Antifungal Activity-Guided Analysis of Actinostemma lobatum Extracts through Serial Sub-fractions
Article information
Abstract
Plants are treasure trove of novel compounds that have potential for antifungal chemicals and drugs. In our previous study, we had screened plant extracts obtained from more than eight hundred plant materials collected in Korea, and found that butanol fraction of the Actinostemma lobatum were most potent in suppressing growth of diverse fungal pathogens of plants. Here in this study, we describe further analysis of the butanol fraction, and summarize the results of subsequent antifungal activity test for the sub-fractions against a selected set of plant pathogenic fungi. This line of analyses allowed us to identify the sub-fractions that could account for a significant proportion of observed antifungal activity of initial butanol fraction from A. lobatum. Further analysis of these sub-fractions and determination of structure would provide the shortlist for novel compounds that can be a lead to new agrochemicals.
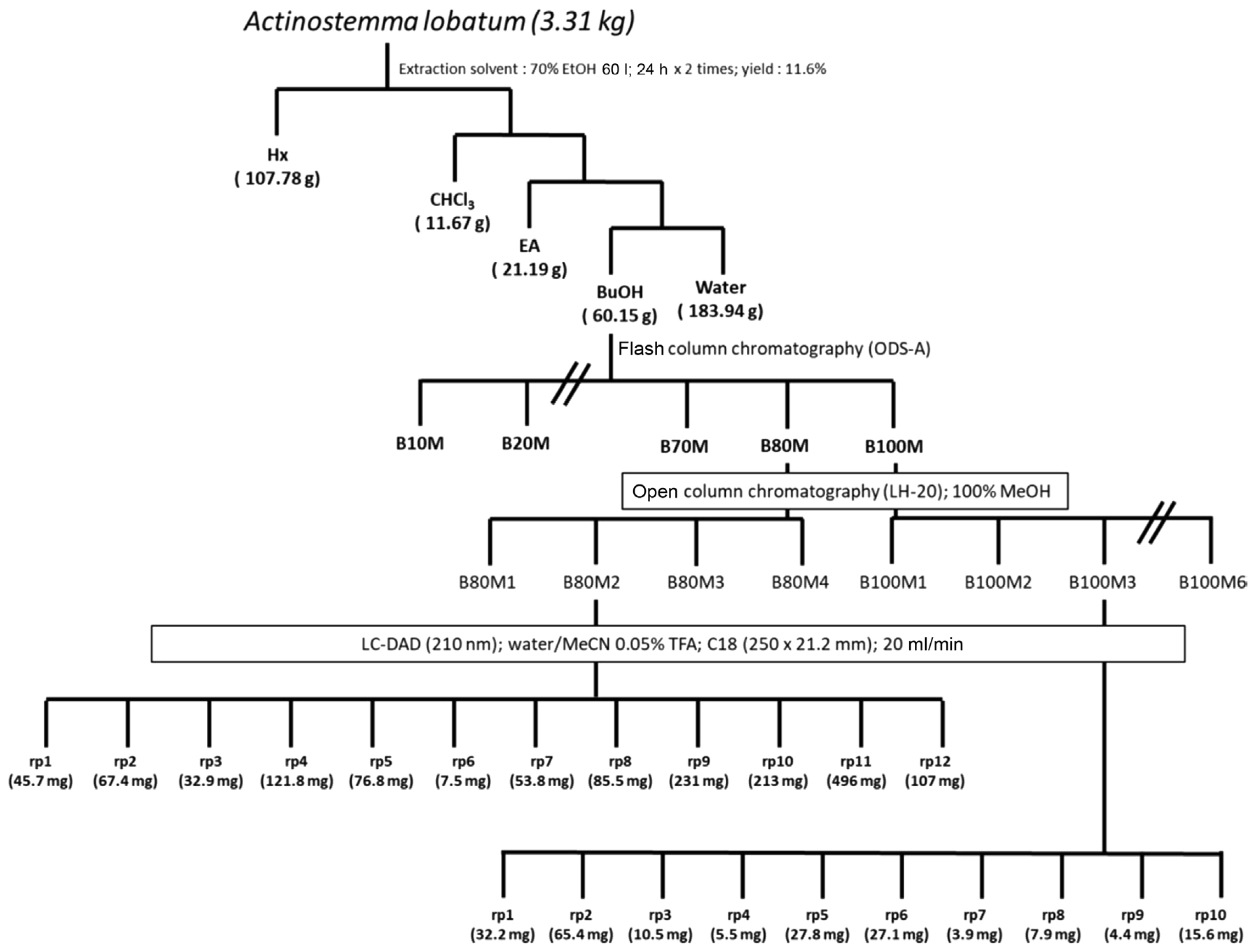
Chemicals derived from plants have played a crucial role in disease control and prevention (Arif et al., 2009). These natural compounds, often referred to as phytochemicals, are found in a wide variety of plants, and have been harnessed for their potent antimicrobial and medicinal properties (Mohammadi et al., 2014; Surapuram et al., 2014). To date, many plants species were exploited to screen for compounds that possess antifungal activity against the fungal pathogens of economically important crops (Choi et al., 2004; Hernández-Ceja et al., 2021; Yusoff et al., 2020). In our previous study, we have screened more than 800 plant extracts, which are obtained from The Nakdonggang National Institute of Biological Resources (NNIBR), for their antifungal activities (Lee et al., 2022). This large-scale screening revealed that the most promising ones are butanol extracts from Actinostemma lobatum (Fig. 1).
Here in this study, we present our follow-up work on the butanol extracts from A. lobatum. The butanol extracts were further fractionated based on antifungal activity assay. For antifungal assay, Aspergillus flavus, Botrytis cinerea, Rhizoctonia solani, Sclerotinia minor, Cochliobolus miyabeanus, Fusarium graminearum (teleomorph: Gibberella zeae GZ3639), Colletotrichum gloeosporioides (MGCF 10, YCJF 10, and YCJF 8), and Fusarium oxysporum (f.sp. conglutinans and f.sp. niveum) strains were grown on potato dextrose agar (PDA) medium at 25°C. Magnaporthe oryzae KJ201 and Phytophthora infestans strains were grown on oatmeal agar [OMA; 5% oatmeal (w/v) and 2% agar (w/v)] medium at 25°C and V8 agar medium at 16°C, respectively. The fungal strains were inoculated on a PDA, OMA, and V8 agar medium plate (90 mm × 15 mm Petri dish) at 25°C, 25°C, and 16°C, respectively. Antifungal assays were performed as described in our previous study (Lee et al., 2022).
The butanolic layer (ca. 7 g) was collected and subjected to chromatographed on a reversed-phase silica column. Elution was performed using two mixed solvents, H2O and MeOH, with decreasing polarity (MeOH/H2O = 1/9; 2/8; 3/7; 4/6; 1/1; 6/4; 7/3; 8/2; 1/0) to give nine fractions (B10M-B100M). Assays with those nine fractions showed that antifungal activities reside in three fractions (B70M, B80M, and B100M) (Fig. 2A, Supplementary Fig. 1). The three fractions were further chromatographed on a Sephadex LH-20 open column and eluted with 100% MeOH yielding sub-fractions (Fig. 1). Subsequent biological activity evaluation confirmed the efficacy of the re-fractions, B80M2 and B100M2 (Fig. 2B, Supplementary Figs. 2 and 3). The re-fractions were analyzed via reversed-phase high-performance liquid chromatography using a C18 column (250 × 21.2 mm ID, Phenomenex, Torrance, CA, USA). Elution was carried out using water (mobile phase A) and acetonitrile (B), both containing 0.05% trifluoroacetic acid, at a flow rate of 20 ml/min. The following gradient was applied: 5% B over 2 min, 5–45% B over 25 min, and 100% B over 5 min, using a 210 nm ultraviolet detector to yield 22 sub-fractions (B80M2-rp1 to B80M2-rp12 and B100M2-rp1 to B100M2-rp10) (Fig. 1). Antifungal assays with the 22 sub-fractions revealed that many of sub-fractions from B80M2 have strong antifungal activities (Fig. 2C), while antifungal activity of B100M3 appears to reside largely in rp1, rp2, and rp3 (Fig. 2D, Supplementary Fig. 4).
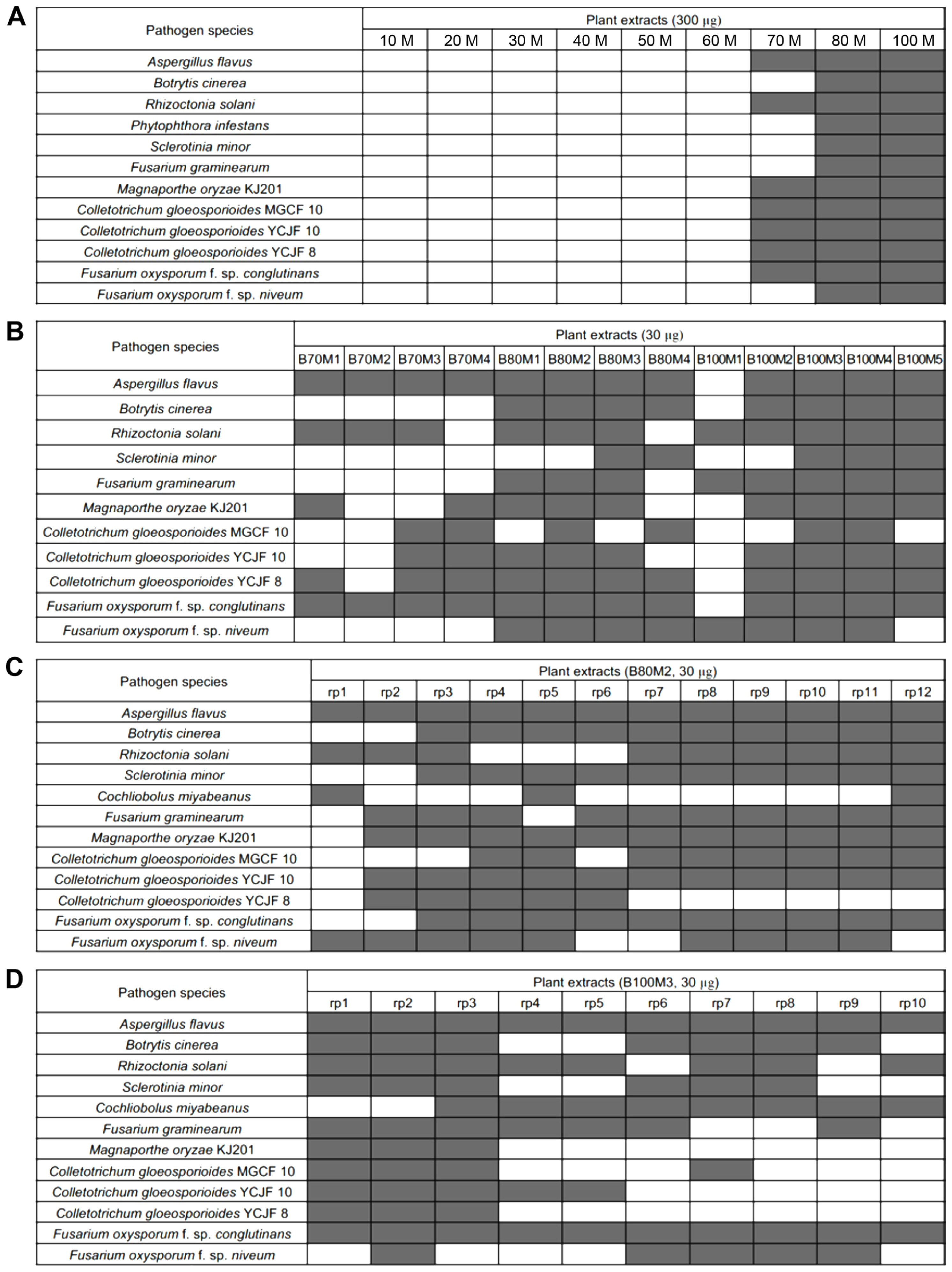
Antifungal activity of Actinostemma lobatum extracts against a set of common plant pathogens. (A) A heatmap summarizing antifungal activity test results of A. lobatum extracts, which are 10 M to 100 M. (B) A heatmap summarizing antifungal activity test results of A. lobatum extracts, which are B70M1 to 4, B80M1 to 4 and B100M1 to 5. (C) A heatmap summarizing antifungal activity test results of sub-fractions, which are B80M2 rp1 to rp12. (D) A heatmap summarizing antifungal activity test results of sub-fractions, which are B100M3 rp1 to rp10. Results were shown as binary outcome: inhibition, gray-filled block; no inhibition, white-block.
As the antifungal activity of sub-fractions were measured in terms of mycelial growth inhibition, it is not clear how effectively they can inhibit the fungal development and pathogenesis. In order to gauge the effect of sub-fractions on fungal development and pathogenicity in comparison to a well-known systemic fungicide, benomyl (Srivastava et al., 2017), a few sub-fractions (rp10 and rp11 from B80M2, and rp1 and rp2 from B100M3) were selected based on the antifungal activities and tested for their activities against the rice blast pathogen, Magnaporthe oryzae. M. oryzae is a causal agent of the rice blast disease, which is one of the most serious threats to food security in the rice-cultivating countries where the consumption of rice accounts for the major proportion of calorie intakes (Talbot, 2003). M. oryzae is a model pathogenic fungus for studying fungal development and pathogenesis, as it undergoes pathogenic development that is common to many plant-pathogenic fungi (Dean, 1997; Ryder et al., 2022). The fungus produces asexual spores called conidia that act as secondary inoculi. The conidium can germinate in presence of water, and upon recognition of surface properties, differentiates an infection-specific structure, appressorium at the tip of germ tube (Howard and Valent, 1996). By building up turgor pressure within appressorium, the fungus mechanically penetrates into the plant cells. The germination of conidium and subsequent appressorium formation are such critical steps for host infection that these processes have been targeted for development of chemical control measures.
For assay, the M. oryzae KJ201 strain was cultured on OMA medium at 25°C under constant light to promote conidium production. Conidia were harvested from 9-day-old culture using 5 ml of sterilized distilled water. The number of conidia was counted using a hemocytometer under a light microscope. To monitor germination and appressorium formation, the concentration of conidial suspension was adjusted to ~5 × 104 conidia/ml. A 40 μl suspension was placed on a hydrophobic surface of plastic coverslips and then incubated in a humidity chamber at 25°C. The germination and appressorium formation rate were calculated by counting the number of germinating spores and appressorium-forming conidia, respectively, out of at least 100 spores at 2, 4, and 6 h post-incubation. All the assays were conducted with three technical replicates in three independent experiments. Slides were observed under a DM2500 microscope (Leica, Wetzlar, Germany) and photographed with a DFC7000 T digital camera (Leica) using LAS X software (Leica). Our assay clearly showed that all the sub-fractions are able to quite effectively inhibit the germination (Fig. 3). In contrast to the DMSO-treated control, benomyl treatment conidia did not show any germination. Both the rp10 and rp11 fractions of B80M2 were also capable of inhibiting conidial germination and appressorium formation (Fig. 3A–F). In particular, rp11 showed inhibition at lower concentration (7.5 μg/ml) than the benomyl, suggesting the presence of more potent chemical(s) in rp11 than the benomyl (Fig. 3D–F). We obtained the similar results for rp1 and rp2 fractions of M100M3 (Fig. 3G–L). In our previous study, complete inhibition of conidial germination was achieved when at least 150 μg/ml of butanol fraction was used (Lee et al., 2022). Therefore, our data indicate that all the sub-fractions are likely to contain the chemical(s) with strong inhibitory effect on fungal development.

Effects of Actinostemma lobatum extracts on fungal development. (A) Images showing the effects of varying concentration of B80M2 rp10 on spore germination and appressorium of Magnaporthe oryzae KJ201 at 2, 4, and 6 h post-incubation of spores on hydrophobic surface. (B) Quantitative measurement of germination under B80M2 rp10 treatment. (C) Quantitative measurement of appressorium formation rates under B80M2 rp10 treatment. (D) Images showing the effects of varying concentration of B80M2 rp11 on spore germination and appressorium of Magnaporthe oryzae KJ201 at 2, 4, and 6 h post-incubation of spores on hydrophobic surface. (E) Quantitative measurement of germination under B80M2 rp11 treatment. (F) Quantitative measurement of appressorium formation rates under B80M2 rp11 treatment. (G) Images showing the effects of varying concentration of B100M3 rp1 on spore germination and appressorium of M. oryzae KJ201 at 2, 4, and 6 h post-incubation of spores on hydrophobic surface. (H) Quantitative measurement of germination under B100M3 rp1 treatment. (I) Quantitative measurement of appressorium formation rates under B100M3 rp1 treatment. (J) Images showing the effects of varying concentration of B100M3 rp2 on spore germination and appressorium of M. oryzae KJ201 at 2, 4, and 6 h post-incubation of spores on hydrophobic surface. (K) Quantitative measurement of germination under B100M3 rp2 treatment. (L) Quantitative measurement of appressorium formation rates under B100M3 rp2 treatment. Different letters on the bars indicate significant difference in mean values (P < 0.001, Tukey’s honestly significant difference test, n = 3).
To test whether the sub-fractions is indeed able to suppress disease development, we carried out pathogenicity assay based on wound inoculation method. Three- to four-leaf stages of rice plants (Oryza sativa cv. Dongjin) were used for pathogenicity assay. To carry out wound inoculation, 40 μl of spore suspension (~5 × 105 spores/ml) was directly placed onto wounded site of rice leaves. Inoculated plants were incubated in a humidity chamber without light for 24 h, and then transferred to an incubator for growth at 25°C with a 16 h photoperiod of fluorescent light. At 7 days post inoculation, lesion development was assessed and photographed. Experiments were done in three biological replicates, each of which involves plants grown in three pots.
Addition of benomyl (10 μg/ml) to the spore suspension prevented the development of disease lesions, unlike control (KJ201 + DMSO) (Fig. 4A and B). All the sub-fractions were comparable to the benomyl in suppressing disease development at concentrations of 15 and 30 μg/ml. However, at lower concentration (7.5 μg/ml), all but rp11 failed to curb the disease (Fig. 4A and B). Treatment with a low concentration of rp11 suppressed the disease lesion development as effectively as that with benomyl. Considering that rp11 was the only sub-fraction which showed inhibition of germination and appressorium formation at 7.5 μg/ml (Fig. 3), our pathogenicity assay data reflect the results on inhibition of germination and appressorium formation by sub-fractions. Although other sub-fractions than rp11 appear to be less potent in disease suppression than the benomyl, it should be noted that sub-fractions are still likely to contain multiple compounds in them, suggesting the presence of single more potent compound.
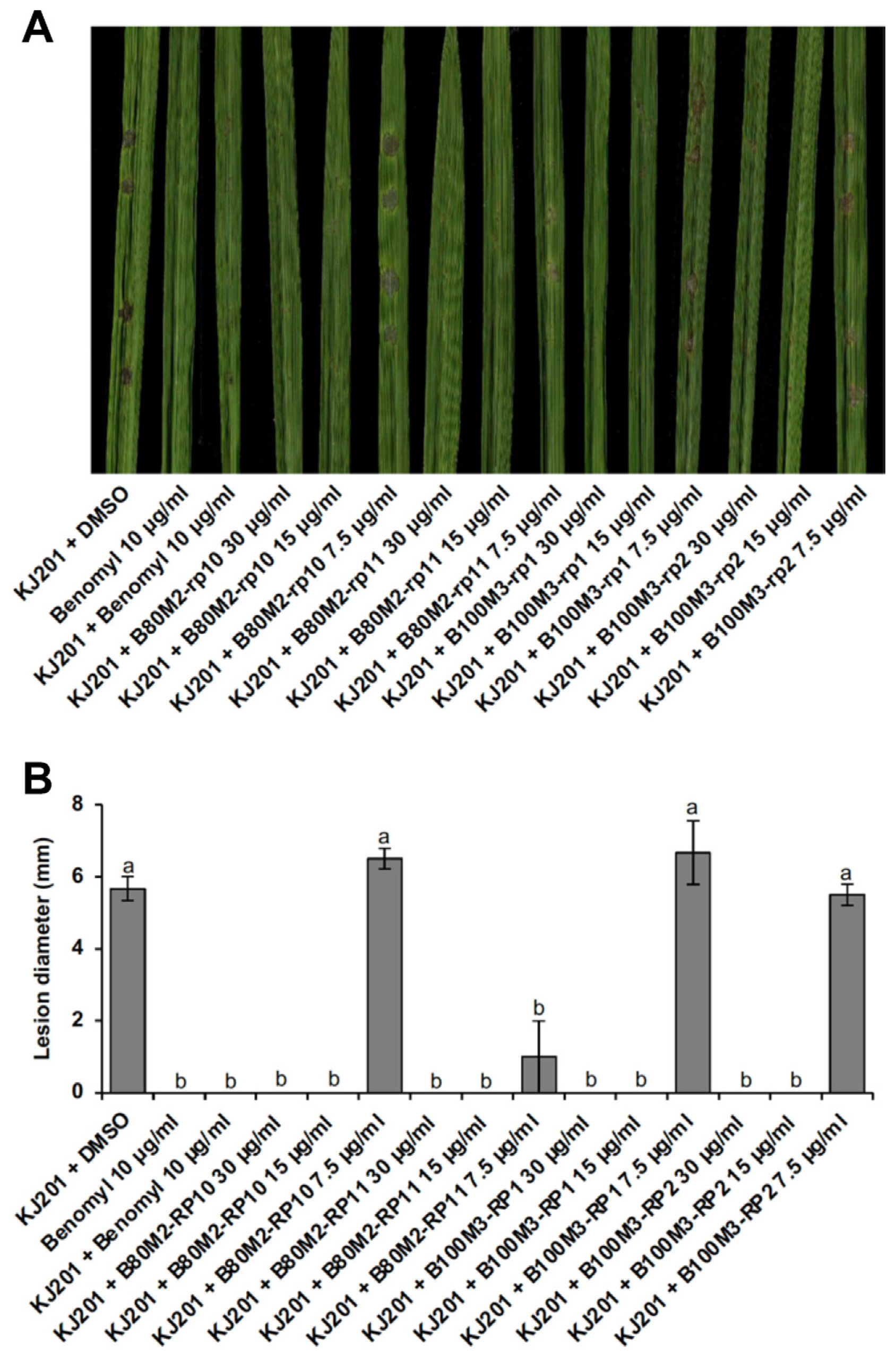
Effects of sub-fractions on fungal pathogenicity. (A) Assessment of pathogenicity of Magnaporthe oryzae KJ201 using wound inoculation with or without the sub-fractions, which are B80M2 rp10, B80M2 rp11, B100M3 rp1, and B100M3 rp2. (B) Quantitative analysis of pathogenicity assay results obtained by measurement of lesion length. Different letters on the bars indicate statistically significant difference in mean values (P < 0.001, Tukey’s honestly significant difference test, n = 3).
In this study, we described how we performed serial sub-fractions that are guided by biological activity. The butanol fractions that we started with contain obviously a mixture of large number of compounds in them. Using our approach in this study, we were able to narrow down to the sub-fractions that are expected to contain only a few compounds. Our antifungal activity assay clearly showed that these sub-fractions are much more potent than the initial butanol fractions, suggesting that those sub-fractions contain key compound(s) with antifungal activity comparable to the widely used fungicide, benomyl. Identification of the single active compound(s) in the sub-fractions would lead to the discovery and development of new fungicides(s) in the future.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Electronic Supplementary Material
Supplementary materials are available at The Plant Pathology Journal website (http://www.ppjonline.org/).
Acknowledgments
This work was supported by a grant from the Nakdonggang National Institute of Biological Resources (NNIBR), funded by the Ministry of Environment (MOE) of the Republic of Korea (NNIBR202102101).