 |
 |
| Plant Pathol J > Volume 40(2); 2024 > Article |
|
Abstract
In this study, the efficacy of the essential oil of Mentha longifolia, Achillea arabica and Artemisia absinthium plants were evaluated against important soil-borne fungal pathogens as Verticillium dahliae, Rhizoctonia solani, and Fusarium oxysporum. Essential oils were obtained from plants by hydrodistillation method and the chemical components of essential oils were determined by analyzing by gas chromatography-mass spectrometry. The main components found as piperitone oxide (13.61%), piperitenone oxide (15.55%), pulegone (12.47%), 1-menthone (5.75%), and camphor (5.75%) in M. longifolia, á-selinene 13.38%, camphor 13.34%, L-4-terpineneol 8.40%, (−)-á-Elemene 7.01%, 1,8-cineole 4.71%, and (−)-spathulenol 3.84% in A. arabica, and á-thujone (34.64%), 1,8-cineole (19.54%), pulegone (7.86%), camphene (5.31%), sabinene (4.86%), and germacrene-d (3.67%) in A. absinthium. The antifungal activities of the oils were investigated 0.05, 0.1, 0.25, 0.5, 1.00, and 2.00 μl/ml concentrations with the contact effect method. M. longifolia oil (1.00 and 2.00 μl/ml) has displayed remarkable antifungal effect and provided 100% inhibition on mycelial growth of V. dahliae, R. solani and F. oxysporum. The results obtained from this study may contribute to the development of new alternative and safe methods against soil-borne fungal pathogens.
Fungal diseases are responsible for 30% of all plant diseases and cause significant yield losses and disruption of the natural ecosystem (Davari and Ezazi, 2022; Punja, 2004). Verticillium dahliae Kleb., Rhizoctonia solani Kühn and Fusarium oxysporum Schltdl. are soil-borne plant pathogenic fungus and generally cause significant yield and quality losses in many parts of the world (Agrios, 2005; Ajayi Oyetunde and Bradley, 2018; Delgado-Baquerizo et al., 2020; Siddiqui-Jain et al., 2002). These pathogens attach a wide range of host including tomato, potato, rice, maize, bean, and strawberry that cause infection in the root and vascular systems of plants (Agrios, 2005; Siddiqui-Jain et al., 2002). They have the ability to survive thanks to special restring structure called microsclerotia, sclerotia, chlamydospore, and oospore on plant debris or in the soil for a long periods (Panth et al., 2020). In Turkey, V. dahliae, R. solani, and F. oxysporum have been reported to cause root rot and wilt disease in many plant species (Demirci, 1998; Eken and Demirci, 2003; Kesimci and Demirci, 2020; Teniz and Demirer Durak, 2023).
Although control methods of these pathogens were grouped as cultural, physical, chemical and biological, the chemical control is considered to be a prominent method with high effect in a short time (Greff et al., 2023). Despite all its known harms, chemical control continues global use by increasing with a total of over 1,000 pesticides (Choudhury and Saha, 2020) which this widespread use of pesticides causes harm to non-target organisms. Ashraf and Zuhaib (2013) indicated that only 1% of the pesticides reach the target pathogen, the remaining 99% reach the non-target organism and harm the environment and other organism. The effects of toxic residues of pesticides on human health and the environment were limitated the use of chemicals and increased scrutiny and questioning of fungicides (Elad et al., 2004). Also, pesticides used against soil-borne fungal pathogens are very difficult and dangerous to apply due to environmental, social, economic, and technical problems on a global (Eryılmaz and Kılıç, 2018). All these reasons have led researchers to develop alternative methods in plant diseases control (Duniway, 2002).
Essential oils stand out among the alternative methods that ensure sustainability in nature. The oils are complex mixtures obtained from the flowers, leaves, fruits, root, wood, and bark of plants that are volatile substances usually colorless, with a special scent, liquid at room temperature, soluble in alcohol, and insoluble in water (Ahmed et al., 2023; Dhifi et al., 2016; Falleh et al., 2020). Over the past decade, essential oils are of increasing interest in plant protection that considered as antimicrobial and antioxidant substances (Appendini and Hotchkiss, 2002; Burt, 2004). It has been identified in many studies that different concentrations of essential oils obtained from different plants showed antifungal effects against soil-borne fungal pathogens (Ahmed et al., 2023; Park et al., 2022).
The antifungal effect of essential oils has been connected with several compounds (Kahramanoğlu et al., 2022; Nazzaro et al., 2017). Mentha, Achillea, and Artemisia are three important genus and it has been reported that these plants contain abundant antifungal compound content (Bai et al., 2020; Barış et al., 2006; Okut et al., 2017; Sharopov et al., 2012; Vieira et al., 2017; Yıldırım et al., 2019). Components and amounts of essential oils are known to create remarkable variations depending on part of the plant, manufacturing process, climate, geographical region, and different locations (Bailen et al., 2013; Kakhky et al., 2009; Özgüven and Kırıcı, 1999). Turkey has a very rich flora with geographical location, climate, and plant diversity (İpek and Gürbüz, 2010). This richness provides a significant advantage for obtaining different essential oils and their components (Guzmán and Lucia, 2021). This study was conducted to determine the chemical components of essential oils obtained from Mentha longifolia L., Achillea arabica Kotschy and Artemisia absinthium L. in Iğdır province, Turkey and to determine antifungal activities against V. dahliae, R. solani, and F. oxysporum.
The isolates of V. dahliae (SOR-8) and R. solani (S-TR-6) obtained from strawberry plants with F. oxysporum (HMK2-6) obtained from tomato plants were supplied from the culture collection of Department of Plant Protection, Faculty of Agriculture, Iğdır University (39°48′45″N and 44°04′43″E, 1,112 m). Disease severity values of isolates SOR-8, S-TR-6 and HMK2-6 were determined in previous studies (Aşkar, 2019; Kesimci and Demirci, 2020; Kesimci et al., 2022). The pathogens were kept on potato dextrose agar (PDA) slants at 5°C.
The aerial part of M. longifolia, A. arabica, and A. absinthium plants were collected at different times in Iğdır province (Bayraktutan, Calpala and Küllük) between June and July 2019. M. longifolia and A. arabica were collected in the generative period, and A. absinthium was collected in the vegetative period. Botanical classification of the plants was presented in Table 1 (Altundağ, 2009; TÜBİVES, 2021).
Aerial parts of the plants were dried and ground into powder in the shade. The powdered plants were placed in cloth bags and kept in cool storage conditions. The dried plant samples were subjected to hydro distillation for 2-8 h using a Clevenger-type apparatus (EM1000/CE, Biblo Scientific, Staffordshire, UK). The essential oils were placed in amber vials and stored in a refrigerator at 4°C (Kılıç, 2008).
The yields oil of the plants was calculated using to following formula (Özbek, 2019): Yield% = [Volume of essential oil (ml) obtained/Amount of raw material (g) used × 100]. The dried plant samples/water ratio was adjusted to be 1:10 and by using 60 g of the dried plant samples.
The chemical compositions of the essential oil obtained from M. longifolia, A. absinthium, and A. arabica plants were made by Iğdır University, Research Laboratory Application and Research Center (ALUM). It was determined by analysis using thermo gas chromatography-mass spectrometry (GC/MS) trace ultra system (Table 2).
The antifungal effects of essential oils were determined with the contact effect method in in vitro conditions. The PDA was sterilized at 121°C for 15 min and cooled to 45-50°C, in Erlenmeyer flask. Essential oils prepared at concentrations of 0.05, 0.1, 0.25, 0.5, 1.00, and 2.00 μl/ml (dissolved with dimethyl sulfoxide at a ratio of 1:2) were added to this medium and 20 ml were added to each 9 cm Petri plates. The medium was solidified at 23°C for 1 h. Agar discs with mycelia (0.5 cm diameter) of V. dahliae, R. solani, and F. oxysporum isolates were cut from the periphery of actively growing regions of the 7-10 day cultures and placed in the center of each Petri plates containing essential oil and PDA (Soliman and Badeaa, 2002). Control plates (without oil) were prepared with sterile water. The Petri plates were tightly closed with paraffin and incubated at 25°C. The experiments were set up with four replications. Evaluations were made after 3rd, 5th, and 7th days by measuring colony diameters (in cm) in perpendicular directions using calipers. The mycelial growth inhibition of each essential oil at different concentrations was calculated at 7th day using the following formula (Soylu et al., 2005). Mycelial growth inhibition % = [(C − T/C) × 100]. Where, C: The mean radial diameter of the control, T: The mean radial diameter of the essential oil-treated.
The results obtained from this study were analyzed using the SPSS program. The data were evaluated according to the Factorial Experiment Design. Normality and homogeneity tests were performed to test the suitability of the data for analysis. Levene homogeneity was performed for the 3rd, 5th, and 7th days and it was statistically determined that the data were homogeneously distributed (P ≥ 0.05). The effect of essential oil and concentration applications on colony diameter was tested by analysis of variance, and mycelial growth inhibition of essential oil and concentrations was analyzed by ANOVA test (P ≤ 0.05). Differences between applications were evaluated with Duncan Multiple Comparison tests.
The oil yields of M. longifolia, A. arabica, and A. absinthium were found as 0.25%, 0.10%, and 0.20% (v/w), respectively. According to the results of GC/MS analysis, 40 different essential oil components were detected in the M. longifolia. Among these components, the main components were found as piperitone oxide 15.5%, piperitenone oxide 13.61%, pulegone 12.47%, 1-menthone 5.75%, camphor 5.57%, and 1,8-cineole 5.73% (Table 3, Fig. 1).
Many studies have determined different the number of chemical components in the M. longifolia. Components of the essential oil in M. longifolia were reported 34 components by Yıldırım et al. (2019), 38 components by Akkuş et al. (2021), 39 components by Bai et al. (2020), 26 components by Kaya (2014), and 40 components by Okut et al. (2017). In the studies, the main components of M. longifolia were identified as piperitone oxide (Golparvar et al., 2013; Kakhky et al., 2009; Yıldırım et al., 2019), piperitenone oxide (Abedi et al., 2015; Golparvar et al., 2013; Kakhky et al., 2009; Mkaddem et al., 2009; Saeidi et al., 2012; Yıldırım et al., 2019), pulegone (Abdel-Gwad et al., 2022; Abedi et al., 2015; Akkuş et al., 2021; Golparvar et al., 2013; Kakhky et al., 2009; Mahmoudi, 2014; Mkaddem et al., 2009; Okut et al., 2017; Saeidi et al., 2012), menthone (Abdel-Gwad et al., 2022; Akkuş et al., 2021; Kakhky et al., 2009; Kaya, 2014; Okut et al., 2017; Saeidi et al., 2012; Yıldırım et al., 2019) and 1,8-cineole (Abdel-Gwad et al., 2022; Abedi et al., 2015; Golparvar et al., 2013; Kakhky et al., 2009; Kaya, 2014; Mahmoudi, 2014; Mkaddem et al., 2009; Okut et al., 2017). In this study, piperitone oxide, piperitenone oxide, pulegone, and 1,8-cineole were similar to the main components. However, camphor was not obtained as the main component in other studies.
In A. arabica, 43 different essential oil components were found and á-selinene 13.38%, camphor 13.34%, L-4-terpineneol 8.40%, (−)-á-Elemene 7.01%, 1,8-cineole 4.71%, and (−)-spathulenol 3.84% were determined as the main components (Table 3, Fig. 2). Previous studies reported that may vary greatly according to origin. For example, it was displayed p-cymene, piperitone, camphor, and 1,8-cineole as main compounds in Iran (Mottaghi et al., 2016). 1,8-cineole and camphor were reported as main component in the essential oils of 19 Achillea species in Turkey (Başer, 2016). This result was found to be similar with camphor, 1,8-cineole, and á-selinene.
Our results revealed that in A. absinthium were determined 32 different essential oil components. á-thujone (34.64%), 1,8-cineole (19.54%), pulegone (7.86%), camphene (5.31%), sabinene (4.86%), and germacrene-d (3.67%) were found as the main components (Table 3, Fig. 3). The number of components of A. absinthium essential oil was determined as 63 by Vieira et al. (2017), 41 by Sharopov et al. (2012), 28 by Rezaeinodehi and Khangholi (2008), 14 by Morteza-Semnani and Akbarzadeh (2005), and 68 by Ghannadi et al. (1999). When analyzed in all studies, the 1,8-cineole component is similar to the study conducted. But, Ghannadi et al. (1999), and Morteza-Semnani and Akbarzadeh (2005) identified p-cymene as the common major component in their studies. When the results of the studies are compared, it is seen that there are similarities in the findings as well as some differences.
The major components of essential oils have a very important role in the antifungal effect. It has been suggested that the major components are effective in inhibiting fungal pathogens, but the synergistic effect of the minor compounds of each oil with each other also affects this result (Moghaddam et al., 2013). Essential oils and component have been used for chemotaxonomic purposes, but the different methods used for isolation and analysis, the difference in the growth stage of the plant and the fact that samples are taken from different plant parts make it difficult to compare the essential oil component detected in studies. In addition, in some species, the composition of the essential oil varies at different times of the year. So, it is important to collect plants at the right time (Figueiredo et al., 2008).
The results of the antifungal assays of essential oils M. longifolia, A. arabica, and A. absinthium against three soil-borne fungal pathogens are summarized in Table 4 and Fig. 4. The essential oils have a fungicidal or fungistatic effect with different ratios depending on the plant species and pathogen. Generally increasing concentrations of essential oils show the effect on mycelium growth of V. dahliae, R. solani, and F. oxysporum, except A. arabica. As a result of study, it has been found that the M. longifolia essential oil to be the most effective oil against these three pathogens. The 0.5, 1.00, and 2.00 μl/ml concentrations of M. longifolia inhibited 100% mycelial growth of R. solani, while 1.00 and 2.00 μl/ml concentrations inhibited 100% mycelial growth of V. dahliae and F. oxysporum. A. absinthium essential oil was found to be the most effective concentrations (2.00 μl/ml concentrations) against V. dahliae, R. solani and F. oxysporum with inhibition of mycelial growth for 53.80%, 86.62% and 98.38%, respectively (Fig. 5). A. arabica essential oil (1.00 μl/ml concentrations) have fungistatic effect against V. dahliae and F. oxysporum, 52.17%, 58.97% respectively. However, all concentrations of the essential oil A. arabica did not inhibit the mycelial growth of R. solani.
Essential oils have different of action as inhibiting fungal growth (fungistatic activity), causing fungal mortality (fungicidal activity) and promoting plant growth in control of pathogenic fungi (Ahmed et al., 2023). In this study, it has been determined that M. longifolia is the most effective oil with high antifungal effect against the pathogens V. dahliae (100%), R. solani (100%) and F. oxysporum (100%) and have fungicidal activity. Mentha spp. showed fungicidal and fungistatic activity against important fungal pathogens and are the most popular and widely used essential oil for the control of fungal pathogens in many studies (Moghaddam et al., 2013; Saharkhiz et al., 2012). Leblalta et al. (2020) found that antifungal effects of Mentha rotundifolia against F. oxysporum and indicates as a natural fungicidal material. Moghaddam et al. (2013) emphasized that it showed high antifungal against Drechslera spicifera, F. oxysporum f. sp. ciceris and Macrophomina phaseolina of essential oil Mentha piperita. Similarly, Koçak and Boyraz (2006) reported that the essential oil obtained from M. piperita showed high antifungal properties against F. oxysporum, Botrytis cinerea, Sclerotinia sclerotiorum and Colletotrichum circinans. Djordjevic et al. (2013) stated that the essential oil obtained from M. piperita had a fungicidal effect against F. oxysporum. Bajpai and Kang (2012) reported that essential oils obtained from Mentha liliflora showed antifungal effect against B. cinerea, Colletotrichum capsici, F. oxysporum, F. solani, Phytophthora capsici, R. solani and S. sclerotiorum.
The essential oil of Achillea arabica inhibited V. dahliae and F. oxysporum at 1.00 μl/ml dose. Although 2.00 μl/ml dose of this essential oil was effective compared to the control, it was found to be less effective on mycelial growth compared to 1.00 μl/ml dose. Also, no antifungal activity was found against R. solani. In a similar study, it was reported that Achillea biebersteinii essential oil did not show antifungal activity against Colletotrichum acutatum, Colletotrichum fragariae and Colletotrichum gloeosporioides pathogens (Tabanca et al., 2011). However, Kordali et al. (2009) emphasized that A. biebersteinii essential oil was successful by inhibiting R. solani by 80.7% and F. oxysporum by 49.8%. It was stated that this oil did not have antifungal activity by promoting the mycelial growth of Fusarium equiseti compared to the control in the same study. The 2.00 μl/ml dose of A. absinthium provided fungistatic effect 98.38%, 86.62%, and 53.80% mycelial growth inhibition on the R. solani, F. oxysporum and V. dahliae respectively. Kordali et al. (2005) and Torres Juan (2017) reported that antifungal activity of essential oil A. absinthium against V. dahliae, R. solani and F. oxysporum. Our results revealed that inhibition rates against V. dahliae, F. oxysporum and R. solani were found to be different from each other according to the plant species and the dose. Moghaddam et al. (2013) emphasized that an increase in the concentration of the essential oil causes an increase in the antifungal activity of the oil.
It is known that variations occur among fungal species in the antifungal effect of essential oils. Therefore, it is quite difficult to compare the results obtained from this study with the results of other studies. One of the most important reasons for this is that the techniques used in the studies are not fully standardized. Also, the genotype characteristics of the plant, the difference in the geographical region and the different dates of collection of the plants cause variations (Çopuroğlu, 2013). In these variations, the resistance of fungi against essential oil is effective. Depending on the morphological and physiological characteristics of the fungal hyphae may change the sensitivity of pathogens. The cell wall of microorganisms acts as a barrier against antimicrobial agents and main components (chitin is one of the important) of the cell wall are important targets of essential oils. Therefore, essential oils affect the normal function of cells by changing the structure and permeability of the cell membrane, reduced membrane potential, change the composition of membrane fatty acids. Also, essential oils can damage significantly inhibiting the mitochondrial ATP enzyme activity, DNA damage, lipid, protein and nucleic acid metabolism (Mani-López et al., 2021; Wu et al., 2022).
The presented studies showed the perspective of using essential oils against soil-borne fungal pathogens. Results demonstrated that M. longifolia, A. arabica and A. absinthium essential oils have contain various main compounds as piperitone oxide, piperitenone oxide, pulegone, 1-menthone, camphor and 1,8-cineole, á-selinene, camphor, l-4-terpineneol, (−)-á-Elemene, 1,8-cineole, (−)-spathulenol, á-thujone, 1,8-cineole, pulegone, camphene, sabinene and germacrene-d. The results also showed that the essential oils have an antifungal effect V. dahliae, F. oxysporum, and R. solani. Especially, M. longifolia essential oil (1.00 μl/ml dose and 2.00 μl/ml dose) serves as an alternative to fungicides for controlling soil-borne fungal pathogens. In order to obtain environmentally friendly plant protection products is necessary to develop extraction methods, toxicological, and stabilization.
Acknowledgments
This article was prepared from the Master Thesis of the first author and supported by Scientific Research Projects Coordination Unit of Iğdır University (Project number 2020-ZİF0520Y05). We are grateful to Assist. Prof. Dr. Belkıs Muca YİĞİT (Iğdır University, Vocational School of Technical Sciences) for identification of plant species and Assist. Prof. Dr. Sezgin SANCAKTAROĞLU for the evaluation of essential oil components. We would like to thank Assoc. Prof. Dr. Cem TIRINK and Assoc. Prof. Dr. İbrahim KAHRAMANOĞLU for help in statistical analysis.
Fig. 1
GC/MS gas chromatogram-mass spectrometry (A) and main components (B) of essential oil of Mentha longifolia.
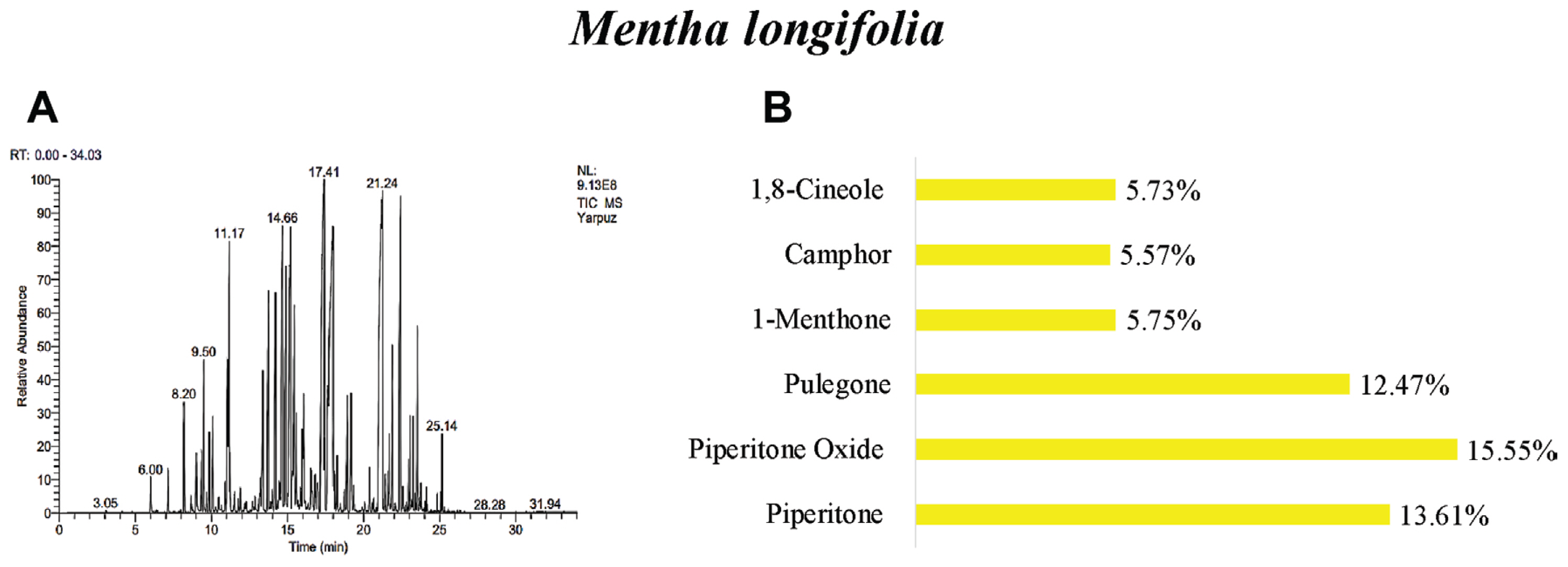
Fig. 2
GC/MS gas chromatogram-mass spectrometry (A) and main components (B) of essential oil of Achiella arabica.
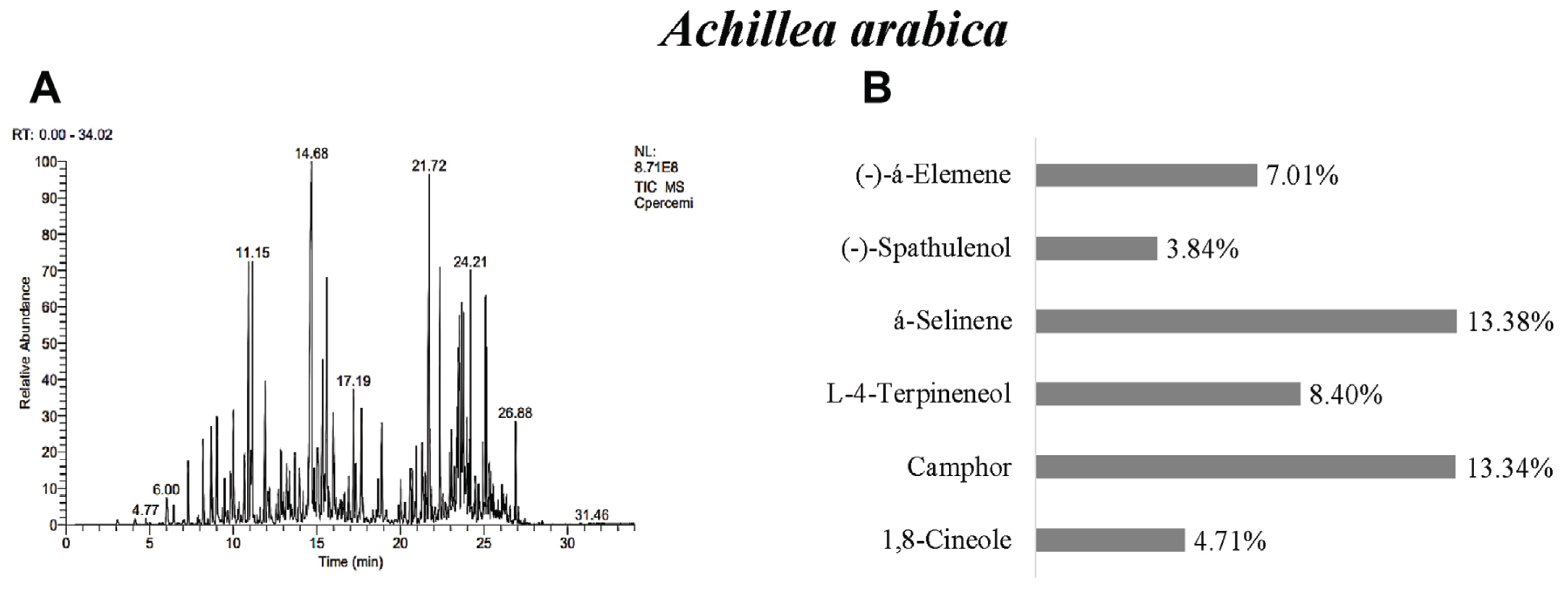
Fig. 3
GC/MS gas chromatogram-mass spectrometry (A) and main components (B) of essential oil of Artemisia absinthium.
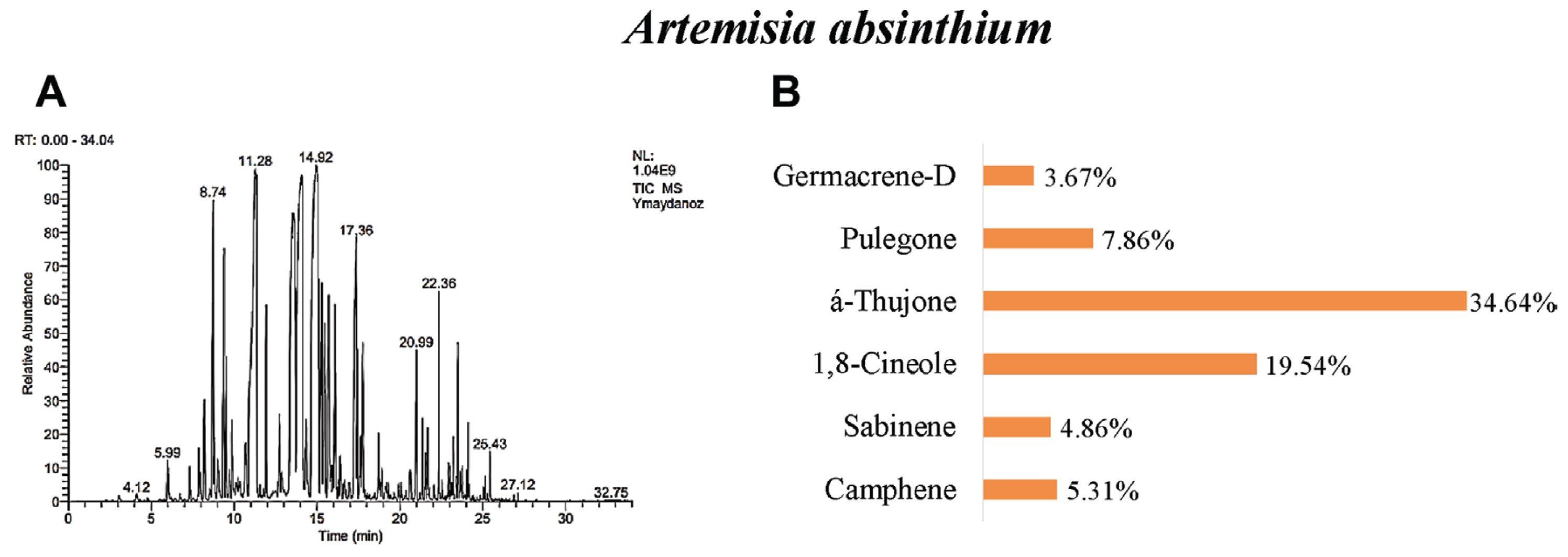
Fig. 4
Effect of different application concentrations of Mentha longifolia, Achillea arabica, and Artemisia absinthium essential oils on the mycelial growth (colony diameter, cm) of Verticillium dahliae, Rhizoctonia solani, and Fusarium oxysporum on potato dextrose agar after seven days of incubation.
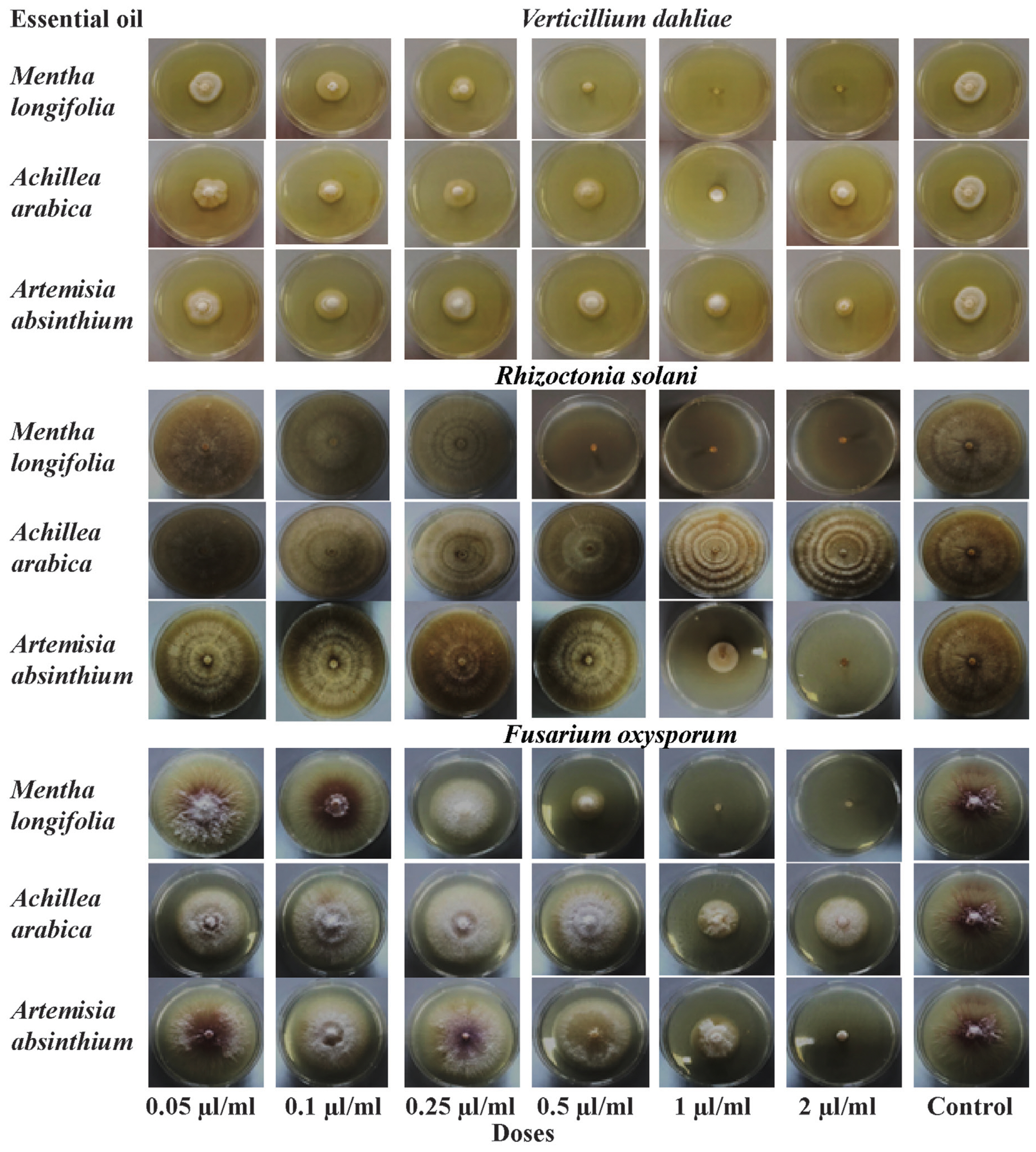
Fig. 5
Inhibitory effect of different application concentrations of Mentha longifolia, Achillea arabica and Artemisia absinthium essential oils on the mycelial growth of Verticillium dahliae, Rhizoctonia solani, and Fusarium oxysporum on potato dextrose agar after seven days of incubation. There is a statistical difference between numbers shown with different letters (P ≤ 0.05).
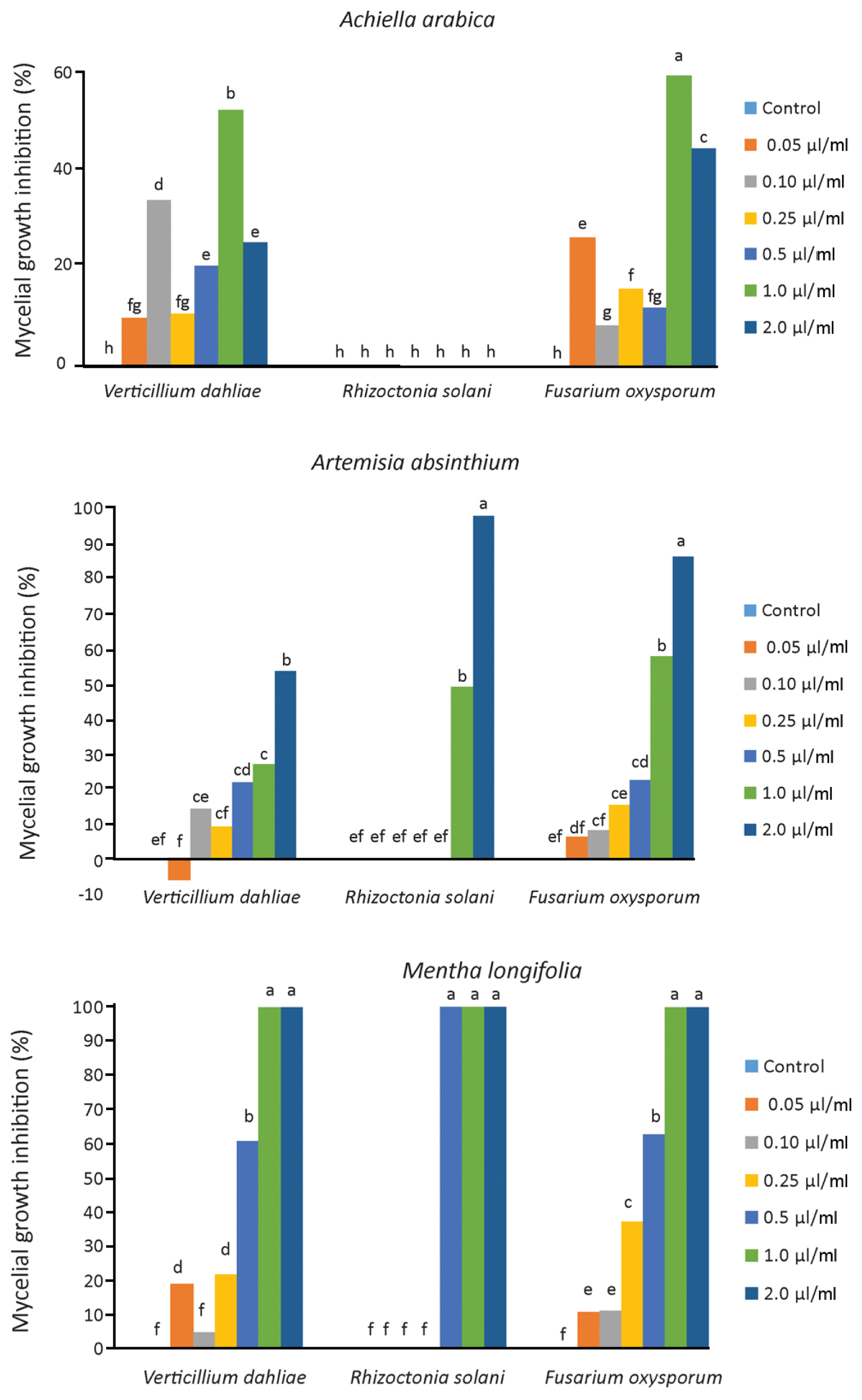
Table 1
Botanical classification of Mentha longifolia L. Achillea arabica Kotschy and Artemisia absinthium L.
Table 2
Gas chromatography-mass spectrometry analysis conditions
Table 3
Chemical composition of the essential oil Mentha longifolia, Achillea arabica and Artemisia absinthium
| No. | Compounds | Mentha longifolia | Achiella arabica | Artemisia absinthium | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| RTa | %b | RT | % | RT | % | ||
| 1 | Furan,2-ethyl | 3.05 | 0.06 | 3.06 | 0.12 | 3.06 | 0.10 |
| 2 | 2-Hexenal | 6.00 | 0.50 | 6.00 | 0.99 | - | - |
| 3 | Furan,2,5,-Diethyltetrahydro | 7.14 | 0.39 | - | - | - | - |
| 4 | 1-Phellandrene | 7.99 | 0.03 | 10.34 | 0.31 | - | - |
| 5 | á-Pinene, (−) | 8.20 | 0.98 | 8.20 | 1.11 | 8.21 | 1.14 |
| 6 | Camphenec | 8.68 | 0.15 | 8.68 | 1.52 | 8.73 | 5.31 |
| 7 | 2-á-Pinene | 9.50 | 2.63 | 9.49 | 0.80 | - | - |
| 8 | á-Myrcene | 9.84 | 0.63 | - | - | - | - |
| 9 | 3-Octanol | 10.08 | 1.02 | - | - | - | - |
| 10 | 2,4-Heptadienal | 10.47 | 0.19 | - | - | - | - |
| 11 | 1,8-Cineolec | 11.16 | 5.73 | 11.14 | 4.71 | 11.27 | 19.54 |
| 12 | Cyclononanone | 11.52 | 0.18 | - | - | - | - |
| 13 | Ç-Terpinene | 11.89 | 0.15 | - | - | 11.94 | 1.68 |
| 14 | Linalool oxide cis | 12.28 | 0.16 | - | - | - | - |
| 15 | Benzene,1-methyl-4-(1-methylethenyl) | 12.85 | 0.24 | 12.85 | 1.24 | - | - |
| 16 | á-Thujonec | 13.73 | 2.56 | 13.69 | 0.66 | 14.09 | 34.64 |
| 17 | Myrtenol | 13.99 | 0.15 | - | - | - | - |
| 18 | 4(5)-Acetyl-2-ethyl-1H-imidazole | 14.20 | 2.88 | - | - | - | - |
| 19 | Camphorc | 14.65 | 5.57 | 14.68 | 13.34 | - | - |
| 20 | 1-Menthonec | 15.19 | 5.75 | - | - | - | - |
| 21 | Isopulegone | 15.43 | 2.63 | - | - | - | - |
| 22 | 3-Cyclohexen-1-ol,4-methyl-1-(1-methylethyl) | 15.57 | 0.82 | - | - | - | - |
| 23 | á-Terpineol | 16.05 | 2.28 | - | - | - | - |
| 24 | Benzofuran,4,7-dimethyl | 16.53 | 0.62 | - | - | - | - |
| 25 | Phenol, 2-methyl-6-(2-propenyl) | 16.80 | 0.32 | - | - | - | - |
| 26 | (4as,5S,8as)-Perhydro-4a,5-dimethyl-2H,4H-[1.3.]benzodioxin-4-one | 16.96 | 0.53 | - | - | - | - |
| 27 | Pulegonec | 17.39 | 12.47 | 17.19 | 1.60 | 17.34 | 7.86 |
| 28 | Piperitone oxidec | 17.97 | 15.55 | 17.66 | 1.78 | 17.75 | 3.12 |
| 29 | 2-Hydroxy-4-methylacetophenone | 18.25 | 0.44 | - | - | - | - |
| 30 | Dihydroedulan II | 18.91 | 1.38 | - | - | - | - |
| 31 | 2-Cyclohexen-1-one,2-hydroxy-3-methyl-6-(1-methylethyl) | 19.18 | 1.63 | - | - | - | - |
| 32 | 2H-2,4a-Ethanonaphthalene, 1,3,4,5,6,7-hexahydro-2,5,5-trimethyl | 20.05 | 0.10 | - | - | - | - |
| 33 | Verbenone | 20.38 | 0.45 | - | - | 16.37 | 0.47 |
| 34 | á-Gurjunene | 20.63 | 0.14 | - | - | - | - |
| 35 | Piperitenone Oxide | 21.22 | 13.61 | 20.93 | 0.96 | 20.99 | 1.59 |
| 36 | 3-(Hydroxymethyl)-6,7-dihydrobenzofuran-4(5H)-One | 21.87 | 2.30 | - | - | - | - |
| 37 | Trans-caryophyllenec | 22.41 | 4.79 | 22.35 | 3.24 | 22.36 | 1.98 |
| 38 | Germacrene-D | 23.52 | 3.17 | - | - | 23.51 | 3.67 |
| 39 | ë-Cadinene | 24.11 | 0.22 | - | - | - | - |
| 40 | (−)-Caryophyllene oxide | 25.14 | 0.57 | - | - | - | - |
| 41 | Hexanal | - | - | 4.77 | 0.10 | - | - |
| 42 | 5-Methyl-3-(1-methylvinyl)-1,4-hexadiene | - | - | 7.31 | 0.79 | 7.32 | 0.35 |
| 43 | Tricyclene | - | - | 7.88 | 0.14 | 7.89 | 0.87 |
| 44 | Benzaldehyde | - | - | 9.00 | 2.00 | 9.02 | 0.81 |
| 45 | 9-Oxabicyclo[6.1.0]nonane | - | - | 10.01 | 2.57 | - | - |
| 46 | á-Terpinolene | - | - | 10.66 | 0.82 | - | - |
| 47 | Benzene,1-methyl-4-(1-methylethyl) | - | - | 10.91 | 3.69 | - | - |
| 48 | Ç-Terpinene | - | - | 11.89 | 2.09 | - | - |
| 49 | 2-(1-Bromo-1-methylethyl)pyrimidine | - | - | 12.18 | 1.11 | - | - |
| 50 | Linalool | - | - | 13.19 | 1.64 | - | - |
| 51 | Pinocarvone | - | - | 15.02 | 1.64 | - | - |
| 52 | L-4-Terpineneolc | - | - | 15.60 | 8.40 | - | - |
| 53 | á Terpineol | - | - | 15.98 | 1.79 | 16.07 | 2.98 |
| 54 | E-3,5-Dimethylhex-2-en-1,2-dicarboxylic acid | - | - | 16.91 | 0.41 | - | - |
| 55 | Benzaldehyde, 4-(1-methylethyl) | - | - | 17.31 | 0.90 | - | - |
| 56 | Phenol, 5-Methyl-2-(1-Methylethyl) | - | - | 18.88 | 2.37 | - | - |
| 57 | Isolongifolone | - | - | 20.02 | 0.49 | - | - |
| 58 | 13,14,15,16,17-Pentanorlabda-7,9 (11)diene | - | - | 20.55 | 1.20 | - | - |
| 59 | (−)-á-Elemenec | - | - | 21.71 | 7.01 | - | - |
| 60 | á-Humulene | - | - | 23.44 | 2.16 | - | - |
| 61 | 13,14,15,16,17-Pentanorlabda-7,9 (11)diene | - | - | 21.31 | 1.30 | - | - |
| 62 | á-Selinenec | - | - | 23.66 | 13.38 | - | - |
| 63 | á-Sesquiphellandrene | - | - | 24.20 | 3.45 | - | - |
| 64 | Methoxy-pivalonitrile | - | - | 24.91 | 0.54 | - | - |
| 65 | (−)-Spathulenol | - | - | 25.07 | 3.84 | - | - |
| 66 | (−)-Caryophyllene oxide | - | - | 25.41 | 0.67 | 25.14 | 0.13 |
| 67 | Oxacyclotetradecan-2-one, 13-methyl | - | - | 26.07 | 0.94 | - | - |
| 68 | Heliannuol K | - | - | 26.88 | 0.76 | - | - |
| 69 | 2-Butanone,3,3-dimethyl-1-(methylsulfonyl)-,O-[(methylamino)carbonyl]oxime | - | - | - | - | 4.12 | 0.14 |
| 70 | Cis-Salvene | - | - | - | - | 5.99 | 0.63 |
| 71 | 9-Oxabicyclo[6.1.0]Non-4-Ene | - | - | - | - | 6.73 | 0.10 |
| 72 | Sabinenec | - | - | - | - | 9.39 | 4.86 |
| 73 | 4,7,7-Trimethylbicyclo[4.1.0]Hept-4-En-3-Ol | - | - | - | - | 9.86 | 1.69 |
| 74 | á Terpinene | - | - | - | - | 10.68 | 0.99 |
| 75 | Endobornyl acetate | - | - | - | - | 18.71 | 1.19 |
| 76 | Phenol,2-Methyl-5-(1-methylethyl) | - | - | - | - | 19.20 | 0.43 |
| 77 | (E)-1-(P-Methoxyphenyl)-2-methoxyethylene | - | - | - | - | 19.90 | 0.13 |
| 78 | Isolongifolene | - | - | - | - | 20.05 | 0.15 |
| 79 | á-Terpinenyl acetate | - | - | - | - | 20.60 | 0.47 |
| 80 | á-Copaene | - | - | - | - | 21.37 | 0.59 |
| 81 | -á-Elemene | - | - | - | - | 21.68 | 1.16 |
| 82 | á-Copaen-4 á-Ol | - | - | - | - | 22.02 | 0.13 |
| 83 | Trans-Á-lonone | - | - | - | - | 25.43 | 0.28 |
| 84 | Chrysanthenyl acetate | - | - | 20.71 | 0.48 | - | - |
Table 4
Effect of different application concentrations of Mentha longifolia, Achillea arabica, and Artemisia absinthium essential oils on the mycelial growth (colony diameter, cm) of Verticillium dahliae, Rhizoctonia solani, and Fusarium oxysporum during three, five, and seven days of incubation
| Essential oil | Dose (μl/ml) | Mycelial growth (cm)a | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Verticillium dahliae | Rhizoctonia solani | Fusarium oxysporum | ||||||||
|
|
|
|
||||||||
| 3 days | 5 days | 7 days | 3 days | 5 days | 7 days | 3 days | 5 days | 7 days | ||
| Mentha longifolia | Control | 0.79 ± 0.06 cde | 1.59 ± 0.06 de | 2.30 ± 0.05 fgh | 8.19 ± 0.25 ı | 8.50 ± 0.00 f | 8.50 ± 0.00 f | 3.46 ± 0.02 jk | 6.11 ± 0.04 ı | 8.50 ± 0.00 j |
| 0.05 | 0.74 ± 0.06 cde | 1.26 ± 0.08 bcde | 1.86 ± 0.11 bcdefg | 7.70 ± 0.20 iı | 8.50 ± 0.00 f | 8.50 ± 0.00 f | 3.11 ± 0.08 hiı | 5.84 ± 0.06 hiı | 7.59 ± 0.11 hiı | |
| 0.1 | 0.84 ± 0.02 cde | 1.65 ± 0.05 d | 2.19 ± 0.04 efgh | 5.34 ± 0.34 fgh | 8.50 ± 0.00 f | 8.50 ± 0.00 f | 3.03 ± 0.09 hi | 5.63 ± 0.17 ghiı | 7.55 ± 0.11 hiı | |
| 0.25 | 0.70 ± 0.06 cde | 0.98 ± 0.09 bcde | 1.80 ± 0.07 bcdefg | 2.44 ± 0.17 cd | 6.55 ± 0.52 de | 8.50 ± 0.00 f | 1.78 ± 0.03 de | 3.63 ± 0.09 e | 5.33 ± 0.09 de | |
| 0.5 | 0.53 ± 0.18 bcd | 0.75 ± 0.07 bc | 0.90 ± 0.10 b | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.86 ± 0.13 b | 2.01 ± 0.10 c | 3.15 ± 0.12 c | |
| 1 | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | |
| 2 | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | |
| Sig | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Achillea arabica | Control | 0.79 ± 0.06 cde | 1.59 ± 0.06 de | 2.30 ± 0.05 fgh | 8.19 ± 0.25 hiı | 8.50 ± 0.00 f | 8.50 ± 0.00 f | 3.46 ± 0.02 jk | 6.11 ± 0.04 ı | 8.50 ± 0.00 j |
| 0.05 | 0.80 ± 0.04 cde | 1.48 ± 0.05 cde | 2.06 ± 0.08 cdefgh | 4.25 ± 0.26 def | 8.50 ± 0.00 f | 8.50 ± 0.00 f | 2.34 ± 0.09 f | 4.50 ± 0.09 f | 6.26 ± 0.10 f | |
| 0.1 | 0.55 ± 0.19 bc | 1.53 ± 0.10 cde | 1.51 ± 0.05 bcdef | 3.09 ± 0.13 cde | 7.99 ± 0.19 ef | 8.50 ± 0.00 f | 3.48 ± 0.03 jk | 5.45 ± 0.07 hiı | 7.78 ± 0.17 hiıj | |
| 0.25 | 0.84 ± 0.02 cde | 1.49 ± 0.07 cde | 2.05 ± 0.07 cdefgh | 3.36 ± 0.32 cde | 7.50 ± 0.53 ef | 8.50 ± 0.00 f | 3.03 ± 0.01 hi | 5.18 ± 0.04 g | 7.18 ± 0.05 gh | |
| 0.5 | 0.71 ± 0.01 cde | 1.74 ± 0.10 e | 1.81 ± 0.09 bcdefg | 2.39 ± 0.31 cd | 6.41 ± 0.65 de | 8.50 ± 0.00 f | 3.39 ± 0.12 ıjk | 5.31 ± 0.08 gh | 7.48 ± 0.05 hi | |
| 1 | 0.78 ± 0.03 cde | 0.94 ± 0.05 bcde | 1.10 ± 0.04 bcd | 1.86 ± 0.31 bc | 5.04 ± 0.52 cd | 8.50 ± 0.00 f | 1.48 ± 0.06 cd | 2.61 ± 0.08 d | 3.49 ± 0.11 c | |
| 2 | 0.70 ± 0.05 cde | 1.21 ± 0.03 bcde | 1.71 ± 0.02 bcdefg | 4.05 ± 1.98 def | 5.31 ± 0.20 cd | 8.50 ± 0.00 f | 1.74 ± 0.04 de | 3.29 ± 0.11 e | 4.74 ± 0.18 d | |
| Sig | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Artemisia absinthium | Control | 0.79 ± 0.06 cde | 1.59 ± 0.06 de | 2.30 ± 0.05 fgh | 8.19 ± 0.25 i | 8.50 ± 0.00 f | 8.50 ± 0.00 f | 3.46 ± 0.03 jk | 6.11 ± 0.04 ı | 8.50 ± 0.00 j |
| 0.05 | 0.88 ± 0.05 cde | 1.71 ± 0.06 e | 2.44 ± 0.07 fgh | 6.30 ± 0.42 ghiı | 8.50 ± 0.00 f | 8.50 ± 0.00 f | 3.53 ± 0.09 jk | 5.70 ± 0.12 ghiı | 7.93 ± 0.14 hiıj | |
| 0.1 | 0.70 ± 0.04 cde | 1.55 ± 0.19 de | 1.96 ± 0.11 cdefg | 4.60 ± 0.04 efg | 8.50 ± 0.00 f | 8.50 ± 0.00 f | 3.53 ± 0.04 jk | 5.86 ± 0.04 hiı | 7.79 ± 0.07 hiıj | |
| 0.25 | 0.72 ± 0.04 cde | 1.47 ± 0.14 cde | 2.08 ± 0.12 cdefgh | 3.20 ± 0.14 cde | 8.50 ± 0.00 f | 8.50 ± 0.00 f | 2.98 ± 0.05 hi | 5.16 ± 0.17 g | 7.18 ± 0.10 gh | |
| 0.5 | 0.73 ± 0.11 cde | 1.20 ± 0.14 bcde | 1.79 ± 0.18 bcdefg | 3.53 ± 0.11 cde | 8.50 ± 0.00 f | 8.50 ± 0.00 f | 2.51 ± 0.11 fg | 4.59 ± 0.14 f | 6.58 ± 0.26 fg | |
| 1 | 0.65 ± 0.02 cde | 1.18 ± 0.08 bcde | 1.66 ± 0.01 bcdefg | 0.38 ± 0.38 ab | 2.09 ± 1.37 b | 4.31 ± 1.94 b | 1.34 ± 0.24 c | 2.35 ± 0.46 cd | 3.54 ± 0.62 c | |
| 2 | 0.39 ± 0.23 abc | 0.83 ± 0.12 bcd | 1.06 ± 0.12 bc | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.14 ± 0.14 a | 0.30 ± 0.17 a | 0.55 ± 0.34 b | 1.14 ± 0.36 b | |
| Sig | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
References
Abdel-Gwad, N. M., Mahmoud, E. A. M., Al-Askalany, S. A. and Hanafy, E. A. 2022. Antioxidant, antibacterial and cytotoxic effect of Cymbopogon citratus, Mentha longifolia, and Artemisia absinthium essential oils. Egypt. J. Chem. 65:287-296.

Abedi, R., Golparvar, A. R. and Hadipanah, A. 2015. Identification of the essential oils composition from four ecotypes of Mentha longifolia (L.) Huds. growing wild in Isfahan province, Iran. J. Biosci. Biotechnol. 4:117-121.
Agrios, G. N. 2005. Plant pathology. 5th ed. Elsevier Academic Press, San Diego, CA, USA. pp. 948.
Ahmed, H. F. A., Seleiman, M. F., Mohamed, I. A. A., Taha, R. S., Wasonga, D. O. and Battaglia, M. L. 2023. Activity of essential oils and plant extracts as biofungicides for suppression of soil-borne fungi associated with root rot and wilt of Marigold (Calendula officinalis L.). Horticulturae 9:222.

Ajayi-Oyetunde, O. O. and Bradley, C. A. 2018.
Rhizoctonia solani: taxonomy, population biology and management of Rhizoctonia seedling disease of soybean. Plant Pathol. 67:3-17.


Akkuş, S., Gözüaçık, C. and Gültekin, L. 2021. Fumigant effects of essential oils of Thymus sipyleus (Boiss.) subsp. rosulans (Borbas) Jalas and Mentha longifolia subsp. longifolia (Lamiaceae) against some stored product pests. J. Inst. Sci. Technol. 11:2487-2497.

Altundağ, E. 2009. Uses of the wild plants in Iğdir province (East Anatolia). Ph.D. thesis. İstanbul University, İstanbul, Türkiye.
Appendini, P. and Hotchkiss, J. H. 2002. Review of antimicrobial food packaging. Innov. Food Sci. Emerg. Technol. 3:113-126.

Ashraf, S. and Zuhaib, M. 2013. Fungal biodiversity: a potential tool in plant disease management. In: Management of microbial resources in the environment, eds. by A. Malik, E. Grohmann and M. Alves, pp. 69-90. Springer, Dordrecht, The Netherlands.

Aşkar, E. 2019. Pathogenicity and Fusarium species isolated from tomato (Solanum lycopersicon Mill) plant in Iğdir province. Ms. thesis. Iğdir University, Iğdir, Türkiye.
Bai, X., Aimila, A., Aidarhan, N., Duan, X. and Maiwulanjiang, M. 2020. Chemical constituents and biological activities of essential oil from Mentha longifolia: effects of different extraction methods. Int. J. Food Prop. 23:1951-1960.

Bailen, M., Julio, L. F., Diaz, C. E., Sanz, J., Martínez-Díaz, R. A., Cabrera, R., Burillo, J. and Gonzalez-Coloma, A. 2013. Chemical composition and biological effects of essential oils from Artemisia absinthium L. cultivated under different environmental conditions. Ind. Crops Prod. 49:102-107.

Bajpai, V. K. and Kang, S. C. 2012.
In vitro and in vivo inhibition of plant pathogenic fungi by essential oil and extracts of Magnolia liliflora Desr. J. Agric. Sci. Technol. 14:845-856.
Barış, Ö, Güllüce, M., Şahin, F., Özer, H., Kılıç, H., Özkan, H., Sömen, M. and Özbek, T. 2006. Biological activities of the essential oil and methanol extract of Achillea biebersteinii Afan. (Asteraceae). Türk. J. Biol. 30:65-73.
Başer, K. H. C. 2016. Essential oils of Achillea species of Turkey. Nat. Volatiles Essent. Oils 3:1-14.
Burt, S. 2004. Essential oils: their antibacterial properties and potential applications in foods—a review. Int. J. Food Microbiol. 94:223-253.


Choudhury, P. P. and Saha, S. 2020. Dynamics of pesticides under changing climatic scenario. Environ. Monit. Assess. 192:814.


Çopuroğlu, Ö 2013. Antimicrobial activities of some endemic plant species from Nigde region. M.S. thesis. Nigde University, Niğde, Türkiye.
Davari, M. and Ezazi, R. 2022. Mycelial inhibitory effects of antagonistic fungi, plant essential oils and propolis against five phytopathogenic Fusarium species. Arch. Microbiol. 204:480.



Delgado-Baquerizo, M., Guerra, C. A., Cano-Díaz, C., Egidi, E., Wang, J.-T., Eisenhauer, N., Singh, B. K. and Maestre, F. T. 2020. The proportion of soil-borne pathogens increases with warming at the global scale. Nat. Clim. Change 10:550-554.


Demirci, E. 1998.
Rhizoctonia species and anastomosis groups isolated from barley and wheat in Erzurum, Turkey. Plant Pathol. 47:10-15.

Dhifi, W., Bellili, S., Jazi, S., Bahloul, N. and Mnif, W. 2016. Essential oils’ chemical characterization and investigation of some biological activities: a critical review. Medicines 3:25.



Djordjevic, M., Djordjevic, O., Djordjevic, R., Mijatovic, M., Kostic, M., Todorovic, G. and Ivanovic, M. 2013. Alternative approach in control of tomato pathogen by using essential oils in vitro. Pak. J. Bot. 45:1069-1072.
Duniway, J. M. 2002. Status of chemical alternatives to methyl bromide for pre-plant fumigation of soil. Phytopathology 92:1337-1343.


Eken, C. and Demirci, E. 2003. Identification and pathogenicity of Rhizoctonia solani and binucleate Rhizoctonia anastomosis groups isolated from forage legumes in Erzurum, Turkey. Phytoparasitica 31:76-80.


Elad, Y., Williamson, B., Tudzynski, P. and Delen, N. 2004.
Botrytis: biology, pathology and control. Springer Science & Business Media, Berlin, Germany. pp. 403.
Eryılmaz, G. A. and Kılıç, O. 2018. Sustainable agriculture and good agricultural practices in Turkey. KSU J. Agric. Nat. 21:624-631.
Falleh, H., Jemaa, M. B., Saada, M. and Ksouri, R. 2020. Essential oils: a promising eco-friendly food preservative. Food Chem. 330:127268.


Figueiredo, A. C., Barroso, J. G., Pedro, L. G. and Scheffer, J. J. C. 2008. Factors affecting secondary metabolite production in plants: volatile components and essential oils. Flavour Frag. J. 23:213-226.

Ghannadi, A., Samsam-Shariat, S.-H. and Moattar, F. 1999. Composition of the leaf oil of Salvia hydrangea DC. ex Benth. grown in Iran. J. Essent. Oil Res. 11:745-746.

Golparvar, A. R., Hadipanah, A. and Gheisari, M. M. 2013. Chemical analysis and identification of the components of two ecotypes of (Mentha longifolia L.) in Iran province. Int. J. Agric. Crop Sci. 17:1946-1950.
Greff, B., Sáhó, A., Lakatos, E. and Varga, L. 2023. Biocontrol activity of aromatic and medicinal plants and their bioactive components against soil-borne pathogens. Plants 12:706.



Guzmán, E. and Lucia, A. 2021. Essential oils and their individual components in cosmetic products. Cosmetics 8:114.

İpek, A. and Gürbüz, B. 2010.
Salvia species in flora of Turkey and their status in danger. J. Field Crops Cent. Res. Inst. 19:30-35.
Kahramanoğlu, İ, Panfilova, O., Kesimci, T. G., Bozhüyük, A. U., Gürbüz, R. and Alptekin, H. 2022. Control of postharvest gray mold at strawberry fruits caused by Botrytis cinerea and improving fruit storability through Origanum onites L. and Ziziphora clinopodioides L. volatile essential oils. Agronomy 12:389.

Kakhky, A. M., Rustaiyan, A., Masoudi, S., Tabatabaei-Anaraki, M. and Salehi, H.-R. 2009. Composition of the essential oil of Perovskia abrotanoides Karel. and Mentha longifolia L. from Iran. J. Essent. Oil Bear. Plants 12:205-212.

Kaya, A. 2014. Investigation of antioxidant activity and analysis of chemical composition of essential oils of Calamintha incana (Sm.) Boiss. and Mentha longifolia (L.) Hudson subsp. Longifolia. M.S. thesis. Isparta University, Isparta, Türkiye.
Kesimci, T. G. and Demirci, E. 2020. Vegetative compatibility groups and pathogenicity of Verticillium dahliae isolates from strawberry plants in Erzurum and Erzincan provinces, Turkey. Fresenius Environ. Bull. 29:454-462.
Kesimci, T. G., Durak, E. D. and Demirci, E. 2022.
Rhizoctonia species from strawberry plants in Erzincan, Turkey: anastomosis groups and pathogenicity. J. Anim. Plant Sci. 32:721-728.
Kılıç, A. 2008. Methods of essential oil production. J. Bartin Fac. For. 10:37-45.
Koçak, R. and Boyraz, N. 2006. Fungicidal and fungistatic effects of essential oils of some plants. Selcuk J. Agric. Food Sci. 20:76-81.
Kordali, S., Cakir, A., Akcin, T. A., Mete, E., Akcin, A., Aydin, T. and Kilic, H. 2009. Antifungal and herbicidal properties of essential oils and n-hexane extracts of Achillea gypsicola Hub-Mor. and Achillea biebersteinii Afan. (Asteraceae). Ind. Crops Prod. 29:562-570.

Kordali, S., Cakir, A., Mavi, A., Kilic, H. and Yildirim, A. 2005. Screening of chemical composition and antifungal and antioxidant activities of the essential oils from three Turkish Artemisia species. J. Agric. Food Chem. 53:1408-1416.


Leblalta, A., Daoud, H., Semcheddine, C. and Demirtaş, I. 2020. Antifungal activity of Mentha rotundifolia essential oil against Fusarium oxysporum. Int. J. Innov. Approaches Agric. Res. 4:56-68.
Mahmoudi, R. 2014. Effect of Mentha longifolia L. essential oil on physicochemical properties of the bio-ayran. J. Essent. Oil Bear. Plants 17:56-66.

Mani-López, E., Cortés-Zavaleta, O. and López-Malo, A. 2021. A review of the methods used to determine the target site or the mechanism of action of essential oils and their components against fungi. SN Appl. Sci. 3:44.
Mkaddem, M., Bouajila, J., Ennajar, M., Lebrihi, A., Mathieu, F. and Romdhane, M. 2009. Chemical composition and antimicrobial and antioxidant activities of Mentha (longifolia L. and viridis) essential oils. J. Food Sci. 74:M358-M363.

Moghaddam, M., Pourbaige, M., Tabar, H. K., Farhadi, N. and Hosseini, S. M. A. 2013. Composition and antifungal activity of peppermint (Mentha piperita) essential oil from Iran. J. Essent. Oil Bear. Plants 16:506-512.

Morteza-Semnani, K. and Akbarzadeh, M. 2005. Essential oils composition of Iranian Artemisia absinthium L. and Artemisia scoparia Waldst. et Kit. J. Essent. Oil Res. 17:321-322.

Mottaghi, M., Salehi Shanjani, P., Jafari, A. A., Mirza, M. and Bihamta, M. R. 2016. Essential oil composition of Achillea filipendulina, A. arabica and A. eriophora cultivated under temperate climate in Iran. J. Med. Plants By-prod. 2:153-158.
Nazzaro, F., Fratianni, F., Coppola, R. and De Feo, V. 2017. Essential oils and antifungal activity. Pharmaceuticals 10:86.



Okut, N., Yagmur, M., Selcuk, N. and Yildirim, B. 2017. Chemical composition of essential oil of Mentha longifolia L subsp. longifolia growing wild. Pak. J. Bot. 49:525-529.
Özbek, ÖÇ 2019. Chemical composition and antimicrobial effects of essential oils in some spices. M.S. thesis. Ege University, İzmir, Türkiye.
Özgüven, M. and Kırıcı, S. 1999. Research on yield, essential oil, contents and components of mint (Mentha) species in different ecologies. Turk. J. Agric. For. 23:465-472.
Panth, M., Hassler, S. C. and Baysal-Gurel, F. 2020. Methods for management of soilborne diseases in crop production. Agriculture 10:16.

Park, J. H., Song, M. G., Lee, S. W., Choi, S. H. and Hong, J. K. 2022. Co-treatment with Origanum oil and Thyme oil vapours synergistically limits the growth of soil-borne pathogens causing strawberry diseases. Plant Pathol. J. 38:673-678.




Punja, Z. 2004. Fungal disease resistance in plants: biochemistry, molecular biology, and genetic engineering. Food Products Press, Binghamton, NY, USA. pp. 266.
Rezaeinodehi, A. and Khangholi, S. 2008. Chemical composition of the essential oil of Artemisia absinthium growing wild in Iran. Pak. J. Biol. Sci. 11:946-949.


Saharkhiz, M. J., Motamedi, M., Zomorodian, K., Pakshir, K., Miri, R. and Hemyari, K. 2012. Chemical composition, antifungal and antibiofilm activities of the essential oil of Mentha piperita L. ISRN Pharm. 2012:718645.




Saeidi, Z., Babaahmadi, H., Saeidi, K. A., Salehi, A., Jouneghani, R. S., Amirshekari, H. and Taghipour, A. 2012. Essential oil content and composition of Mentha longifolia (L.) Hudson grown wild in Iran. J. Med. Plants Res. 6:4522-4525.
Sharopov, F. S., Sulaimonova, V. A. and Setzer, W. N. 2012. Composition of the essential oil of Artemisia absinthium from Tajikistan. Rec. Nat. Prod. 6:127-134.
Siddiqui-Jain, A., Grand, C. L., Bearss, D. J. and Hurley, L. H. 2002. Direct evidence for a G-quadruplex in a promoter region and its targeting with a small molecule to repress c-MYC transcription. Proc. Natl. Acad. Sci. U. S. A. 99:11593-11598.



Soliman, K. M. and Badeaa, R. I. 2002. Effect of oil extracted from some medicinal plants on different mycotoxigenic fungi. Food Chem. Toxicol. 40:1669-1675.


Soylu, E. M., Yiğitbaş, H., Tok, F. M., Soylu, S., Kurt, Ş, Baysal, Ö and Kaya, A. D. 2005. Chemical composition and antifungal activity of the essential oil of Artemisia annua L. against foliar and soil-borne fungal pathogens. J. Plant Dis. Prot. 112:229-239.
Tabanca, N., Demirci, B., Gürbüz, İ, Demirci, F., Becnel, J. J., Wedge, D. E. and Can Başer, K. H. 2011. Essential oil composition of five collections of Achillea biebersteinii from central Turkey and their antifungal and insecticidal activity. Nat. Prod. Commun. 6:701-706.



Teniz, N. and Demirer Durak, E. 2023. Diagnosis and pathogenicity of Fusarium spp. and Rhizoctonia spp. from pepper, tomato and melon in Erciş, Gevaş and Edremit districts of Van. Yuzuncu Yil Univ. J. Inst. Nat. Appl. Sci. 28:704-714.
Torres Juan, M. 2017. Chemical composition and antifungal activity of essential oils of Artemisia herba-alba Asso, Artemisia absinthium L. and Mentha longifolia L. Ph.D. thesis. Universitat Politècnica, de València, Spain.
TÜBİVES, 2021 Turkish plants data service URL http://www.tubives.com/ [5 August 2021].
Vieira, T. M., Dias, H. J., Medeiros, T. C. T., Grundmann, C. O., Groppo, M., Heleno, V. C. G., Martins, C. H. G., Cunha, W. R., Crotti, A. E. M. and Silva, E. O. 2017. Chemical composition and antimicrobial activity of the essential oil of Artemisia absinthium Asteraceae leaves. J. Essent. Oil Bear. Plants 20:123-131.

Wu, H., Zhao, F., Li, Q., Huang, J. and Ju, J. 2022. Antifungal mechanism of essential oil against foodborne fungi and its application in the preservation of baked food. Crit. Rev. Food Sci. Nutr. Advanced online publication. https://doi.org/10.1080/10408398.2022.2124950.

- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 472 View
- 48 Download
- ORCID iDs
-
Tuba Genç Kesimci

https://orcid.org/0000-0003-2022-0193 - Related articles
-
Paenibacillus elgii SD17 as a Biocontrol Agent Against Soil-borne Turf Diseases2005 December;21(4)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



