 |
 |
| Plant Pathol J > Volume 33(4); 2017 > Article |
Abstract
Acknowledgments
Fig.┬Ā1

Fig.┬Ā2
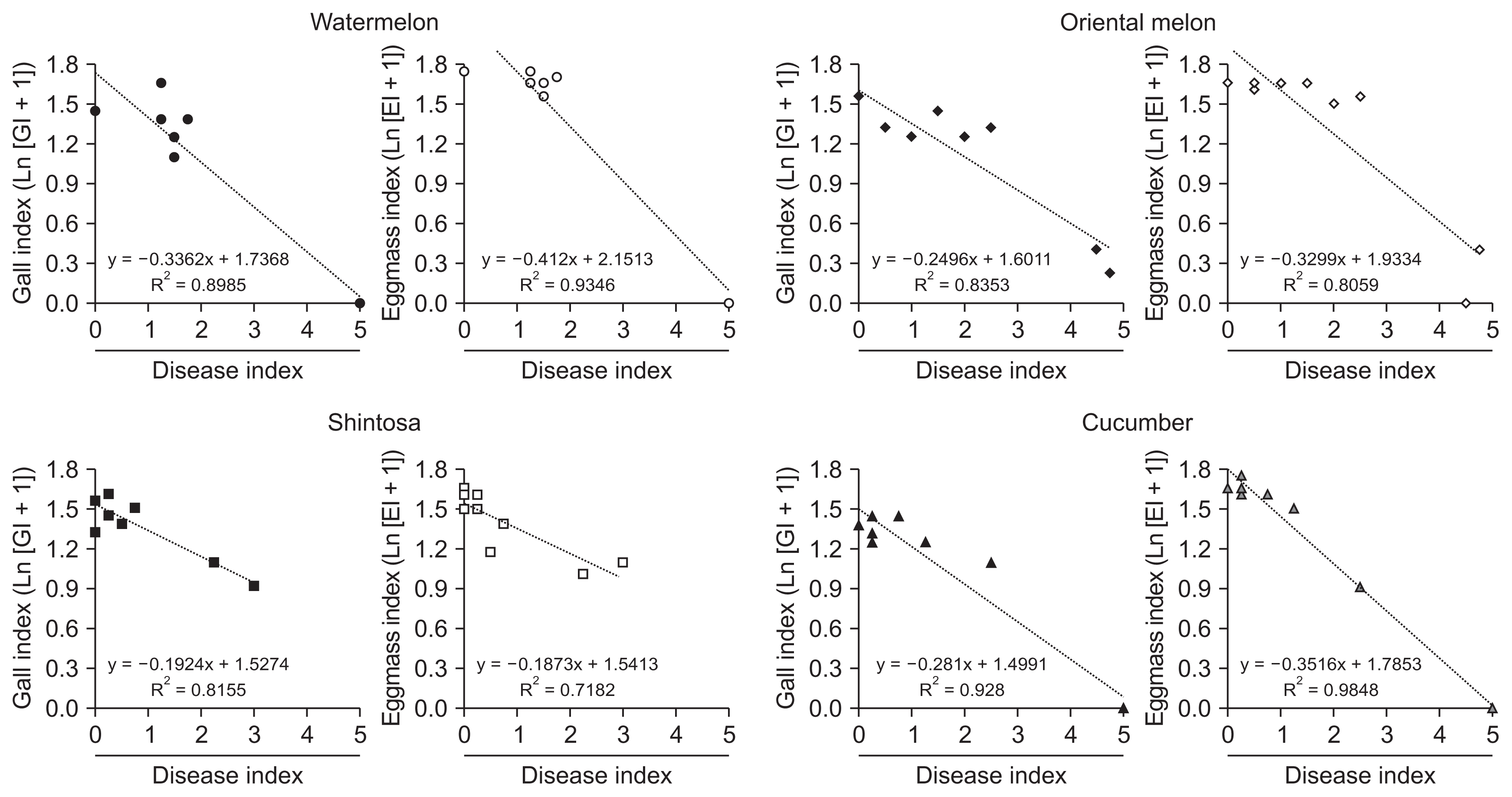
Fig.┬Ā3

Fig.┬Ā4
Fig.┬Ā5

Fig.┬Ā6

Table┬Ā1
| Inoculum* | DIŌĆĀ | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Watermelon | Oriental melon | Shintosa | Cucumber | ||||||
|
|
|
|
|
|
|||||
| Species | Isolate | RKNŌłÆ | RKN+ | RKNŌłÆ | RKN+ | RKNŌłÆ | RKN+ | RKNŌłÆ | RKN+ |
| F. oxysporum (LD) | FOM | 1.00 ┬▒ 0.00bŌĆĪX┬¦ | 1.25 ┬▒ 0.50bX | 1.00 ┬▒ 0.82abX | 1.50 ┬▒ 0.58bX | 0.00 ┬▒ 0.00bX | 0.00 ┬▒ 0.00bX | 0.25 ┬▒ 0.50cX | 0.25 ┬▒ 0.50cX |
| FON | 1.00 ┬▒ 0.00bX | 1.50 ┬▒ 0.58bX | 0.75 ┬▒ 0.50bX | 0.50 ┬▒ 0.58cX | 0.00 ┬▒ 0.00bX | 0.00 ┬▒ 0.00bX | 0.25 ┬▒ 0.50cX | 0.25 ┬▒ 0.50cX | |
| F. proliferatum (LD) | F5 | 0.50 ┬▒ 0.58bY | 1.25 ┬▒ 0.50bX | 2.00 ┬▒ 0.82aX | 2.00 ┬▒ 0.82bX | 0.50 ┬▒ 0.58bX | 0.50 ┬▒ 0.58bX | 1.75 ┬▒ 2.22bX | 1.25 ┬▒ 0.50bX |
| F6 | 5.00 ┬▒ 0.00aX | 5.00 ┬▒ 0.00aX | 2.00 ┬▒ 0.82aY | 4.75 ┬▒ 0.50aX | 2.00 ┬▒ 0.82aX | 2.25 ┬▒ 0.50aX | 5.00 ┬▒ 0.00aX | 5.00 ┬▒ 0.00aX | |
| Total (LD) | 1.88 ┬▒ 0.14Y | 2.25 ┬▒ 0.39X | 1.44 ┬▒ 0.74Y | 2.19 ┬▒ 0.62X | 0.63 ┬▒ 0.35X | 0.69 ┬▒ 0.27X | 1.81 ┬▒ 0.80X | 1.69 ┬▒ 0.38X | |
| F. oxysporum (HD) | FOM | 1.50 ┬▒ 1.00╬▓||X | 1.50 ┬▒ 0.58╬▓X | 1.25 ┬▒ 0.50╬▓ ╬│X | 1.00 ┬▒ 0.82╬│X | 0.25 ┬▒ 0.50╬▓X | 0.25 ┬▒ 0.50╬▓X | 0.50 ┬▒ 0.58╬▓ ╬│X | 0.75 ┬▒ 0.96╬│X |
| FON | 1.00 ┬▒ 0.00╬▓X | 1.50 ┬▒ 0.58╬▓X | 0.50 ┬▒ 0.58╬│X | 0.50 ┬▒ 0.58╬│X | 0.25 ┬▒ 0.50╬▓X | 0.25 ┬▒ 0.50╬▓X | 0.25 ┬▒ 0.50╬│X | 0.25 ┬▒ 0.50╬│X | |
| F. proliferatum (HD) | F5 | 1.50 ┬▒ 0.58╬▓X | 1.75 ┬▒ 0.96╬▓X | 2.25 ┬▒ 0.50╬▓X | 2.50 ┬▒ 0.58╬▓X | 1.00 ┬▒ 0.82╬▓X | 0.75 ┬▒ 0.50╬▓X | 1.50 ┬▒ 0.58╬▓X | 2.50 ┬▒ 1.73╬▓X |
| F6 | 5.00 ┬▒ 0.00╬▒X | 5.00 ┬▒ 0.00╬▒X | 4.50 ┬▒ 1.00╬▒X | 4.50 ┬▒ 1.00╬▒X | 3.50 ┬▒ 1.73╬▒X | 3.00 ┬▒ 1.41╬▒X | 5.00 ┬▒ 0.00╬▒X | 5.00 ┬▒ 0.00╬▒X | |
| Total (HD) | 2.25 ┬▒ 0.39X | 2.44 ┬▒ 0.53X | 2.13 ┬▒ 0.64X | 2.13 ┬▒ 0.74X | 1.25 ┬▒ 0.89X | 1.06 ┬▒ 0.73X | 1.81 ┬▒ 0.41X | 2.13 ┬▒ 0.80X | |
* LD, lower inoculum density with 10 g cultural medium (CM)/pot; HD, higher inoculum density with 20 g CM/pot; FOM, F. oxysporum f. sp. melonis; FON, F. oxysporum f. sp. niveum; F5 and F6, F. proliferatum isolates obtained from oriental melon greenhouses.
ŌĆĀ Wilt symptom development as measured by wilt disease severity index (DI) 0 to 5; 0 = no symptom, 1 = underground stem yellow-brownish discolored, 2 = < 30% aboveground stem brownish discolored, 3 = stem bottom region decayed, 4 = stem darkly discolored and split, 5 = whole plant dead (modified from Bletsos, 2005).
ŌĆĪ Averages with the same letters (a, b, c) denote no significant difference at P Ōēż 0.05 among Fusarium isolates with LD within a column by least significant difference (LSD) test.
Table┬Ā2
| Inoculum* | Watermelon | Oriental melon | Shintosa | Cucumber | |||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|||||
| Species | Isolate | GIŌĆĀ | EIŌĆĪ | GI | EI | GI | EI | GI | EI |
| RKN alone | 3.25 ┬▒ 0.50b┬¦X|| | 4.75 ┬▒ 0.50aX | 3.75 ┬▒ 1.26aX | 4.25 ┬▒ 0.50aX | 3.75 ┬▒ 0.96aX | 4.25 ┬▒ 0.50aX | 3.00 ┬▒ 0.82aX | 4.25 ┬▒ 0.50abX | |
| F. oxysporum (LD) | FOM | 3.00 ┬▒ 0.00b | 4.75 ┬▒ 0.50a | 3.25 ┬▒ 1.26a | 4.25 ┬▒ 0.50a | 3.75 ┬▒ 0.96a | 4.00 ┬▒ 0.00a | 3.25 ┬▒ 0.50a | 4.00 ┬▒ 0.00bc |
| FON | 2.00 ┬▒ 0.82c | 3.75 ┬▒ 1.26a | 2.75 ┬▒ 0.50a | 4.00 ┬▒ 0.00a | 2.75 ┬▒ 0.50ab | 3.50 ┬▒ 0.58a | 2.75 ┬▒ 0.96a | 4.75 ┬▒ 0.50a | |
| F. proliferatum (LD) | F5 | 4.25 ┬▒ 0.96a | 4.25 ┬▒ 0.50a | 2.50 ┬▒ 0.58a | 3.50 ┬▒ 1.00a | 3.00 ┬▒ 0.00ab | 2.25 ┬▒ 0.50b | 2.50 ┬▒ 0.58a | 3.50 ┬▒ 0.58c |
| F6 | 0.00 ┬▒ 0.00d | 0.00 ┬▒ 0.00b | 0.25 ┬▒ 0.50b | 0.50 ┬▒ 1.00b | 2.00 ┬▒ 0.00b | 1.75 ┬▒ 1.26b | 0.00 ┬▒ 0.00b | 0.00 ┬▒ 0.00d | |
| Total (LD) | 2.31 ┬▒ 0.44Y | 3.19 ┬▒ 0.56Y | 2.19 ┬▒ 0.71Y | 3.06 ┬▒ 0.63Y | 2.88 ┬▒ 0.36Y | 2.88 ┬▒ 0.58Y | 2.13 ┬▒ 0.51Y | 3.06 ┬▒ 0.27Y | |
| RKN alone | 3.25 ┬▒ 0.50╬▒┬ČX | 4.75 ┬▒ 0.50╬▒X | 3.75 ┬▒ 1.26╬▒X | 4.25 ┬▒ 0.50╬▒X | 3.75 ┬▒ 0.96╬▒X | 4.25 ┬▒ 0.50╬▒X | 3.00 ┬▒ 0.82╬▒X | 4.25 ┬▒ 0.50╬▒X | |
| F. oxysporum (HD) | FOM | 2.50 ┬▒ 0.58╬▓ ╬│ | 4.25 ┬▒ 0.50╬▒╬▓ | 2.50 ┬▒ 0.58╬▒ | 4.25 ┬▒ 0.50╬▒ | 3.25 ┬▒ 0.50╬▒ | 4.00 ┬▒ 0.00╬▒ | 3.25 ┬▒ 1.26╬▒ | 4.00 ┬▒ 0.00╬▒ |
| FON | 2.00 ┬▒ 0.00╬│ | 3.75 ┬▒ 0.50╬▓ | 2.75 ┬▒ 0.50╬▒ | 4.25 ┬▒ 0.50╬▒ | 4.00 ┬▒ 1.15╬▒ | 3.50 ┬▒ 0.58╬▒ | 2.50 ┬▒ 0.58╬▒ | 4.25 ┬▒ 0.50╬▒ | |
| F. proliferatum (HD) | F5 | 3.00 ┬▒ 0.00╬▒ ╬▓ | 4.50 ┬▒ 0.58╬▒╬▓ | 2.75 ┬▒ 0.50╬▒ | 3.75 ┬▒ 0.50╬▒ | 3.50 ┬▒ 0.58╬▒ | 3.00 ┬▒ 0.82╬▒╬▓ | 2.00 ┬▒ 1.41╬▒ | 1.50 ┬▒ 1.00╬▓ |
| F6 | 0.00 ┬▒ 0.00╬┤ | 0.00 ┬▒ 0.00╬│ | 0.50 ┬▒ 1.00╬▓ | 0.00 ┬▒ 0.00╬▓ | 1.50 ┬▒ 1.29╬▓ | 2.00 ┬▒ 1.41╬▓ | 0.00 ┬▒ 0.00╬▓ | 0.00 ┬▒ 0.00╬│ | |
| Total (HD) | 1.88 ┬▒ 0.14Y | 3.13 ┬▒ 0.39Y | 2.13 ┬▒ 0.64Y | 3.06 ┬▒ 0.38Y | 3.06 ┬▒ 0.88X | 3.13 ┬▒ 0.70Y | 1.94 ┬▒ 0.81X | 2.44 ┬▒ 0.38Y | |
* LD, lower inoculum density with 10 g cultural medium (CM)/pot; HD, higher inoculum density with 20 g CM/pot; FOM, F. oxysporum f. sp. melonis; FON, F. oxysporum f. sp. niveum; F5 and F6, F. proliferatum isolates obtained from oriental melon greenhouses.
ŌĆĀ Gall index (GI): 0-5 rating scale according to the percentage of galled tissue; 0 = 0-10% of galled roots, 1 = 11-20%, 2 = 21-50%, 3 = 51-80%, 4 = 81-90%, 5 = 91-100% (Barker, 1985).
ŌĆĪ Eggmass index (EI) was assigned to each count using a rating of 0 = no masses, 1 = 1-2 egg masses, 2 = 3-10 egg masses, 3 = 11-30 egg masses, 4 = 31-100 egg masses, and 5 = Ōēź 100 egg masses per root system (Taylor and Sasser, 1978).
┬¦ Averages with the same letters (a, b, c) denote no significant difference at P Ōēż 0.05 among Fusarium isolates with LD within a column by least significant difference (LSD) test.



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




