Occurrence of Stolbur Phytoplasma Disease in Spreading Type Petunia hybrida Cultivars in Korea
Article information
Abstract
In January 2012, spreading type petunia cv. Wave Pink plants showing an abnormal growth habit of sprouting unusual multiple plantlets from the lateral buds were collected from a greenhouse in Gwacheon, Gyeonggi Province, Korea. The presence of phytoplasma was investigated using PCR with the primer pairs P1/P6, and R16F1/R1 for nested-PCR. In the nested PCR, 1,096 bp PCR products were obtained, and through sequencing 12 Pet-Stol isolates were identified. Comparison of the nucleotide sequences of 16S rRNA gene of the 12 Pet-Stol isolates with other phytoplasmas belonging to aster yellows or Stolbur showed that Pet-Stol isolates were members of Stolbur. The presence of phytoplasma in petunia was also confirmed by microscopic observation of the pathogens. In this study, Stolbur phytoplasma was identified from spreading type petunia cultivars by sequence analysis of 16S rRNA gene of phytoplasma and microscopic observation of phytoplasma bodies. This is the first report of Stolbur phytoplasma in commercial Petunia hybrida cultivars.
Phytoplasmas are characterized by their lack of cell walls, and a pleiomorphic or filamentous shape, normally with a diameter less than one micrometer (Hopkins, 1977). The 16S rRNA gene is universal in prokaryotes and possesses both conserved and variable regions. Thus, sequence analysis of rRNA gene amplified by PCR has provided the basis for several comprehensive studies on phytoplasma phylogeny and taxonomy (Gundersen et al., 1994; Khadhair et al., 1998; Lee et al., 1998; Lee et al., 1994; Schneider et al., 1993; Seemüller et al., 1994).
Phytoplasmas are known as the causal pathogen of yellowing, stunting, phyllody and witch’s broom diseases in various plants (Bertaccini et al., 1990; McCoy et al., 1989). In Petunia hybrida, phytoplasmal disease was firstly reported in 1964 from plants showing stunting or yellowing symptoms (Doi et al., 1967). Later, an aster yellows (AY) phytoplasma associated with petunia flat stem disease was reported from both China (PFS-C, GenBank accession no. AY283186, unpublished) and Korea (PFS-K, Chung and Huh, 2008).
Petunia is an economically important ornamental plant worldwide. Recently, spreading type petunia plants were introduced into the flower-market in Korea. They are produced for hanging baskets and patio containers because of their cascading habit. They are usually produced by stem cutting of seedlings, so that product quality is even and good during the season. However, it has been widely known to farmers that the commercial value of spreading type petunia plants usually decline throughout the winter by showing typical symptom of phytoplasmal disease such as yellowing and axillary shoot proliferation. Accordingly, they were suspected of phytoplasma infection.
In order to inspect the phytoplasma infection from spreading type petunia plants, in January 2012, cv. Wave Pink plants exhibiting symptoms of unusual formation of multiple plantlets from the lateral buds were collected from a commercial greenhouse located in Gwacheon, Gyeonggi Province, Korea (Fig. 1). To compare them with other sources of spreading type petunia, petunia plants showing abnormal growth habit (Fig. 2), and non-symptomatic petunia cv. Wave Purple plants (Fig. 3) were collected from commercial greenhouses located in Ansan and Hwasung, Gyeonggi Province, Korea. They were kept in an insect proof glasshouse and used for PCR amplification of phytoplasma 16S rRNA sequences and microscopic observation of phytoplasma pathogens.

Symptomatic Petunia hybrida cv. Wave Purple infected with Stolbur phytoplasma, which were collected from a commercial greenhouse located in Gwacheon, Gyeonggi Province. (A) Yellowing along with multiple plantlets and (B–D) unusual sprout of malformed plantlets from the lateral buds of stems.
Symptomatic Petunia hybrida cv. Wave Pink infected with Stolbur phytoplasma, which were collected from a commercial greenhouse located in Hwasung, Gyeonggi Province. Pet-phy-HS1, mosaic like leaf pattern; Pet-phy-HS2 and Pet-phy-HS7-8, leaf narrowing; Pet-phy-HS3, upward rolling of leaf edge; Pet-phy-HS4, yellowing along with necrosis; Pet-phy-HS5, yellowing; Pet-phy-HS9, leaf narrowing along with vein bending.
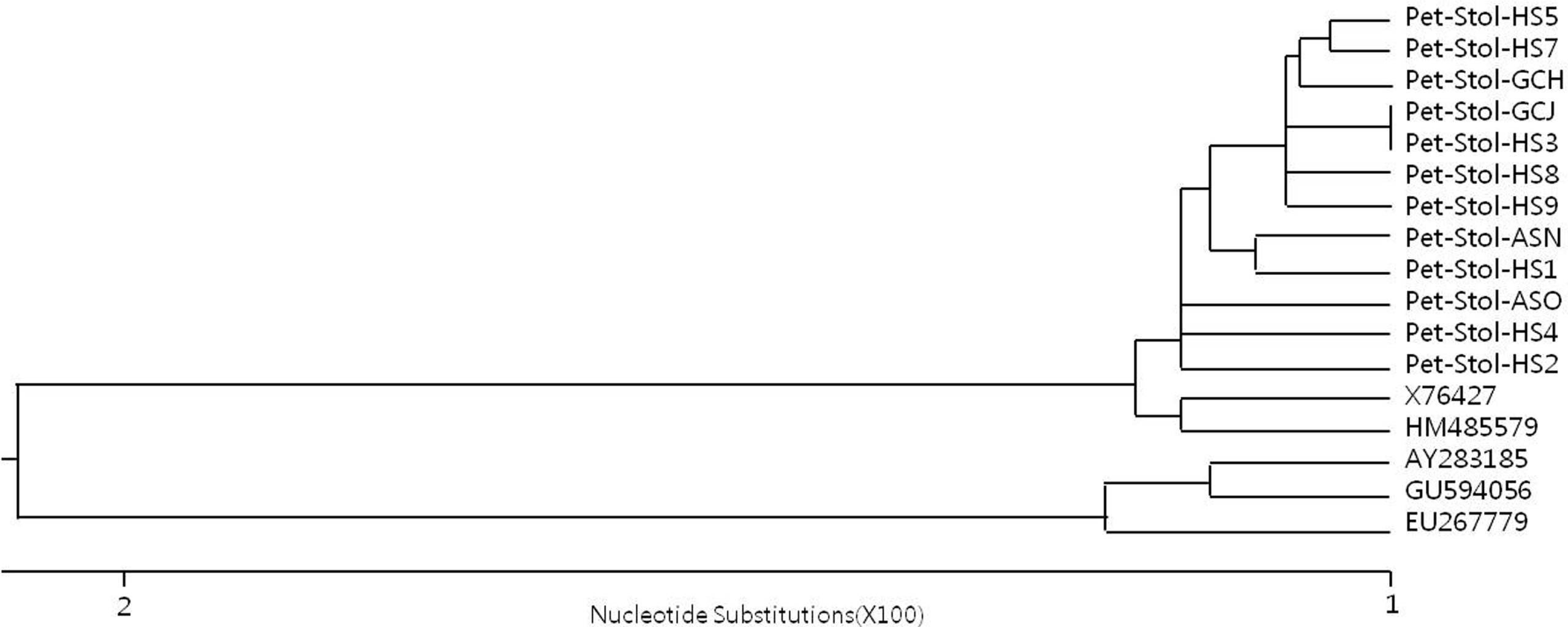
Phylogenetic tree constructed by CLUSTAL method using DNASTAR software version 7.0 (DNASTAR, Madison, WI, USA), comparing 16S rRNA gene sequences of Pet-Stol Stolbur phytoplasma isolates with other Stolbur or AY phytoplasmas registered in GenBank (www.ncbi.nlm.nih.gov). The scale refers to the similarity index.
Phytoplasma infection was determined by PCR. DNA was extracted from leaf veins by a method described previously (Lee and Davis, 1983). Two pairs of primers were used in PCR. Primer pair P1/P6 (Deng and Hiruki, 1991) was used to prime phytoplasma-universal amplification of 16S rRNA gene sequences, and in nested-PCR, primer pair R16F1/R1 was used (Lee et al., 1994).
The PCR reaction mixture contained 20 ng of template DNA, 1×PCR buffer (50 mM KCl, 10 mM Tris-HCl, pH 8.3), 2 mM of each dNTP, 1 μl of 10 pM each primer, 2.5 mM MgCl2 and 2.5 U Taq DNA polymerase (Takara, USA). Thirty-five PCR cycles were conducted in a PTC-0220 Perlitier Thermal Cycler (MJ Research, MA, USA). The thermal conditions for primer sets P1/P6 and 16F1/R1 were as follows: denaturation at 94°C for 30 sec (2 min for the first cycle), annealing at 65°C (45°C for reactions with a 16F1/R1) for 50 sec and extension at 72 °C for 1.5 min. The last cycle was extended for an additional 3 min at 72 °C.
Phytoplasma 16S rDNA, which was amplified in nested PCR primed by R16F1/R1, was cloned using the pGEM-T easy vector (Promega, USA) according to the manufacturer’s instruction. The ligation mixture was used to transform competent cells of Escherichia coli JM 109. Recombinants were screened by the blue and white colony screening method (Sambrook et al., 1989). Nucleotide sequences were determined using ABI Prism BigDye™ Terminator Cycle Sequencing Kit (Applied Biosystems, USA). The 16S rRNA gene sequences were aligned using CLUSTAL method of DNASTAR software version 5.1 (Madison, WI. USA).
An approximately 1.5 kb DNA fragment of the 16S rRNA gene was amplified with the P1/P6 primer set from petunia plants producing unusual malformed plantlets from the lateral buds of stems (Fig. 1) and from plants showing a mosaic-like leaf pattern, upward rolling, leaf yellowing or leaf yellowing along with necrosis, and leaf narrowing along with vein bending (Fig. 2), as well as from plants without symptoms (Fig. 3). In the nested PCR assays with the primer pair 16F1/R1, the expected DNA fragment of 1.1 kb was amplified (data not shown). Nucleotide sequences of 16S rRNA gene of 12 Pet-Stol phytoplasma isolates were compared with other phytoplasmas belonging to AY or Stolbur groups: They had 98.7 to 99.5% identify with sequences from other Stolbur phytoplasmas in the 16S rDNA region, while, they shared 95.4 to 96.2% of identify with sequences of AY phytoplasmas (Table 3). Based on phylogenetic analysis of the 16S rRNA gene sequences, all 12 Pet-Stol isolates identified from the present study were placed in the phylogenetic branch of phytoplasmas belonging to Stolbur (Fig. 4). These results indicated that the phytoplasma isolates of Pet-Stol are members of the Stolbur phytoplasma.

Sequence identity percent of the 16S rDNA of petunia Stolbur phytoplasma isolates (Pet-Stol) with other phytoplasmas registered in GenBank (www.ncbi.nlm.nih.gov)
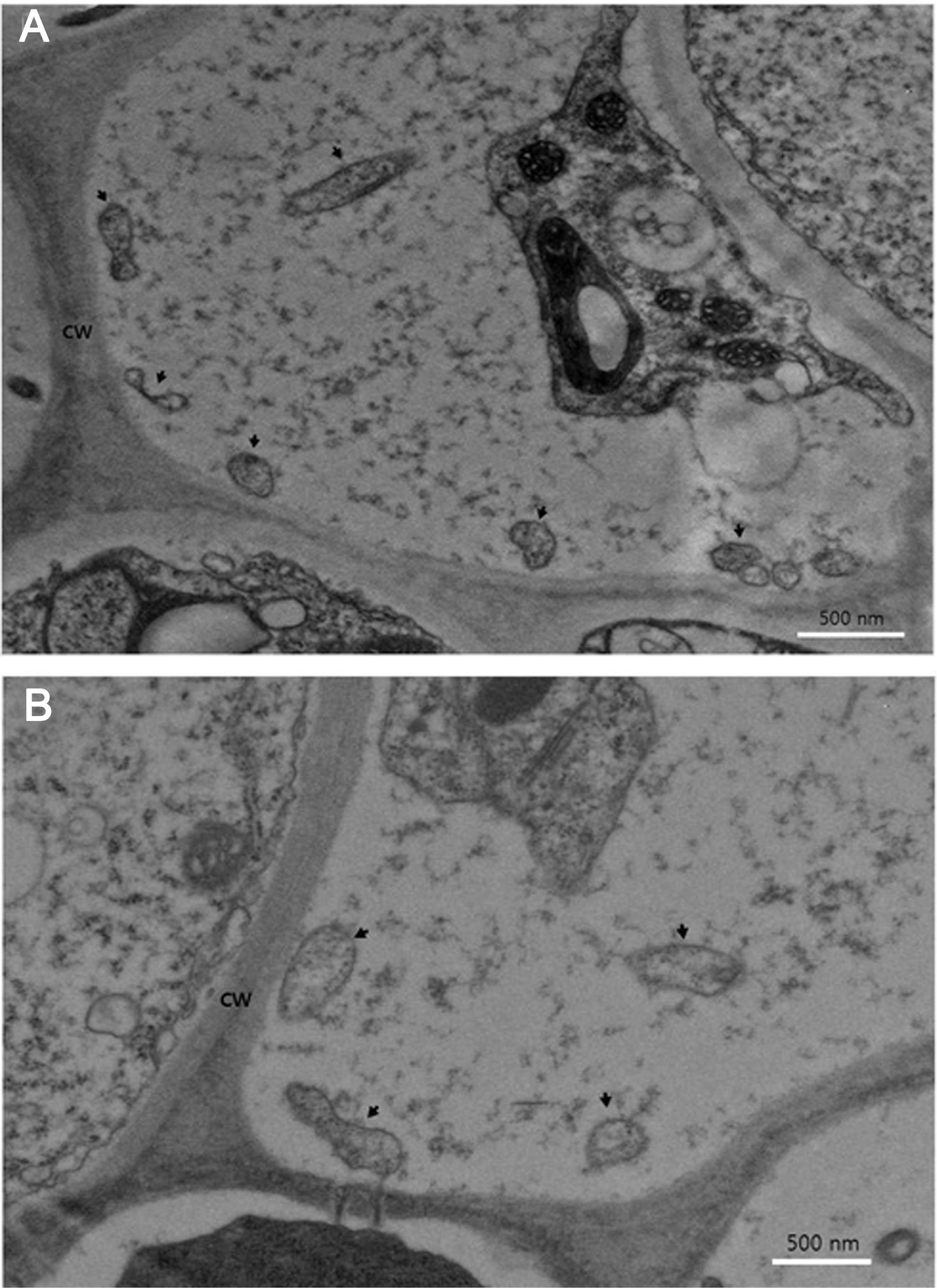
Electron micrographs of the sieve elements of leaf midrib of spreading type Petunia hybrida cv. Wave Purple infected with Stolbur phytoplasma. (A) Membrane-bound phytoplasma-like structures measured 308–890 nm are shown (arrows) from plant sprouting unusual malformed plantlets (Pet-Stol-GCJ) and (B) Membrane-bound phytoplasma-like structures measured 340–636 nm are shown (arrows) from plant infected without symptom (Pet-Stol-ASN). Bar represents 500 nm.
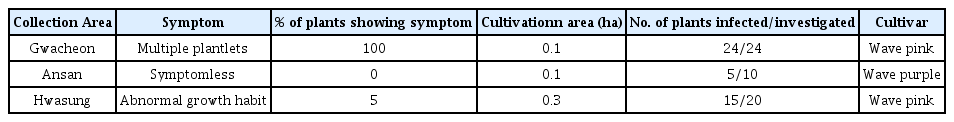
Stolbur phytoplasma was detected from 50 to 100% of the some petunia plants showing multiple plantlets, abnormal growth habit and symptomless plants, depending on the cultivars (Table 1). Phytoplasma isolates identified in this study were designated as listed in Table 2 and nucleotide sequences were registered in the GenBank (Table 2). Previously, AY phytoplasma was detected from seed-propagated standard type petunia plants (Chung and Huh, 2008). They showed stem flattening along with flower malformation or phyllody. In contrast to AY phytoplasma in standard type petunia, Stolbur phytoplasma induced yellowing and produced malformed plantlets or sometimes symptomless in the spreading type petunia plants. Though some of them were symptomless, it is assumed that once they are exposed to below 5 °C for a few days during winter and afterwards as they are invigorated, they will begin revealing typical phytoplasma infection symptoms such as yellowing and axillary shoot proliferation. We speculate that temperature affects the symptomatology of other host plants of phytoplasma as it does with poinsettia (Chung and Choi, 2010). It was known that phytoplasmas cause a desirable growth habit only in poinsettia, but recently it was found that they also showed typical phytoplasma symptoms depending on agronomic practices during the winter. Further studies are required for verifying the association of temperature to symptomatology of the host plants of phytoplasma.
Occurrence of Stolbur phytoplasma in commercial spreading type Petunia hybrida cultivars. Phytoplasma 16S rDNA fragment was amplified in the veins of leaves by PCR
To observe the phytoplasma pathogen from the cells of infected plants, small pieces from the leaf midribs of Pet-Stol-GCJ and -ASN infected petunia plants were prefixed in 1% Karnovsky’s fixative solution, postfixed in 1% osmium tetroxide in cacodylate buffer, pH 7.2, and dehydrated in an ethanol series of 50, 75, 90, 95 and 100% for 30 min at each step. Embedding was conducted in Spurr resin (Electron Microscopy Science, Washington, PA). Ultrathin sections, prepared with an ultramicrotome, were stained with 2% uranyl acetate and 0.08 M lead citrate buffer, pH 12.0. The sections were examined using a Carl Zeiss LEO 906 transmission electron microscope (Germany).
In the electron microscopy of ultrathin sections of the leaf midribs, amorphous phytoplasma-like structures with a diameter ranging from 308–890 nm were rarely observed in sieve tubes of phloem cells (Fig. 4). In many cases of phytoplasmal disease, phytoplasma cells are present in high numbers within sieve elements in plant tissues (Chung et al., 2001, 2007). While, in some other cases, the pathogen is present in very low titer or not be present within tissues that display external symptoms. For example, in papaya plants affected by dieback disease, phytoplasma cells were not observed in transmission electron microscopy, a PCR test indicated the presence of phytoplasma DNA (Siddique et al., 1998). In the report they concluded that the papaya plant limit proliferation of the dieback-associated pathogen as a defense strategy. In a previous report, direct correlation of the distribution and concentration of phytoplasma with expression of symptoms has been proposed (Kuske and Kirkpatrick, 1992). In the present study, phytoplasma present in sieve elements of petunia affected by stolbur phytoplasma is very low. We estimate that the density of phytoplasmas in plant cells appears to be related with either plant species or accumulation of phytoplama pathogen according to the symptom progress. In other words, it appears to be from the host-pathogen interactions.
In this study, Stolbur phytoplasma infection was confirmed in spreading type petunia plants showing an abnormal growth habit of sprouting unusual multiple plantlets from the lateral buds by sequence analysis of 16S rRNA gene of phytoplasma and microscopic observation of phytoplasma bodies. This study revealed that phytoplasma infection is responsible for quality decline of spreading type petunia during winter. This is the first report of Stolbur phytoplasma in commercial Petunia hybrida cultivars.
