Tomato spotted wilt virus (TSWV) was first observed in Australia in 1915 (Brittlebank, 1919), and was identified as a viral disease in 1930 (Samuel et al., 1930). TSWV has a wide host range of more than 800 species in 82 families and is spread throughout the world. In Korea, TSWV was first observed on paprika in Yesan, Chungcheongnam-do Province in 2004 (Kim et al., 2004); at present, it is widespread in open fields of cultivated Capsicum annuum. TSWV is transmitted by thrips. Nine species of thrips are reported as TSWV vectors (Gibbs, 1983). As pesticides become less available, or less effective due to insecticide resistance in the thrips (Gao et al., 2012), alternative strategies are needed for crop protection. One such strategy is to use resistant cultivars.
Resistance of pepper plants to TSWV is conferred in both PI152225 and PI159236 lines by a single dominant gene (Tsw), which is resistant to a broad range of TSWV isolates (Black et al., 1996). The Tsw gene controls systemic spread of TSWV via a hypersensitivity reaction (HR) in the host plants (Black et al., 1991; Boiteux, 1995; Moury et al., 1997). The HR mechanism is known to be strongly influenced by ambient temperature, as well as physiological conditions of plants (Moury et al., 1998; Roselló et al., 1997; Soler et al., 1998).
Climate change models predict a progressive increase of the global average temperature of up to 4.6°C by the year 2100 (IPCC, 2014), with higher latitudes warming faster than lower latitudes (Jones, 2009). The dynamics of plant virus epidemics and the losses they cause are likely to be influenced greatly by the direct consequences of climate change, such as increased temperature, and indirectly by the abundance and activity of pathogen vectors (Jones, 2009). In this study, we investigated the effect of temperature on TSWV-Pap infection in both C. chinense and C. annuum, demonstrating the resistance conferred by the Tsw gene via HR. We found that Tsw-mediated resistance is overcome in both C. chinense and C annuum species at high temperatures (30 ± 2°C).
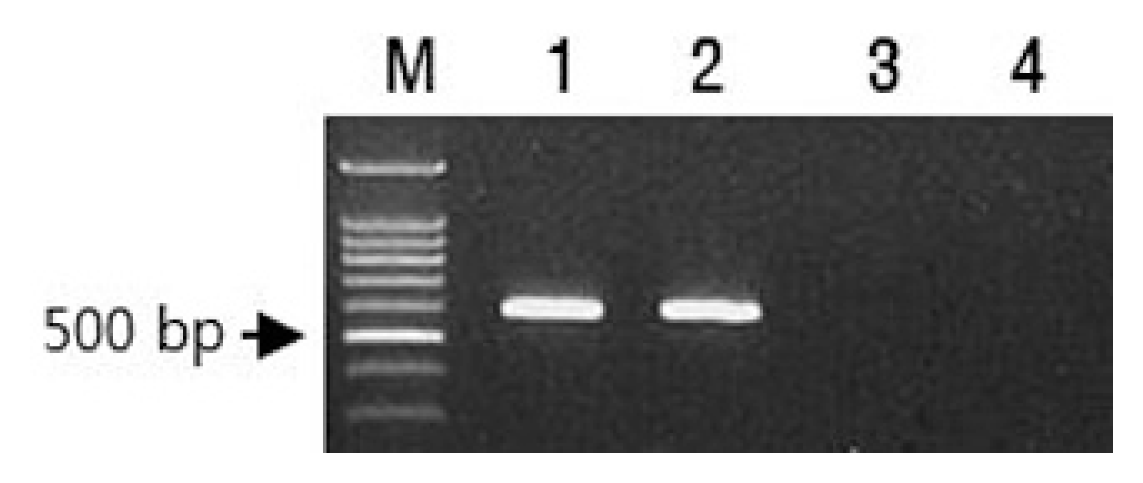
Accessions of C. chinense accession PI152225 and C. annuum S3669, carrying the Tsw gene, were obtained from AVRDC Vegetable Genetic Resources (Taiwan) and the Hana Seed Company (Korea), respectively. ‘Habanero’ and ‘Yeokgangsumumjang’ cultivars were used as TSWV-susceptible controls for C. chinense and C. annuum, respectively. The presence of Tsw gene from both PI152225 and S3669 was confirmed by polymerase chain reaction using a pairs of primer (Tsw 600F/Tsw 600R) (Fig. 1). However, there were no Tsw gene specific 600bp amplicons from both ‘Hanabero’ and ‘Yeokgangsumumjang’ (Fig. 1). The sequence of forward primer (Tsw 600F) was homologous to nucleotides 1501-24 of GenBank accession no. KT751527 and those of reverse primer (Tsw 600R) was complementary to nucleotides 2077-100 of the Tsw accession.
The TSWV inoculum source used in this study was TSWV-Pap isolated from paprika (Chung et al., 2012). Two true leaf stages of pepper seedlings were inoculated with TSWV-Pap-infected Nicotiana rustica crude sap in 0.05 M sodium phosphate buffer, pH 7.0, and were maintained at constant temperatures ranging from 15 ± 2°C to 30 ± 2°C (in 5°C increments) in growth chambers (16 h day/8 h night, 65% relative humidity) until 20 days postinoculation (dpi). Ten and 58 plants were tested for S3669 and PI152225, respectively. Fifty eight plants for PI152225 and 15 plants for Hanabero represent the total number from three experiments. We used mechanical transmission instead of thrips vector to inoculate each test plant with the same virus titer.
TSWV-Pap infection was confirmed by reverse transcription polymerase chain reaction (RT-PCR). Total RNA was isolated from leaf tissue using RNeasy Plant Mini Kit (Qiagen, Netherlands) according to the manufacturer’s instructions. The RT-PCR method and primers were the same as those used in a previous study (Chung et al., 2006). To avoid generation of defective strains that might occur in successive mechanical inoculations (Resende et al., 1991), virus inoculum plants were prepared from the TSWV-Pap isolate source for each experiment.
For Northern blot analysis, total RNA samples were isolated from either mock-inoculated or virus-infected plants using a RNeasy Plant Mini Kit (Qiagen), and were fractionated by electrophoresis in agarose gels under denaturing conditions (6% formaldehyde), blotted to nitrocellulose membranes, and hybridized with the specified digoxigenin-labeled RNA probes for TSWV segment M. Nonradioactive blots were washed, incubated with antidigoxigenin antibody (Roche Diagnostics, UK), and exposed following the manufacturer’s instructions as described in a prior study (Canto and Palukaitis, 2001). The TSWV probe was specific to the sequences encoding the NSm protein in TSWV segment M. Enzyme-linked immunosorbent assay (ELISA) was conducted using Agdia (Indiana, USA) product SRA39300 according to the manufacturer’s instructions.
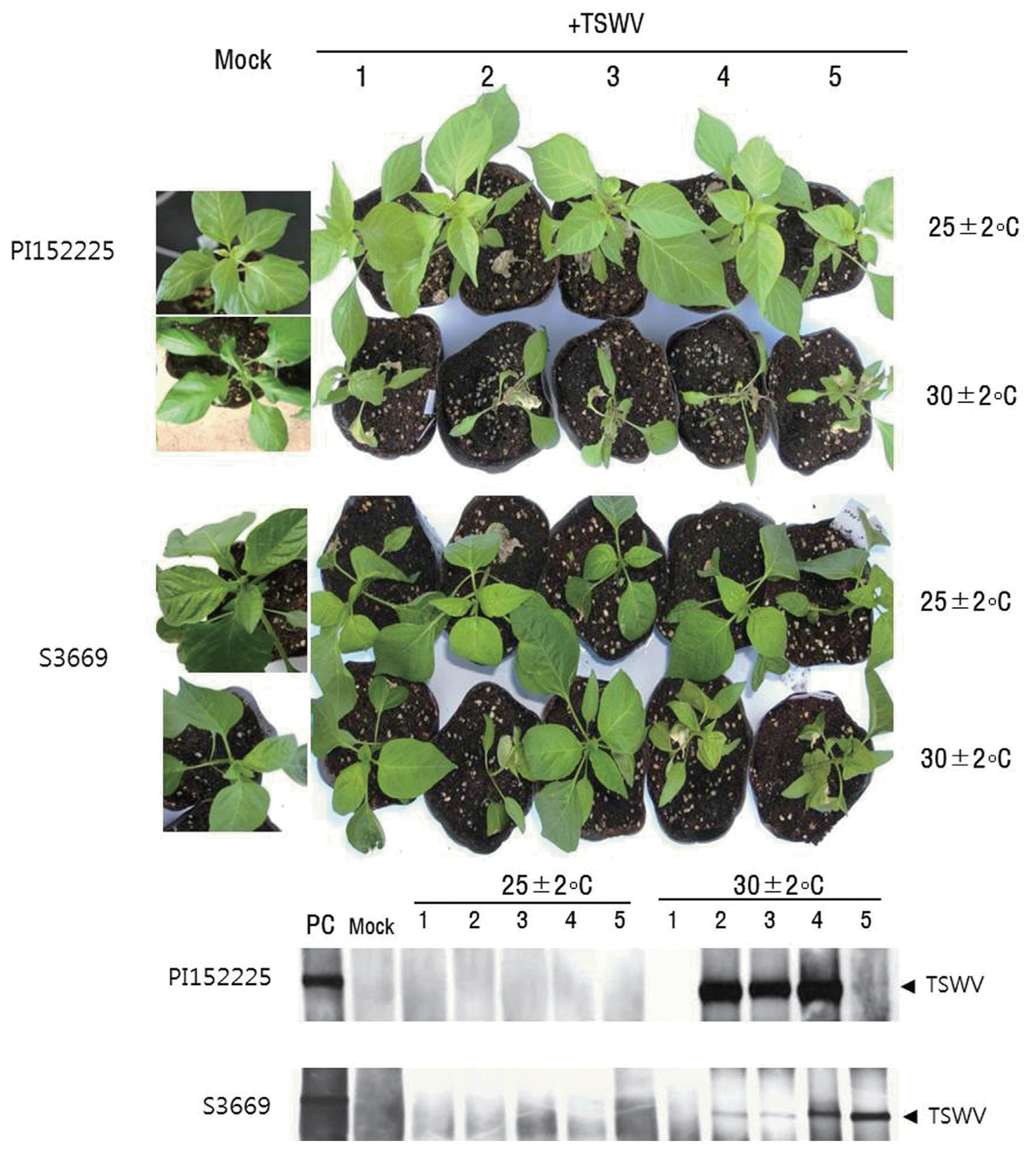
About 73% of PI152225 plants maintained at 25 ± 2°C produced HR on inoculated leaves, while 33% of plants maintained at 30 ± 2°C formed necrotic local lesions in an upward direction from leaf to leaf (non-self-limiting HR), revealing movement of virus to upper leaves (Table 1, Fig. 2). All 17% of PI152225 plants formed HR on inoculated leaves at 30 ± 2°C revealed systemic necrosis. HR reaction was observed in inoculated leaves of 20% of S3669 plants tested at 25 ± 2°C, while systemic infection was observed in 50% of the plants maintained at 30 ± 2°C (Table 1, Fig. 2). Mock plants of both PI152225 and S3669 did not show viral symptom (Fig. 2). Non of the S3669 plants formed HR on inoculated leaves at 30 ± 2°C revealed systemic infection (Table 1). Meanwhile, TSWV-susceptible cultivars ‘Habanero’ and ‘Yeokgang-sumumjang’ revealed systemic infection with 100% and 4-89% depend on temperature, respectively (Table 1).
Systemic infection of both S3669 and PI152225 plants was confirmed by detection of TSWV RNA using northern blot analysis (Fig. 2), and by detection of coat proteins using ELISA (data not shown). TSWV-specific bands were detected in the upper leaves of PI152225 2nd, 3rd, and 4th plants and S3669 2nd to 5th plants, while plants maintained at 25 ± 2°C did not exhibit TSWV-specific bands (Fig. 5). Mock plants of both PI152225 and S3669 did not show TSWV-specific bands (Fig. 2).
PI152225 plants formed necrotic spots in upper leaves at 30 ± 2°C revealed progress of necrotic symptoms from A to C as time passed (Fig. 3). Over time, plants with systemic necrosis withered due to stem necrosis (Fig. 3). HR reaction was observed in inoculated leaves of S3669 plants at 25 ± 2°C at 7 dpi, while systemic mottle infection was observed in plants maintained at 30 ± 2°C (Fig. 4). At 15 dpi, viral symptoms did not appear in leaves that had newly emerged, and the virus was not detected in symptomless upper leaves by RT-PCR (Fig. 4C). At 10 dpi, RT-PCR of 4 plants exhibiting systemic symptoms displayed TSWV-specific bands, while at 15 dpi, TSWV-specific bands were not observed in 2 of 4 plants (Fig. 4D).
Both TSWV-susceptible ‘Habanero’ and ‘Yeokgangsumumjang’ cultivars exhibited systemic mottle symptoms at 15-20 dpi (Fig. 5). ‘Habanero’ exhibited different symptoms depending on the temperatures maintained after inoculation with the virus. More severe symptoms were observed in plants maintained at 25 ± 2°C and 30 ± 2°C than those maintained at 15 ± 2°C and 20 ± 2°C. ‘Yeokgangsumumjang’ exhibited the most severe mottle symptoms, with necrosis of plants maintained at 25 ± 2°C (Fig. 5).
Tsw gene-based resistance was overcome in both C. chinense (PI152225) and C. annuum (S3669) genetic resources at 30 ± 2°C. PI152225 exhibited HR in inoculated leaves, followed by systemic necrotic local lesions, while S3669 displayed systemic mottle symptoms. Generally, necrotic local lesions induced by HR are self-limiting in size (Pennazio, 1995; Solymosy, 1970); however, in this study, local lesions were formed systemically in the upper leaves at high temperatures, showing that the virus was no longer confined to local lesions but able to spread to upper leaves. Our study demonstrated that the manifestation of systemic infection symptoms differs depending on the genetic resources of Tsw-carrying Capsicum species being used. PI152225 exhibited non-self-limiting necrotic spots in the upper leaves, while S3669 displayed systemic mottle symptoms.
The PI152225 and PI159236 C. chinense accessions are known to exhibit TSWV resistance; they develop HR without systemic infection (Dufour et al., 1989; Makkouk and Kumari, 1993). In this study, maintaining PI152225 plants at high temperatures (30 ± 2°C) after mechanical TSWV-Pap inoculation led to a modification of the virus symptom pattern in plant tissues, resulting in HR in inoculated leaves, followed by necrotic local lesions in the upper leaves. Similar breaking of TSWV resistance due to high temperatures has been previously reported. Roggero et al. (1996) found a break in TSWV resistance when C. chinense plants were maintained at a constant 33°C. Virus movement in PI159236 was faster when C. chinense plants were kept at a 30/18°C day/night temperature regime (Soler et al., 1988). The same phenomenon has been observed with the PI152225 accession, so increased viral accumulation may allow the virus to reach the phloem when the leaf acts as a photosynthate exporter (Soler et al., 1988). Breaking of Tobacco mosaic virus (TMV) resistance at high temperatures has also been reported (Weststeijn, 1984). During HR in tobacco expressing the N gene, tissue necrosis and resistance to TMV were overcome at temperatures above 28°C, followed by systemic infection without HR. Zhu et al. (2010) showed that mutation of the N gene and another gene SNC1 could provide HR at higher temperatures. In the current study, C. annuum S3669 inoculated with TSWV-Pap similarly displayed systemic mottle symptom without HR at high temperatures.
Although HR occurred on inoculated PI152225 leaves, viruses were not confined to the necrotic legions but moved systematically to the upper leaves. This finding supports the hypothesis that necrosis and virus localization are controlled by two different mechanisms (Pennazio, 1995). Pennazio (1995) proposed that high temperatures result in the blocking of virus localization, which is known to confer extreme resistance to the whole plant, leading to interference with virus replication by antiviral factors synthesized during HR, and inhibition of the cell-to-cell spread of virus infection. Samuel (1930) also found that higher temperatures inhibit HR. Taliansky et al. (1994) suggested that the ability of the virus to overcome HR could be related to high virus replication capacity in the host cells.
We assume that the differences that we observed in upper leaf symptoms between S3669 and PI152225 at high temperatures were due to differences in heat tolerance. A recent study (Chung et al., 2016) found that Potato virus Y-O and Potato virus A tended to accumulate at higher temperatures (30 ± 2°C) in early stages of infection, but that as time passed, virus accumulation was reduced, indicating an increase of host plant resistance over time. The modification of virus distribution has also been observed in wheat plants resistant to Wheat mosaic virus as temperature increases (Myers et al., 1993), as high temperatures affect the tridimensional structure of coat proteins, which act as elicitors of HR in TMV.
In this study, 25% of the S3669 plants that displayed systemic infection symptoms at 30 ± 2°C recovered from infection as time passed. Newly emerged leaves were symptomless and virus-free. This suggests that different resistance genes will behave differently at higher temperature. Symptom recovery in virus-infected plants is characterized by the emergence of asymptomatic leaves after systemic symptomatic infection, and has been linked to induction of RNA silencing (Baulcombe, 2004; Macdiarmid, 2005). Szittya et al. (2003) suggested that RNA silencing-mediated plant defenses are temperature-dependent and that levels of siRNA increase gradually with rising temperatures. One recent study (Ghoshal and Sanfacon, 2014) found that plant growth at lower temperatures (21°C rather than 27°C) alleviated the recovery of ToRSV-infected plants. This recovery was associated with AGO1 siRNA, which plays a central role in RNA silencing.
The purpose of this study was to investigate if the virus could overcome TSWV resistance conferred by the Tsw gene in Capsicum species including C. annuum at elevated temperature in the future. Thus, to determine the critical temperature at which virus resistance conferred by the Tsw gene be overcome, this study was conducted at constant temperature not at diurnal-nocturnal variation temperature. Conclusively we found that TSWV resistance shown from pepper plants possess the Tsw gene could reveal systemic infection at 30 ± 2°C. Our results indicated that TSWV resistance shown from pepper plants possess the Tsw gene could be overcome at high temperature in the future according to climate change scenarios (IPCC, 2014). Thus, breeders should conduct evaluation of TSWV resistance in pepper cultivars at high temperature (30°C) than at ambient.






 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Supplement
Supplement Print
Print






