 |
 |
| Plant Pathol J > Volume 29(4); 2013 > Article |
Abstract
In 2011-2012, sixty nine samples were collected from alfalfa plants showing viral infection symptoms in Riyadh region. Mechanical inoculation with sap prepared from two collected samples out of twenty five possitive for Alfalfa mosaic virus (AMV) by ELISA were produced systemic mosaic on Vigna unguiculata and Nicotiana tabacum, local lesion on Chenopodium amaranticolor and C. quinoa. Vicia faba indicator plants that induce mosaic and mottle with AMV-Sagir isolate and no infection with AMV-Wadi aldawasser isolate. Approximately 700-bp was formed by RT-PCR using AMV coat protein specific primer. Samples from infected alfalfa gave positive results, while healthy plant gave negative result using dot blot hybridization assay. The nucleotide sequences of the Saudi isolates were compared with corresponding viral nucleotide sequences reported in GenBank. The obtained results showed that the AMV from Australia, Brazil, Puglia and China had the highest similarity with AMV-Sajer isolate. While, the AMV from Spain and New Zealaland had the lowest similarity with AMV-Sajer and Wadi aldawasser isolates. The data obtained in this study has been deposited in the GenBank under the accession numbers KC434083 and KC434084 for AMV-Sajer and AMV- Wadialdawasser respectively. This is the first report regarding the gnetic make up of AMV in Saudi Arabia.
Generally, alfalfa ranks among the top crops in Saudi Arabia and is cultivated as a forage crop throughout the Kingdom with an average annual cultivation exceeds 122.536 thousand hectares and the productivity from this area is more than 252.844 thousand tons (Agriculture Statistical Year Book, 2010). Thirty one different viruses from thirteen different virus groups have been reported infecting alfalfa (Erwin et al., 1990). Some of which appear to have a world-wide distribution, but more studies are needel to validate this. Alfalfa mosaic virus (AMV) was first reported by Weimer, 1931 and is considered the most important viral pathogen in alfalfa (Avgelis, 2008; Parella, 2000). AMV is one of the most important viruses worldwide and has a very wide host range (Jaspers and Bos, 1980). This virus can naturally infect many herbaceous and some woody plant hosts (Jaspars and Bos, 1980; Xu and Nie, 2006). AMV belongs to the Alfamoviruses genus and is made up of particles that are composed of icosahedral capsids measuring 30-57 nm in length with a diameter of 18 nm. AMV is one of the most biologically variable plant viruses with numerous natural variants having different pathogenicity (Crill et al., 1970; Hajimorad and Francki, 1988; Hiruki and Miczynski, 1987; Walter et al., 1985). To date, AMV has been recorded throughout North America, England, France, Italy, Greece, Iran, Australia, New Zealand, Egypt and Saudi Arabia (Al-Shahwan and Abdulla, 1998; Al-Shahwan, 2002; Cook et al., 1984; El-Helaly et al., 2012; Massumi and Hosseini Pour1, 2007; Sawalha and Mansour, 1996a, b). AMV is transmitted in a non-persistent manner by numerous aphid species (Jeffries, 1998). The genome of AMV consists of three single-stranded RNA molecules of plus-sense polarity, designitedted as RNA1 to RNA3 in decreasing order of molecular size, encapsidated into B, M and Tb components, respectively (Jaspars, 1985). RNAs 1 and 2 encode the 1a and 2a subunits of the replicase proteins (Bol, 1999). RNA 3 is bicistronic and codes for a movement protein (Langereis et al., 1986) and the coat protein, which is translated from a subgenomic messenger, RNA 4 (Bol, 1999; Brederode et al., 1980; Tenlladoand Bol, 2000; Van der Vossen et al., 1994). In Saudi Arabia, alfalfa crop is nowadays showing peculiar types of symptoms along with the mottling, mosaic, yellowing, calico and stunting which are spreading in a several fields in different locations and causing drastic effect on the crop suggesting occurrence of new viruses which are yet to be determined or new strains of AMV which was already reported (Al-Shahwan, 2002; Cook et al., 1984). Since the isolation and the molecular characterization of AMV virus were not fully described in Saudi Arabia, here we report survey of AMV from a fields-grown alfalfa in Riyadh region in the Kingdom of Saudi Arabia characterizing using biological, serological and molecular techniques.
Sixty nine diseased leaves of alfalfa plants growing under field conditions and showing a mottling and mosaic symptoms suspected to be virus infections (Fig. 1) were collected from different location in Riyadh region. Commercial ELISA kits for alfalfa viruses (agdia Co France and AC diagnostic, USA) was used to serologically identify the viruses infecting alfalfa [AMV, Bean common mosaic virus (BCMV), Bean leaf roll virus (BLRV), Bean yellow mosaic virus (BYMV), Cucumber mosaic virus (CMV), Lucerne transient streak virus (LTSV), Pea streak virus (PSV), Red clover vein mosaic virus (RCVMV), Tobacco streak virus (TSV) and White clover mosaic virus (WCMV)]. The positive ones for AMV from each region were homogenized in a mortar, after adding phosphate buffer (1:3 w/v, 0.1 M, pH 7.2, Xu and Nie, 2006), then the extracted sap was passed through a double layer of cheesecloth and mechanically inoculated to selected herbaceous hosts including: Chenopodium amaranticolor Cost & Reyn, Ch. quinoa, Vigna unguiculata, Pisum sativum, Vicia faba, Nicotiana tabacum, Nicotiana benthamiana, Capsicum annum and Cucumis sativus. For biological purification, single local lesion technique was carried out (Kahn and Monroe, 1963) using C. amaranticolor as a local lesion host, whereas N. tabacum was used as propagative host for the following experiments. Five seedlings of each host were inoculated and observed daily for symptoms expression. A number of healthy seedlings of the same species and age were inoculated with buffer to serve as a control. Inoculated plants were kept in the greenhouse and used as a source of infection in the following experiments with temperature ranged between 25-32°C for 2-3 weeks. Symptoms expressions on each species were described and recorded.
Ten samples out of twenty six samples positive for AMV by ELISA were chosen as represent the two main regions in Riyadh for RT-PCR characterization. Total RNA from infected and uninfected alfalfa plants collected from Riyadh region (Wadi aldawasser and Sagir) using Isolate plant RNA Mini Kit was extracted (Bioline, A meridian Life Science Company). The oligonucleotide primers designed according to (Xu and Nie, 2006) was asfollows, the upstream primer AMVcoat-F: 5â˛-GT GGT GGG AAA GCT GGT AAA-3â˛] and downstream primer AMVcoat-R: 5â˛-CAC CCA GTG GAG GTC AGC ATT-3Ⲡwere used. RT-PCR was performed using the one Step RT-PCR Kit (Bioline). The reaction was set up according to manufacturerâs recommendations. A total of 1 Îźl (100 ng) of RNA was added to the one-step RT-PCR reaction mixture and amplified using the following cycling parameters: hold at 50 °C 30 minutes (RT step), hold at 95 °C 15 minutes (hot start to PCR), the tubes were heated at 94 °C for 2 min and then subjected to 35 cycles of amplification: 30 s at 94 °C for denaturation, 30 s at 58 °C for annealing, and 30 s at 74°C for extension, followed by a final hold at 72 °C for 10 minutes. Five Îźl aliquots from each of RT-PCR amplified DNA products were mixed with gel loading buffer (0.25% bromophenol blue, 0.25% xylene cyanol, and 30% glycerol). Separation was done on a 1% agarose gel in 1 Ă TBE buffer pH 8.3 (1 Ă = 89 mM Tris, 89 mM borate, and 2 mM EDTA). DNA was stained with ethidium bromide added to the gel at a concentration of 0.5 Îźg/ml. DNA was visualized on a UV transilluminator and photographed using DNA documentation gel analysis (IN GENIUS, Syngene Bio Imaging, UK). HyperLadder II DNA marker (Bioline) was used to determine the size of RT-PCR amplified cDNA products.
The probe was synthesized by using PCR DIG Probe Synthesis Kit (Roche) through PCR amplification. 1 Îźl of the DNA (the amplified 700 bp fragment from the virus was eluted and purified from agarose gel electrophoresis) was used as template DNA for the probe preparation. The master mix contains 5 Îźl of PCR buffer, 5 Îźl of PCR DIG probe synthesis Kit, 0.75 Îźl of the PCR enzyme mix, 1 Îźl of the template DNA, 2 Îźl of of the 10 ÎźM the reverse and forwared primers (AMVcoat-F and AMVcoat-R) and 34.25 Îźl of water PCR grade. The total volum of a PCR reaction tube was 50 Îźl. The thermocycling schedule was done as above. The probe quality was checked by running 5 Îźl of the DIG-labeled PCR product via gel electrophersis on 1% agarose gel using ethidium bromide. By comparing to the unlabelled PCR product, the DIG labeled PCR products showed a significant greater molecular weight than the unlabeled products. This is due to high density labeling with DIG. The samples were prepared for dot blot on nitro-cellulose membrane according to Podleckis et al., 1993; Pallas et al., 1998. Prehybridization, hybridization, and colorimetric detection were carried out using a hybridization oven (Amersham Biosciences, Piscataway, NJ, USA) following the protocol recommended by Boehringer Mannheim (Germany). The results were documented by photographing the wet filter.
Two samples out of ten samples positive fo AMV by RT-PCR were chosen as represent the two main regions in Riyadh for nucleotide sequence characterization. Virus-specific RT-PCR products from two isolates of AMV-SA (WD and Saj), collected naturally infected alfalfa plants with mosaic symptoms, were sequenced in the King Faisal Specialist Hospital and Research Centre, Riyadh-KSA, using an Applied Biosystem AB3730xI DNA analyzer (Life Technologies, USA). Partial nucleotide sequence of the CP gene was subjected to a BlastN search for comparison with published AMV CP gene sequences retrieved from GenBank (Table 2). A phylogenetic tree was constructed with CP sequences using DNAMAN trial version 5.2.10 program (Lynnon Biosoft, Canada). Nucleotide sequencing of the RT-PCR amplified fragment in the RT-PCR was completed to determine if these isolates were AMV or not and to compared with those of other AMV isolates available in GenBank. Nucleotide sequence of the CP gene of the two isolates from Saudi Arabia alfalfa was determined. The nucleotide sequence of the two Saudi isolates of AMV was submitted in the Gen-Bank under Accession No. KC434083 and KC434084 for AMV-Saj and AMV-Wd respectively. Multiple sequence alignment of the nucleotide sequence of the coat protein gene of Saudi isolates AMV (KC434083 and KC434084) with the corresponding sequences of twenty four different AMV isolates available in GenBank were analyzed using DNAMAN software.
Twenty five out of the 69 samples tested positive for AMV by ELISA, however, variable results were obtained when the samples were tested for the presence of other viruses infecting alfalfa (BLRV, BCMV, BYMV, CMV, LTSV, PeSV, RCVMV, TSV and WCMV). The symptoms induced by the two Saudi AMV isolates can be divided into four types. The first group includes the variable systemic symptom exhibited by the, V. unguiculata and N. tabacum. The second group included chlorotic local lesions exhibited by some plants (C. quinoa, C. amaranticolor), the third group included the rest of the plants that were not infected by these isolates (P. sativum, N. benthamiana, C. annum, C. sativus) and the fourth group include V. faba indicator plant that induce mosaic and mottle with AMV Saj isolate and no infection with AMV-Wd isolate (Fig. 2 and Table 1).
The size of the major product from AMV-infected tissue was identical to that of the 700 bp from the CP gene of AMV; the specific primers did not amplify viral cDNA from extracts of uninfected alfalfa plants. RT-PCR products obtained from RNAs extracted from AMV - infected alfalfa plants. All RT-PCR tests were performed using primer set AMV-F/AMV-R (L1, 2, 3, 4, (Wadi aldawasser), 6, 7, 8, 9, 10, 11 (Sagir), Total RNA extracted from a healthy alfalfa plant was used in the RT-PCR (lane 5) as a negative control (Fig. 3).
The probe was synthesized using PCR DIG Probe Synthesis Kit (Roche). The labeled and unlabeled products migrate as separate bands on an electrophoretic gel (Fig. 4). The shift in molecular weight and the intensity of the labeled band is equal to (or lightly less than) the intensity of the unlabeled product, the labeling reaction was successful. From Figure 5, spot in Row A, B and C shows positive reaction, with AMV infected alfalfa collected from Wadi aldawasser and sajer locations. (Row C: 1, 2), RT-PCR product DNA as positive control No hybridization reaction was observed with healthy samples as a negative control (Row D: 3, 4, 5).
Sequence comparisons showed the percentage of similarity ranged from 90.3-99.3% of the twenty four reported isolates of AMV with the Saudi isolates of AMV. The obtained results showed that the AMV strains from Australia, Brazil and Puglia) had the highest similarity with AMV-SA-Sajer isolate (99.2%- 99.3%) and AMV strains from China had the highest similarity with AMV-SA-Wadi aldawasser isolate (94%). while, the AMV isolates from Spain (AMV-VIRB isolate) and New Zeala (NZ2) had the lowest similarity with AMVSA-Sajer and Wadi aldawasser isolates (90.7% and 90.3%) respectevilly (Fig. 6 and Table 2).
AMV is distributed world-wide and causes diseases of many economically important crops including the families Solanaceae and Leguminosae (Crill et al., 1970; Hiruki and Miczynski, 1987; Smith, 1972). This virus was isolated from naturally infected alfafa in Saudi Arabia (Alshahwan et al., 2002; Cook et al., 1984). In the present study two different AMV isolates were detected and characterized from naturally infected alfalfa in different locations in Riyadh (Wadi aldawasser and Sagir). Positive results were obtained for all the tested samples with AMV by DASELISA. These isolates were characterized by host reactions, RT-PCR and nucleotide sequence.The isolated virus easily transmitted by mechanical means to different hosts. These results harmonized with their reported (Al-Shahwan, 2002; Jana et al., 2009; Mervat, 1999; Parrella and Acanfora, 2010; Shafie et al., 1997; Xu and Nie, 2006). The most characteristic symptom produced by AMV-SA isolates were severe yellow mosaic accompanying chlorotic and/or chloritic local lesions spots in C. ammaranticolor and C. quinoa. Symptoms as well as systemic responses were also characteristic for some other Alfamovirus (Oswald, 1950; Valkonen et al., 1992). A close relationship between each isolate was established based on biological assay, serological reaction and molecular works including RT-PCR, nucleic acid hybridization and sequence analysis for compositional properties of genomic RNA and coat protein. Host range studies and serological reaction with AMV antisera showed that virus was one of the Alfamovirus, previously reported on several crops (Hull, 1969; Paliwal, 1982; Valkonen et al., 1992; Weimer, 1931, 1934). The symptoms on some test plants including D. stramonium, G. globosa, N. rustica, and N. tabacum inoculated with AMVSA showed some differences as compared to that of previously described AMV isolated from soybean in Korea (Kudela and Gallo, 1995; Lee, 1985). Lee (1985) reported that D. stramonium infected with AMV from soybean developed severe mosaic symptom systemically while no symptom was apparent in N. rustica. In contrast, AMVKR induced systemic mosaic symptoms on D. stramonium and N. rustica. Similarly, AMV from Soybean induced necrotic spots only on inoculated N. tabacum leaves (Knorr et al., 1983; Paliwal, 1982) and systemic symptoms on Chenopodiacea plants while AMV-KR1 caused systemic symptoms on N. tabacum and chlorotic and/or necrotic spots on Chenopodiacea plants. However, AMV-KRs and Az could be clearly differentiated by host reactions in some indicator plants such as N. glauca, C. quinoa and S. tuberosum. From these results, although these two isolates have almost identical serological, they have distinctive biological and molecular characteristics. The present study demonstrated the successful use of RT-PCR and nucleic acid hybridization to detect AMV in total nucleic acid extracts from infected alfalfa plants, only 1-2 days are required for positive identification of the virus from infected tissue, amplified products for AMV was established. Total RNAs extracted from alfalfa leaves confirmed to be positive for AMV by ELISA, bioassay, RT-PCR, was used for amplified and identification of the precent AMV isolates with primer set AMV-coatF and AMV-coatR, which amplified with the CP gene of AMV, resulting in PCR amplicons of 700 bp. Also nucleic acid hybridization was used for detection of these AMV isolates using specif probe for AMV. Barker et al. (1983) reported that the RT-PCR. PCR primers for AMV were designed based on the sequence of the AMV strain 425 Madison RNA-3 assembled from data in GenBank accession number K02703 Down stream primer (AlMV-3pr) is complementary of nucleotides 2,014 to 2,037 of RNA-3, while the upstream primer (AMV-5pr) is located between nucleotides 1,136 and 1,159, giving an expected amplification product of 901 bp. The primers are corresponding to intergenic and 3Ⲡnon-coding regions covering full-length of CP gene on RNA-3. RT-PCR reaction was performed as described previously (Neeleman et al., 1991; Vossen et al., 1995). Dot blot spot hybridization is based on the principle of duplex formation (hybridization) between a âreporterâ nucleic acid (probe) and the nucleic acid of the virus or virus-like agentâs target. This method can allow for detection of both viruses and virus-like agents without nucleic acid purification. The development of a RTPCR assay by Kawasaki in 1990 provided a way to potentially detect RNA viruses present in very low titers. Molecular detection of AMV using RT-PCR is widely used and has been previously described (Koper-Zwarthoff et al., 1980; Neeleman et al., 1991; Vossen et al., 1995). Performing RT-PCR allows for the detection of viruses or virus-like agents in large numbers of samples in a fast, sensitive manner.
The nucleotide sequences of the coat protein gene (700 bp) were determined for the two Saudi isolates (AMV-SA-Wd and AMV-Saj) using DNAMAN program. Nucleotide sequence of the CP gene of AMV isolated from Brazil alfalfa cultivars (accession no. FJ858265) was determined and was closely related (99.2%) in the nucleotide sequences of their CP gene and gene products from AMVSA-Saj isolate. Nucleotide sequence of the CP gene of AMV isolated from China alfalfa cultivars were determined and were closely related (94%) in the nucleotide sequences of their CP gene and gene products from AMVSA-Wd isolate. The CP gene sequence of two Saudi Arabia alfalfa isolates of AMV was deposited in NCBI GenBank (Accession No. KC434083 and KC434084). The AMV isolated from Italy tomato cultivars (accession no. Y09110) was closely related (99.2%) in the nucleotide sequences of their CP gene and gene products from AMV-SA-Saj. AMV-SA-Saj was slightly different from the other AMV-SA-Wd Saudi isolate wherese the identity sequence between them is (92.8%). AMV isolate was detected in alfalfa plants in Saudi Arabia and characterized at the biological, serological and molecular level in this study and their genetic relationships with other known AMV strains and isoaltes were established. Sequence analysis of nucleotides showed that the Saj and Wadeldwasser isolates (Saudi Arabia) were slight differences in nucleotides sequences in their CP gene (Fig. 6) and Table 2. DNA sequences for the CP gene was determined for the first time for two Saudi isolates of AMV (AMV-SA-Wd, AMVSA-Saj) in Riyadh governorate. AMV isolates were detected in alfalfa plants in Saudi Arabia and characterized at the biological, serological and molecular level in this study and their genetic relationships with other known AMV strains were established. Further details of survey are needed to determine which other alfalfa viruses affect alfalfa crop and study their incidence as well as their relative importance using modern diagnostic tests and molecular characteristics.
Acknowledgments
The authors are grateful to the Agricultural Research Center (ARC) at the College of Food and Agricultural Sciences, King Saud University for the financial support.The authors are grateful for Dr. Ahmed Ali Al-Qahtani at King Faisal Specialist Hospital and Research Center for cooperation in sequencing.
Fig. 1.
Natural infection of AMV: mosaic and mottling symptoms (A and B from Wadi aldawasser and C from Sagir) and D: healthy on alfalfa plants.
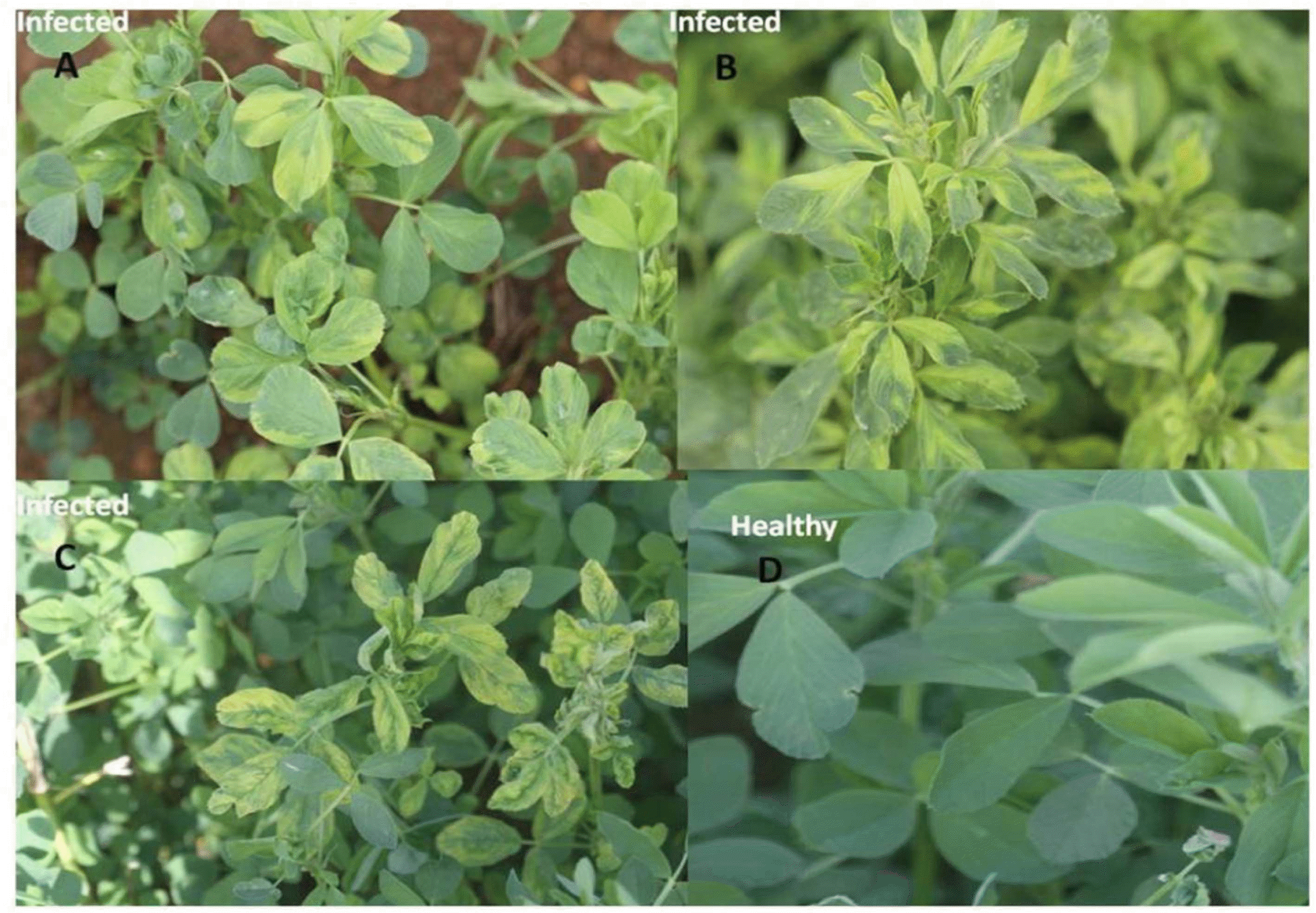
Fig. 2.
Reaction of selected indicator plants to mechanical inoculation with the the two isolats of AMV (Wadi aldawasser and Saj):Chorotic local lesion on C. amaranticolor (A) and Ch. quinoa (B), mosaic on N. tabacum (C), V. unguiculata (D) and V. faba (E)
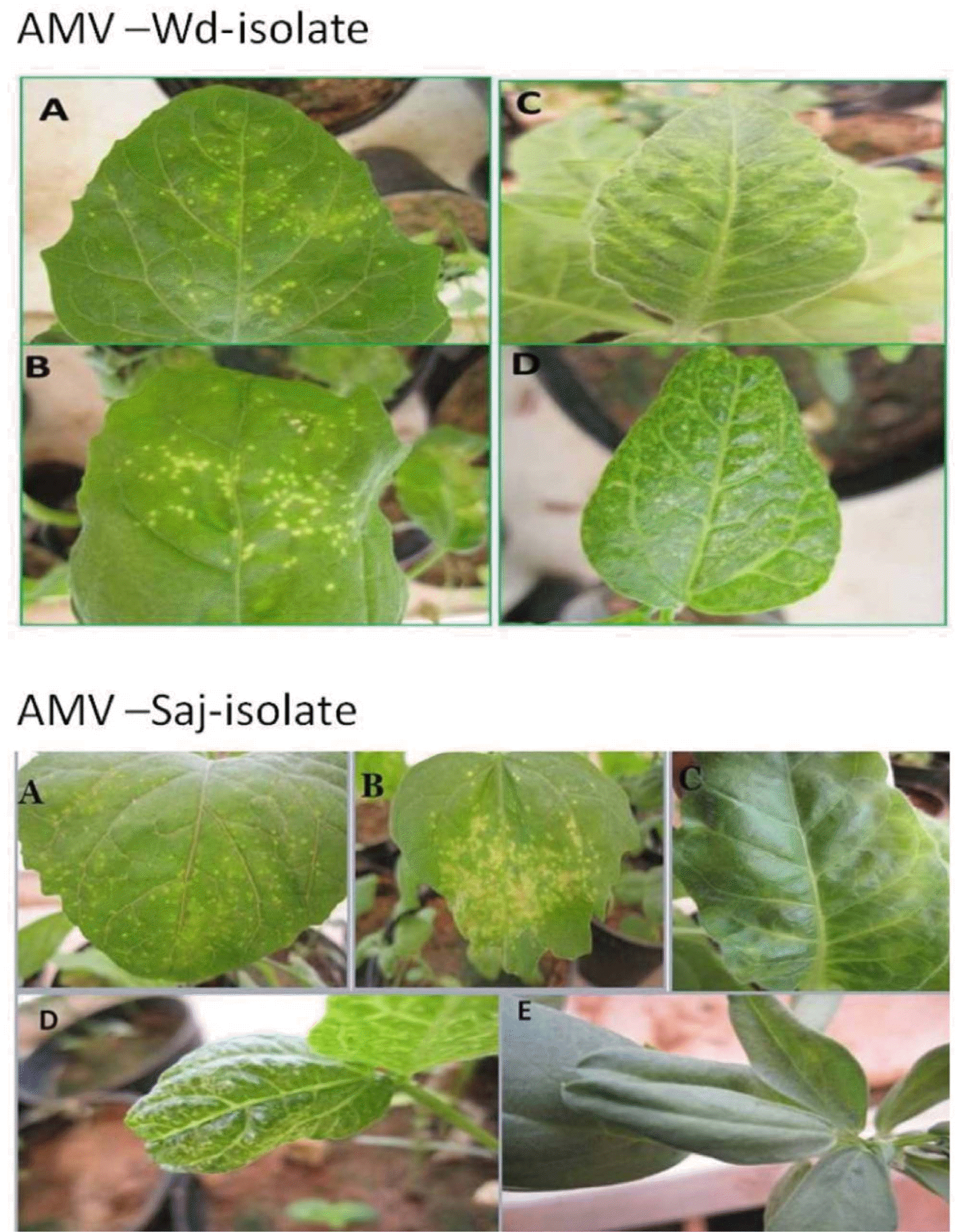
Fig. 3.
Gel electrophoresis on RT-PCR amplification of a fragment from AMV genome using specific primer pair designed to amplify 700 bp fragment of CP gene. Lane M represents Hyper-Laddert II (Bioline), Lane 1-4 (Sample 1-4 from infected alfalfa plants collected from Sajer location), Lane 6-11 (Leaf sample from symptomatic alfalfa plants collected from Wadi aldawasser location), and Lane 5 (healthy alfalfa as a negative control.
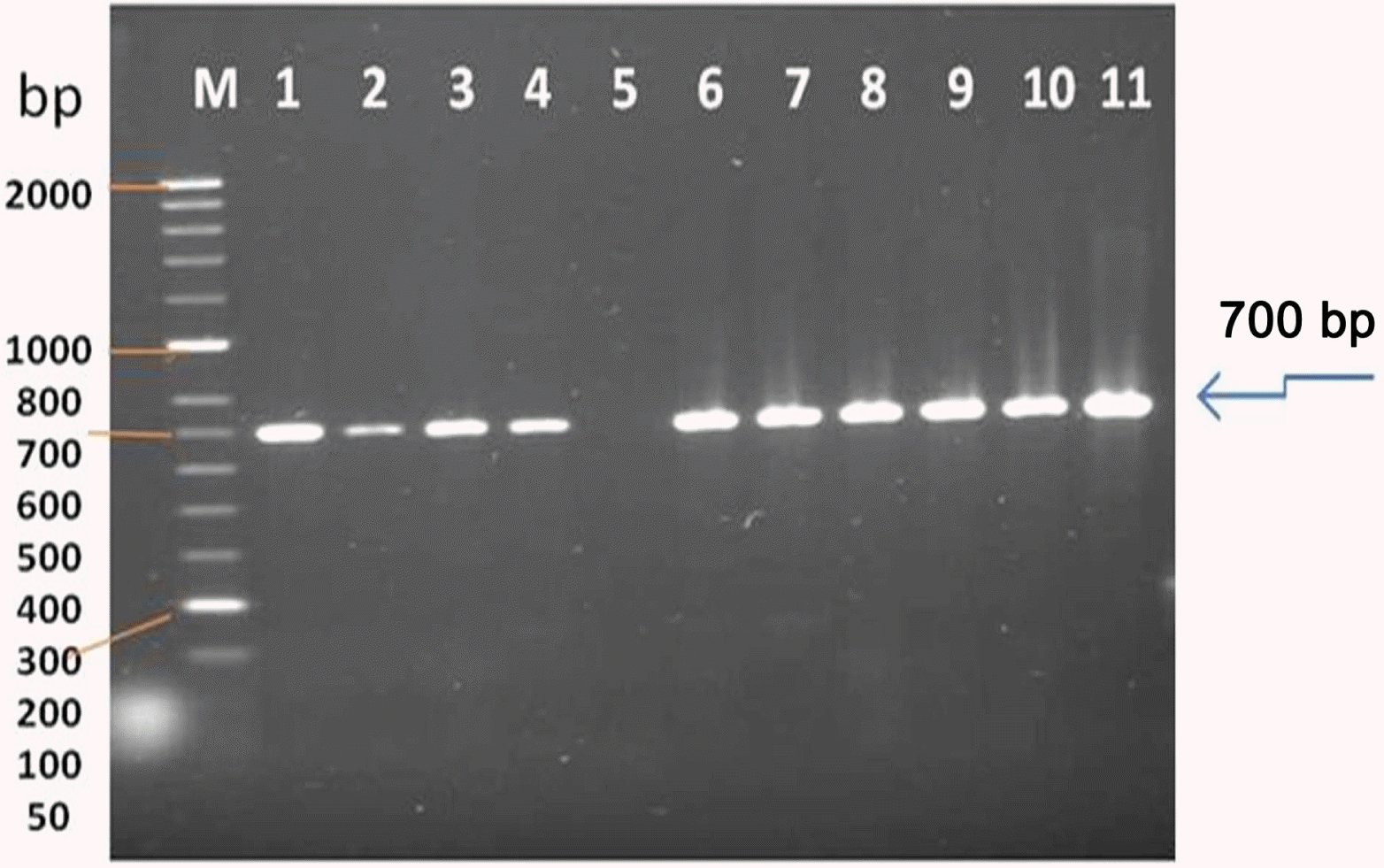
Fig. 4.
Evaluation of PCR-labeled probes by agarose gel electrophoresis. Lanes 1 and 5: unlabeled PCR product Lanes 2, 3, and 4: corresponding DIG-labeled PCR products, Lane 6 healthy alfalfa plant,M: Hyperladder DNA marker.
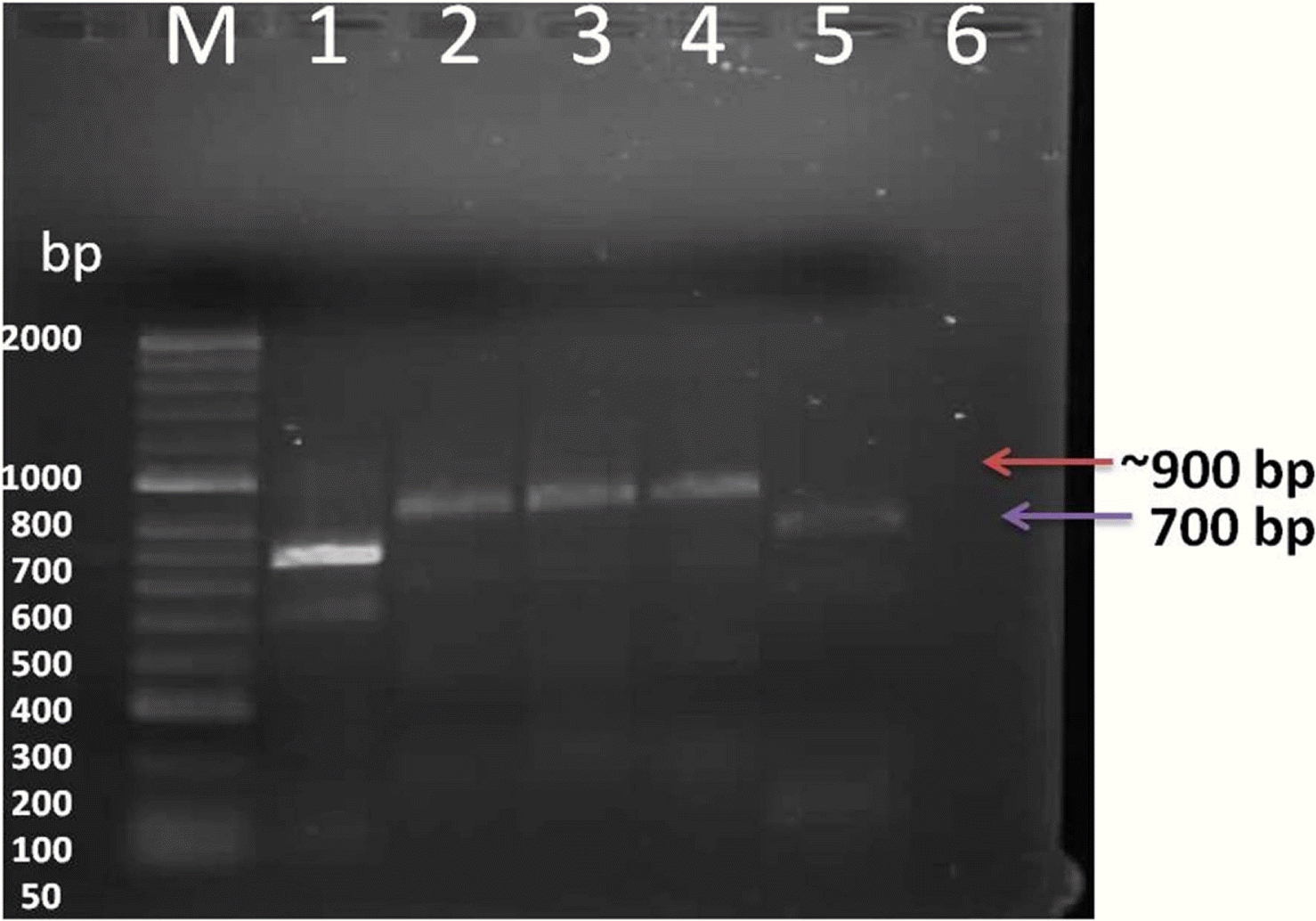
Fig. 5.
Results of dot blot hybridization assay of several infected alfalfa samples collected from Wadi aldawasser (Row A:1, 2, 3, 4 and 5) and Sajer (Row B: 1, 2, 3, 4 and 5) using AMV DIG-cDNA probe. (Row C:1, 2),RT-PCR product DNA as positive control. (No hybridization reaction was observed healthy alfalfa samples (Row C: 3, 4 and 5).
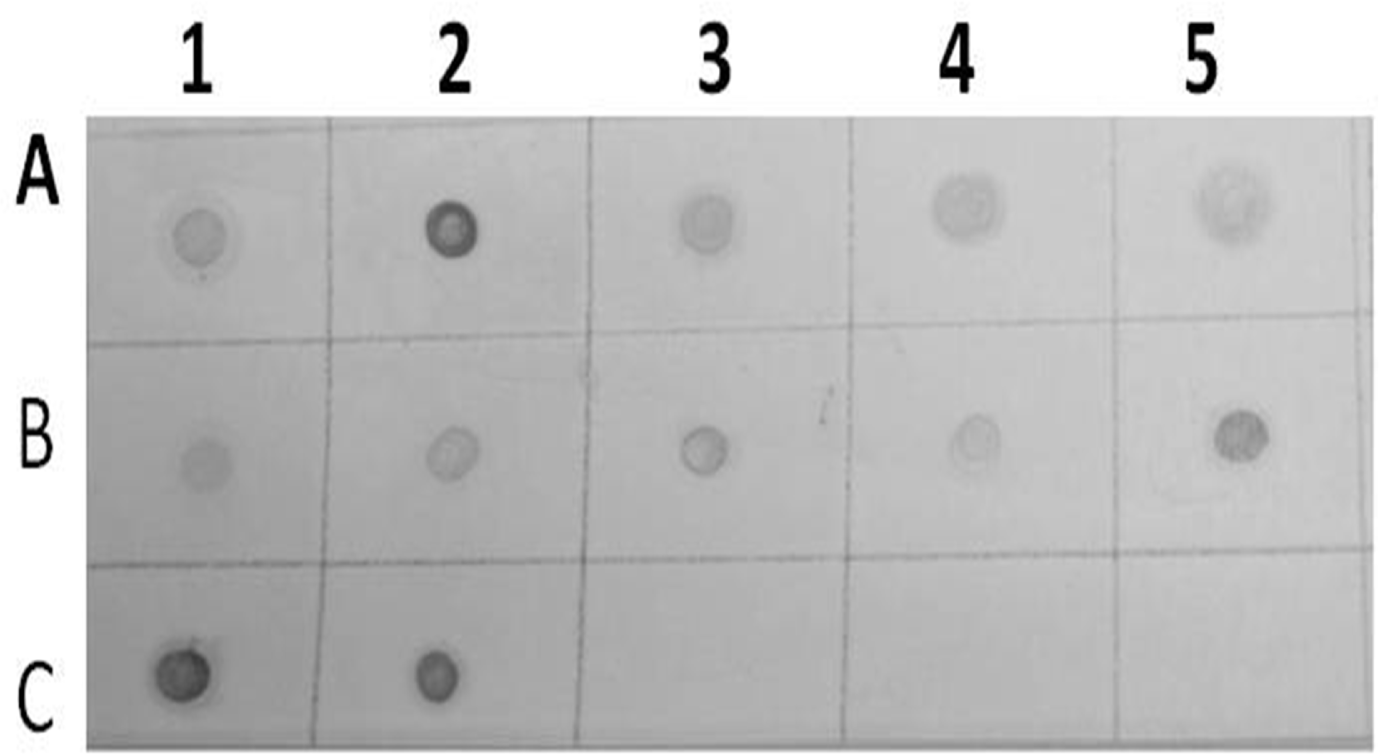
Fig. 6.
A phylogenetic tree constructed from the alignment of nucleotide sequences of the coat protein gene of of AMV Saudi Arabian isolates and 24 AMV isolates obtained from GenBank (Table 1) using DNAMAN based on the nucleotide sequences.
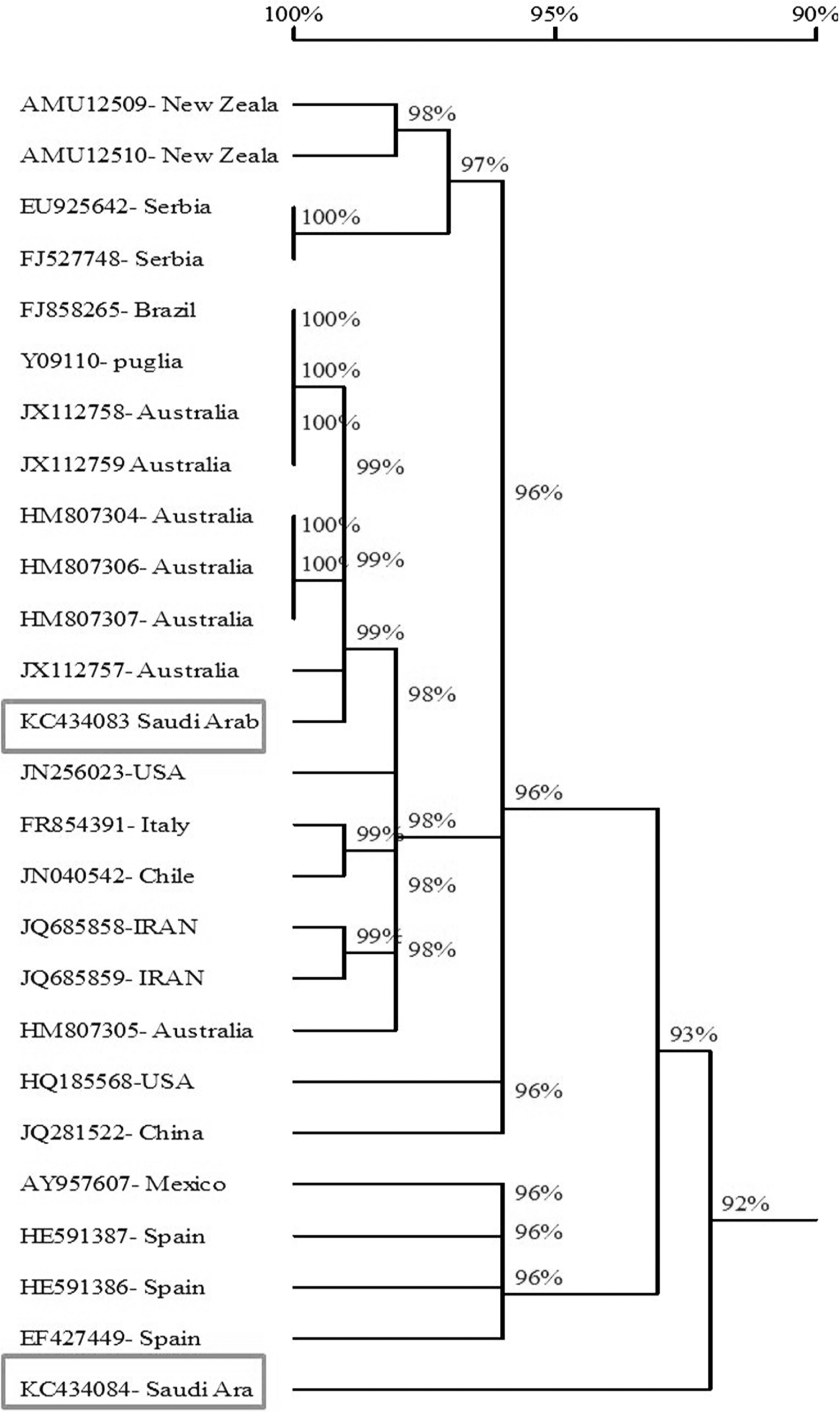
Table 1.
Reactions of indicator plants to mechanical inoculation of the AMV isolates
Table 2.
Nucleotide identities percentage of CP gene sequences between AMV Saudi Arabia isolates with those of AMV isolates originating in different countries
Refrences
Avgelis, N and Katis, N 2008. Identification of Alfalfa mosaic virus in Greek Alfalfa Crops. J Phytopathol. 125:231-237.

Al-Shahwan, IM 2002. Alfalfa mosaic virus (AMV) on alfalfa (Medicago sativa L.) in Saudi Arabia. Assuit J Agric Sci. 33:21-30.
Barker, RF, Jarvis, NP, Thompson, DV, Loesch-Fries, LS and Hall, TC 1983. Complete nucleotid sequence of Alfalfa mosaic virus RNA3. Nucleic Acids Res. 11:2881-2891.


Cook, AA, Sharif, M and Abdeen, F 1984. Virus and virus diseases of alfalfa in Saudi Arabia. Symp Biol ASP Saudi Arabia. 7:137-138.
Crill, P, Hagedorn, DJ and Hanson, EW 1970. Alfalfa mosaic, the disease and its virus incitant. Univ. Wisconsin Agric Sci Res Bull. (280):40.
Erwin, DC and Stuterville, LD 1990. Compendium of Alfalfa Diseases. 2nd Edition. APS Press.
El-Helaly, HS, Ahmed, AA, Awad, MA and Soliman, AM 2012. Biological and Molecular Characterization of Potato Infecting Alfalfa mosaic virus in Egypt. Inter J Virol. 8:106-113.

Hiruki, C and Miczynski, KA 1987. Severe isolate of alfalfa mosaic virus and its impact on alfalfa cultivars grown in Alberta. Plant Dis. 71:1014-1018.

Hajimorad, MR and Francki, RIB 1988. Alfalfa mosaic virus isolates from lucerne in South Australia: biological variability and antigenic similarity. Ann Appl Biol. 113:45-54.

Hiruki, C and Miczynski, KA 1987. Severe isolate of Alfalfa mosaic virus and its impact on alfalfa cultivars grown in Alberta. Plant Dis. 71:1014-1018.

Jaspars, EMJ 1985. Interaction of alfalfa mosaic virus nucleic acid and protein. eds. by JW Davies, In: Molecular plant virology, CRC Press, New York. 155-221.

Jaspars, EM and Bos, L 1980. Alfalfa mosaic virus. (229):Descriptions of plant viruses. Commonwealth Mycology Institute/Association of Applied Biologists, Kew, England.
Jana, F, Tatiana, S, Karel, P, Jakesova, H, Beckova, M and et al 2009. Cultivated and wild growing forage crops-reservoirs of viruses and phytoplasmas. In: Proceeding of the 15th European Grassland Federation Symposium; September; Brno, Czech Republic. pp 106-108.
Koper-Zwarthoff, EC and Bol, JF 1980. Nucleotide sequence of the putative recognition site for coat protein in the RNAs of Alfalfa mosaic virus and tobacco streak virus. Nucleic Acids Res. 8:3307-3318.



Kudela, O and Gallo, J 1995. Charaterization of the alfalfa mosaic virus strain T6. Acta Virol. 39:131-135.

Kahn, RP and Monroe, RI 1963. Detection of tobacco veinal necrosis (strain of potato virus Y) in Solanum carensaii and S. andigenum introduce into the United States. Phytopathology. 53:1356-1359.
Knorr, DA, Laemmlen, FF and Dawson, WO 1983. Effect of a necrosis inducing isolate of alfalfa mosaic virus on stand loss in tomatoes. Phytopathology. 73:1554-1558.

Kawasaki, ES 1990. Amplification of RNA. 21-27. eds. by MA Innis, DH Gelfand, JJ Sninsky and TJ White, In: PCR Protocols: A Guide to Methods and Applications, Academic Press, San Diego.

Lee, SH, Choi, YM, Kim, JS and Chung, BJ 1985. Identification of alfalfa mosaic virus from soybean. Korean J Plant Pathol. 1:33-37.
Langereis, K, Mugnier, M-A, Cornelissen, BJC, Pink, L and Bol, JF 1986. Variable repeats and poly (A)-stretches in the leader sequence of Alfalfa mosaic virus RNA 3. Virology. 154:409-414.


Massumi, H and Hosseini, AP 2007. Serological Characterization of Alfalfa mosaic virus in Alfalfa (Medicago sativa) in Some Regions of Iran. J Agric Sci Technol. 9:341-347.
Mervat, MF 1999. Plant virus and virus like diseases mosaic and dwarf diseases of alfalfa. Ph.D. Thesis, Faculty of Agriculture, Alexandria, University.
Neeleman, L, Van Der Kuyl, AC and Bol, JF 1991. Role of alfalfa mosaic virus coat protein gene in symptom formation. Virology. 181:687-693.


Oswald, JW 1950. A strain of alfalfa mosaic virus causing vine and tuber necrosis in potato. Phytopathology. 40:973-991.
Paliwal, YC 1982. Virus diseases of alfalfa and biology of alfalfa mosaic virus in Ontario and western Quebec. Canadian J Plant Pathol. 4:175-179.

Parrella, G and Acanfora, N 2010. First record and complete nucleotide sequence of Alfalfa mosaic virus from Lavandula stoechas in Italy. Plant Dis. 94:924-924.

Parrella, G, Acanfora, N, OrĂlio, FA and Navas-Castillo, J 2011. Complete nucleotide sequence of a Spanish isolate of alfalfamosaic virus: evidence for additional genetic variability. Arch Virol. 156:1049-1052.



Parrella, G 2000. Evidence for two distinct subgroups of Alfalfa mosaic virus (AMV) from France and Italy and their relationships with other AMV strains. Arch Virol. 145:2659-2667.



Pesic, Z and Hiruki, C 1988. Comparison of ELISA and dothybridisation for detection of alfalfamosaic virus in alfalfa pollen. Can J Plant Pathol. 10:116-122.
Pallas, V, Mas, P and Sanches-Navarro, JA 1998. Detection of Plant RNA Viruses by Nonisotopic Dot-Blot Hybridization. eds. by GD Foster and SC Taylor, In: Methods in Molecular Biology, 81:461-468.

Podleckis, EV, Hammond, RW, Hurtt, S and Hadidi, A 1993. Chemiluminescent detection of potato and pome fruit viroids by digoxigeninlabelled dot blot and tissue blot hybridization. J Virol Methods. 43:147-158.

Sawalha, HD and Mansour, AN 1996a. Alfalfa mosaic virus in Jordan. MĂştah Journal For Reasearch and Studies. 11:163-174.
Sawalha, HD and Mansour, AN 1996b. Incidence of Alfalfa mosaic virus in alfalfa fields in Jordan. Dirasat, Agricult Sci. 23:81-83.
Shafie, MSA, Nahed, ZA, El-Kady, MAS and Abu-Zeid, AA 1997. Natural occurrence of Alfalfa mosaic virus on Basil plants in Egypt. J Appl Sci. 12:15-30.
Smith, KM 1972. A Textbook of Plant Virus Diseases. 3rd ed. Academic Press, New York.
Tenllado, F and Bol, JF 2000. Genetic detection of the multiple functions of Alfalfa mosaic virus coat protein in viral RNA replication, encapsidation, and movement. Virology. 268:29-40.


Vossen, EAG, Notenboom, T and Bol, JF 1995. Characterization of sequences controlling the synthesis of Alfalfa mosaic virus subgenomic RNA in vivo. Virology. 212:663-672.


Van der Vossen, EAG, Neeleman, L and Bol, JF 1994. Early and late functions of Alfalfa mosaic virus coat protein can be mutated separately. Virology. 202:891-903.


Valkonen, JPT, Pehu, E and Watanabe, K 1992. Symptom expression and seed transmission of alfalfa mosaic virus and potato yellowing virus and potato yellowing virus (SB-22) in Solanum brevidens and S. etuberosum. Potato Res. 35:403-410.


Weimer, JL 1931. Alfalfa mosaic virus (type strain). CMI/AAB Description of Plant Viruses, no. 46.
- TOOLS
-
METRICS

-
- 21 Crossref
- 0 Scopus
- 2,103 View
- 19 Download
- Related articles
-
Update on Distribution and Genetic Variability of
Plum pox virus Strains in Bulgaria2019 June;35(3)



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



