key regulator in the ripening process, initiating a series of physiological and biochemical changes that result in the softening, color change, and development of flavor and aroma in fruits (Bleecker and Kende, 2000). Moreover, ET is involved in mediating plant responses to biotic and abiotic stresses, such as pathogen attack and flooding, respectively, highlighting its importance in plant survival and adaptation (Lund et al., 1998).
ABA is a vital phytohormone in plants, known primarily for its role in mediating plant responses to stress, particularly drought, and in regulating developmental processes like seed dormancy and germination (Cutler et al., 2010; Zeevaart and Creelman, 1988). ABA is synthesized from carotenoids in plastids, a process that is essential for plant adaptation to environmental stress conditions. The biosynthetic pathway of ABA involves the conversion of carotenoids to xanthoxin, which is then converted into ABA via several enzymatic steps. The enzyme 9-cis-epoxycarotenoid dioxygenase is a key player in this pathway, catalyzing the cleavage of carotenoids to produce xanthoxin (Schwartz et al., 1997). This step is considered the primary regulatory point in ABA biosynthesis, especially in response to water stress. ABA functions by regulating the closure of stomata in response to water deficiency, thereby reducing water loss through transpiration (Wilkinson and Davies, 2002). In addition to its role in stress responses, ABA also regulates various developmental processes, including seed and bud dormancy, and it plays a role in ensuring plant growth under unfavorable conditions (Finkelstein, 2013). This review summarizes the biosynthesis of various plant hormones, including auxin, SA, GAs, CKs, ET, and ABA, and explores their relationship with GLU metabolism.
Photosynthesis: Biosynthesis of Glucose
Plants produce GLU in chloroplasts, specialized organelles containing the green pigment chlorophyll. Specifically, within the thylakoids of chloroplasts, the light-dependent reaction (LDR) takes place, utilizing water (H2O) and light energy to generate ATP and nicotinamide adenine dinucleotide phosphate (NADPH), both of which are essential for the Calvin cycle (Fig. 2). The Calvin cycle, also known as the dark reactions or light-independent reactions, comprises several steps: (1) Phase I involves carbon fixation, where carbon dioxide (CO2) and ribulose 1,5-bisphosphate (RuBP) are converted to 3-phosphoglycerate (3-PG) by ribulose-1,5-bisphosphate carboxylase-oxygenase (RuBisCo); (2) Phase II entails the reduction of 3-PG to glyceraldehyde 3-phosphate (G3P), facilitated by phosphoglycerate kinase and NADP-glyceraldehyde phosphate dehydrogenase, utilizing ATP and NADPH from the LDR, respectively; and (3) Phase III includes regenerating RuBP from G3P through a series of complex reactions, requiring ATP also from the LDR. Notably, G3P, an intermediate in the Calvin cycle, can be utilized for GLU synthesis (for further details on GLU, sucrose, and starch production from G3P, see Choi and Montemagno, 2013). Importantly, GLU produced from photosynthesis is fed into the respiratory pathway, which involves various stages of glycolysis and the TCA cycle. The intermediate compounds formed in these stages are crucial for the biosynthesis of plant hormones.
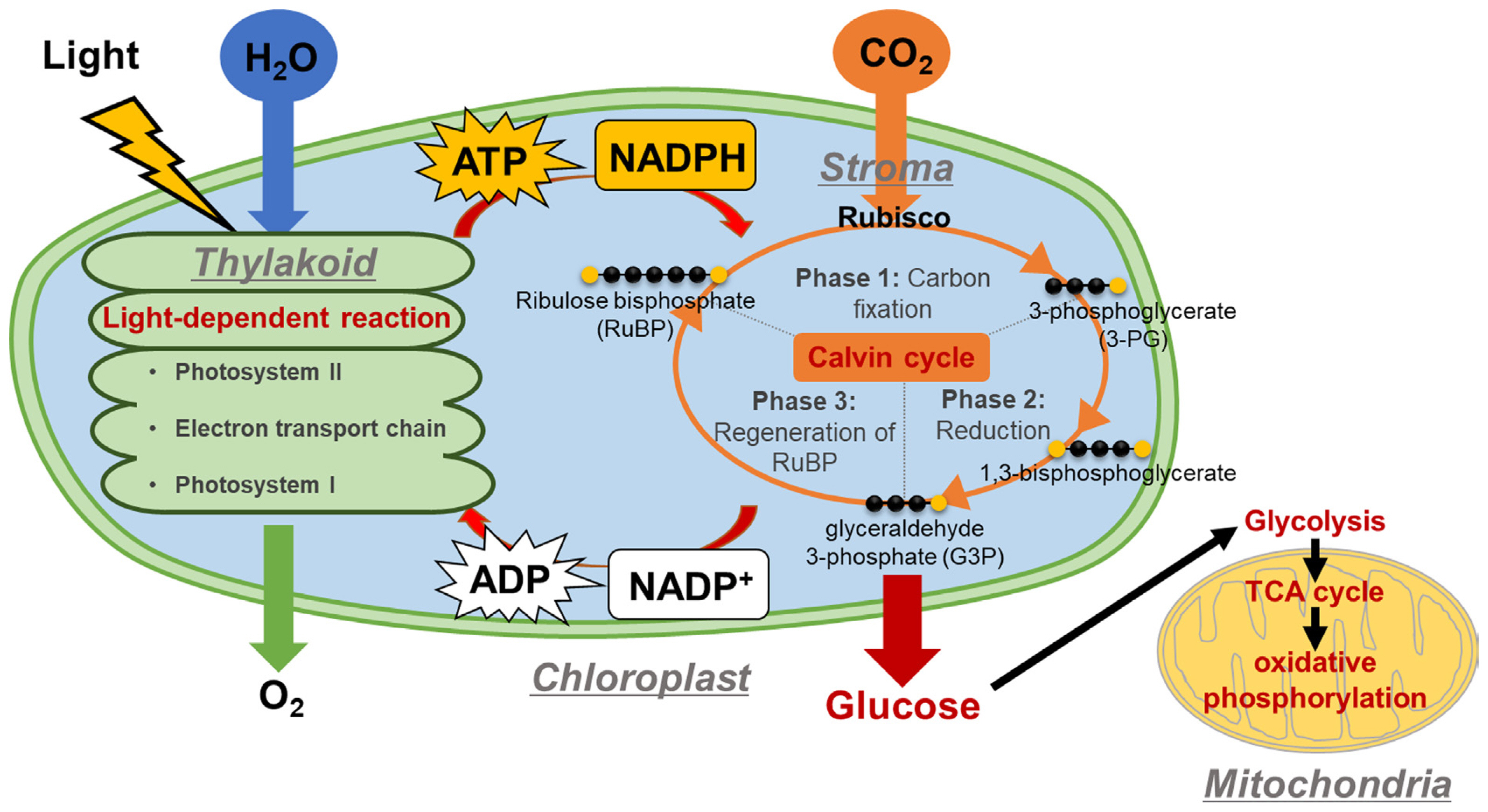
Glucose production in plants via photosynthesis. In light-dependent reaction, H2O molecule is used to produce ATP and NADPH, those of which are used for Calvin cycle (light-independent reaction). In Calvin cycle, CO2 is used to produce glucose via for phase 1, 2, and 3 reactions. RuBP, ribulose 1,5-bisphosphate; TCA, tricarboxylic acid.
Respiration: Glycolysis, TCA Cycle and Oxidative Burst
In plant respiration, GLU, synthesized by condensation and isomerization of G3P, undergoes metabolism to produce cellular energy in the form of ATP through a respiratory pathway. This pathway consists of three major stages: (1) glycolysis, (2) the TCA cycle (also known as the citric acid cycle or Krebs cycle), and (3) oxidative phosphorylation (Fig. 3). Respiration primarily occurs in mitochondria (except for glycolysis) and it generates not only ATP but also various carbon skeletons that are used as precursors of amino acids and hormones. Glycolysis, a cytoplasmic pathway, breaks down a GLU molecule into two three-carbon compounds, pyruvates, while producing ATP and nicotinamide adenine dinucleotide hydrogen (NADH) as byproducts. Pyruvate, the end product of glycolysis, then enters the TCA cycle (details of intermediate metabolic compounds and corresponding enzymes are depicted in Fig. 3). In the TCA cycle, pyruvate is further broken down, releasing CO2 and generating NADH and flavin adenine dinucleotide hydrogen (FADH2). The final stage, oxidative phosphorylation, occurs within the mitochondrial inner membrane. Here, NADH and FADH2 drive the production of a substantial amount of ATP through the electron transport chain and chemiosmosis. This stage is oxygen-dependent and highly efficient in terms of energy production.
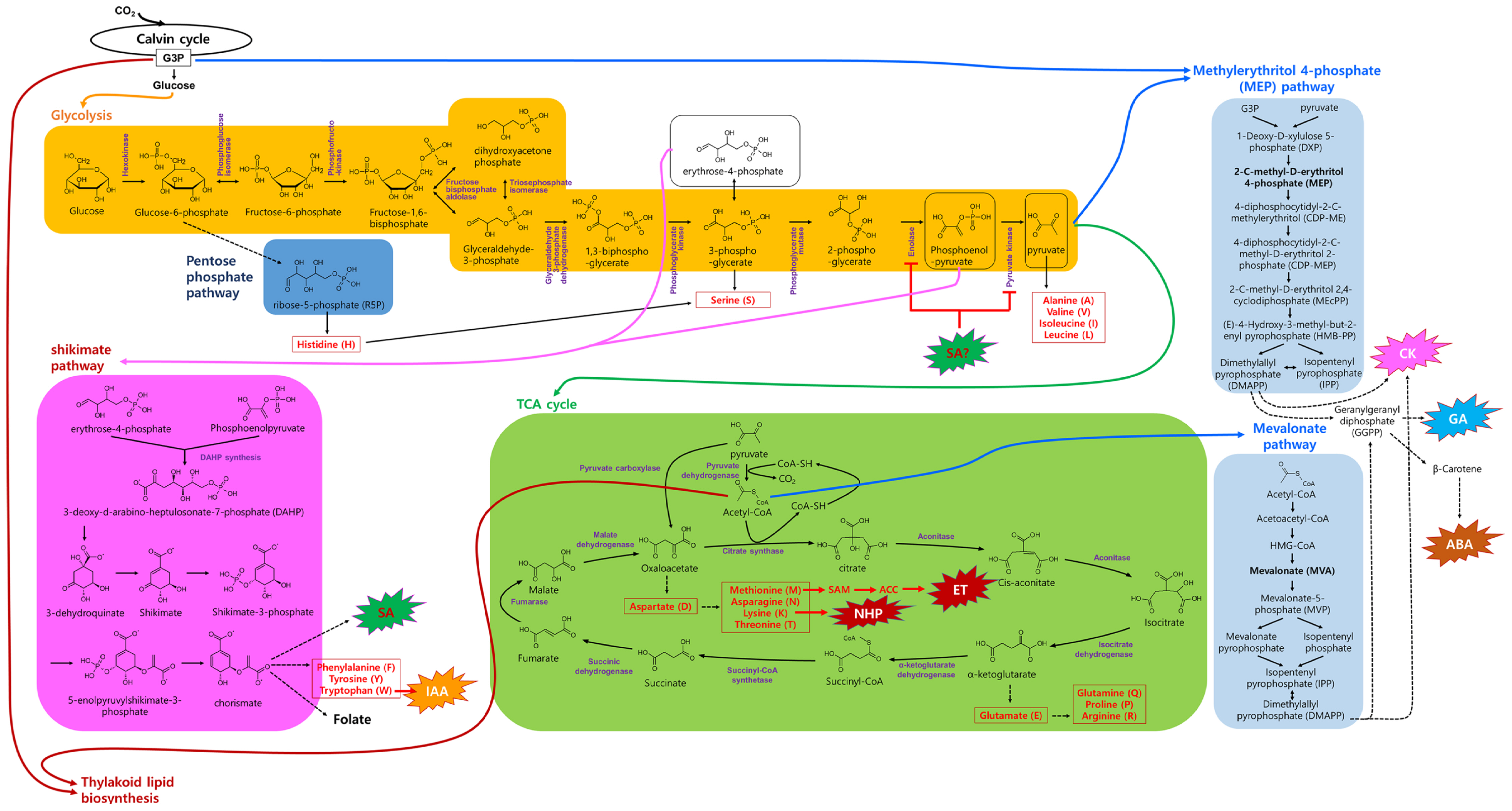
Plant metabolic pathways related with hormone biosynthesis. Glucose is further metabolized into various intermediates, amino acids and hormones. Various compounds produced from glycolysis and tricarboxylic acid (TCA) cycle are fed to shikimate, methylerythritol 4-phosphate (MEP) and mevalonate (MVA) pathways to produce different plant hormones. ABA, abscisic acid; ACC, 1-aminocyclopropane-1-carboxylic acid; ET, ethylene; G3P, glyceraldehyde 3-phosphate; CK, cytokinin; GA, gibberellin; IAA, indole-3-acetic acid; NHP, N-hydroxypipecolic acid; SA, salicylic acid; SAM, S-adenosyl-L-methionine.
Intermediate compounds of glycolysis for hormone biosynthesis
Several intermediates of glycolysis, including glucose-6-phosphate (G6P), 3-PG, phosphoenolpyruvate (PEP), and pyruvate, are crucial for the biosynthesis of various amino acids and hormones (Fig. 3). For instance, G6P, produced from GLU through hexokinase action, enters the pentose phosphate pathway, contributing to the synthesis of amino acids such as histidine and serine. In this pathway, G6P is converted into ribose-5-phosphate (R5P), essential for the de novo synthesis of nucleotides. R5P can be further metabolized to produce histidine and serine. In addition, serine can also be synthesized from 3-PG through a pathway involving 3-phosphoglycerate dehydrogenase, 3-phosphoserine aminotransferase, and phosphoserine phosphatase.
Erythrose-4-phosphate derived from 3-PG along with PEP serve as a primary substrate for the shikimate pathway. Here, the end product, chorismate, is utilized to produce the plant defense hormone SA and amino acids like phenylalanine, tyrosine, and Trp. Notably, Trp is a precursor of IAA, an active form of auxins. As both SA and IAA share chorismate as a common precursor, increased SA biosynthesis during defense responses may limit resources for IAA production, and vice versa (Koo et al., 2020). Thus, this balance finely tunes the allocation of resources for growth or defense, depending on external and internal conditions.
Pyruvate, the end product of glycolysis, serves as a precursor for various amino acids, including alanine (Ala), valine (Val), isoleucine (Ile), and leucine (Leu). Significantly, pyruvate, along with G3P from the Calvin cycle, also contributes to the MEP pathway (Fig. 3). In this pathway, pyruvate and G3P lead to the production of DMAPP, which is crucial for synthesizing CKs, GAs, and ABA. Generally, CKs and GAs promote plant growth, whereas ABA inhibits it. Thus, the regulation of CKs and GAs versus ABA production from DMAPP is important for controlling plant growth.
Intermediate compounds of TCA cycle for hormone biosynthesis
Pyruvate enters the TCA cycle to generate more ATP and NADPH. In the TCA cycle, acetyl coenzyme A (acetyl-CoA) is produced from pyruvate by the action of pyruvate dehydrogenase (Fig. 3). Acetyl-CoA is a key substrate in fatty acid and sterol synthesis, which are essential for cell membranes and plant hormones. In the MVA pathway, acetyl-CoA serves as the starting material to produce DMAPP, a precursor of CKs, GAs, and ABA.
Another TCA cycle intermediate, oxaloacetate, can be utilized to produce aspartate, a precursor to amino acid of methionine, asparagine, lysine, and threonine. As mentioned above, Met is an upstream precursor of ET. In addition, recent studies suggest that lysine-induced N-hydroxypipecolic acid plays an important role in plant systemic acquired resistance (Hartmann and Zeier, 2018).
Conclusion
This review has comprehensively examined the biosynthesis of various plant hormones, including auxin, SA, GAs, CKs, ET, and ABA, in relation to GLU metabolism. In plants, intricate regulatory interactions between GLU metabolism and various hormones have been reported as crucial for responding to environmental signals, including biotic and abiotic stresses (Rolland et al., 2006; Sami et al., 2019). However, biosynthetic pathways of different plant hormones from GLU are not fully understood. Thus, we have highlighted the intricate hormone biosynthesis processes by which initiated from GLU metabolism. Future research should continue to explore these biosynthetic pathways, particularly focusing on the regulatory mechanisms that govern the GLU-mediated dynamic balance between different hormones. Such studies will deepen our understanding of plant physiology and could lead to innovative strategies for crop improvement and sustainable agriculture. In conclusion, the complex web of GLU metabolism and hormone biosynthesis in plants offers a window into the remarkable adaptability and sophistication of plant life, revealing a multitude of opportunities for future research and application in plant sciences and agriculture.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Acknowledgments
This work was supported by a Research Grant of Andong National University.