|
 |
| Plant Pathol J > Volume 31(3); 2015 > Article |
Abstract
The primary step for efficient control of viral diseases is the development of simple, rapid, and sensitive virus detection. Reverse transcription loop-mediated isothermal amplification (RT-LAMP) has been used to detect viral RNA molecules because of its simplicity and high sensitivity for a number of viruses. RT-LAMP for the detection of Potato virus X (PVX) was developed and compared with conventional reverse transcription polymerase chain reaction (RT-PCR) to demonstrate its advantages over RT-PCR. RT-LAMP reactions were conducted with or without a set of loop primers since one out of six primers showed PVX specificity. Based on real-time monitoring, RT-LAMP detected PVX around 30 min, compared to 120 min for RT-PCR. By adding a fluorescent reagent during the reaction, the extra step of visualization by gel electrophoresis was not necessary. RT-LAMP was conducted using simple inexpensive instruments and a regular incubator to evaluate whether RNA could be amplified at a constant temperature instead of using an expensive thermal cycler. This study shows the potential of RT-LAMP for the diagnosis of viral diseases and PVX epidemiology because of its simplicity and rapidness compared to RT-PCR.
Potatoes can be infected by over 40 viruses in the world including Potato virus X (PVX), Potato virus Y (PVY), Potato virus S (PVS), Potato virus M (PVM), Potato virus A (PVA), Potato leafroll virus (PLRV), and so on (Wang et al., 2011). Among them, PVX is one of the major viruses that infect potato (Aboul-Ata et al., 2011). PVX infection of potato plants induces disease symptoms including crinkling, browning of leaf tissue, plant death, and tuber necrosis depending on biological and environmental factors (Jones, 1985).
Several diagnostic methods have been developed for the diagnosis of viral diseases in potatoes (El-Araby et al., 2009). Major potato viruses such as PVA, PVS, PVX, PVY, and PLRV can be detected at the same time by employing multiplex RT-PCR (Wang et al., 2005). A system for simultaneous detection (in real-time) has been developed for several potato viruses including PLRV, PVA, PVX, and PVY (Agindotan et al., 2007). In addition, PVX, PVS, PLRV, and TSWV from potato tubers can be detected at the same time by real-time RT-PCR using four different fluorescent reagents (Mortimer-Jones et al., 2009).
Recently, a detection system using nucleic acid sequence-based amplification known as the isothermal amplification method has been developed and successfully used to detect several viruses including Prunus necrotic ringspot virus (PNRV), Prune dwarf virus (PDV), Plum pox virus (PPV), Citrus tristeza virus (CTV), and Lettuce mosaic virus (LMV) (Adkar-Purushothama, 2011; Helguera et al., 2001; Moreno et al., 2007; Olmos et al., 1999; 2002). As another isothermal amplification method, a novel technique for the amplification of nucleic acids has been described and named loop-mediated isothermal amplification (LAMP) (Notomi et al., 2000). It is a simple method for diagnostic purposes and has the advantage of high sensitivity, speed, and low cost (Parida et al., 2008; Tomita et al., 2008). LAMP will be useful not only for the detection of infected plants but also quarantine. The reactions are easily monitored by detecting the turbidity caused by the production of a large amount of target DNA (Webster et al., 2004). The reaction time for RT-LAMP is less than 60 min and this time can be further reduced by adding two more loop primers (Ju, 2011; Nagamine et al., 2002). In addition, if a fluorescent dye like SYBR Green is present in the reaction, PCR products in the reaction tubes can be seen with the naked eye under a UV lamp (Cardoso et al., 2010).
Viruses infecting potatoes including PLRV and PVY have been detected by RT-LAMP (Ju, 2011; Nie, 2005). The RT-LAMP method has not been reported for the diagnosis of PVX. The purpose of this study was to develop a system to diagnose PVX by RT-LAMP based on its unique nucleotide sequences encoding a coat protein. The specificity, sensitivity, and rapidity of RT-LAMP were also assessed to optimize the detection of PVX.
A pSPVXp31 binary vector, containing PVX full-length cDNA, was kindly provided by Dr. Kim from Seoul National University, Seoul Korea (Park and Kim, 2006). An Agro-infiltration method previously described by English et al. (1997) has been applied for PVX infection to Nicotiana benthamiana. After two or three weeks, the treated-leaves were collected from healthy and diseased plants.
Total RNA was extracted from the leaves of PVX-infected or non-infected N. benthamiana using the easy-Blue RNA extraction kit (Intron, Republic of Korea) as directed by the manufacturer’s instructions.
Primer sets were designed from the coat protein (CP) gene of PVX (Fig. 1). Three primer sets were designed using Primer Explorer V4 (Eiken Chemical Co. Ltd., Japan), and the other three primer sets were designed using Lamp Designer (Premier Biosoft, USA). All primers were purified by HPLC.
The RT-LAMP reaction was conducted by incubating a total reaction mixture of 25 μl at 65°C for 60 min. A homemade mixture (Table S1) and two commercial mixtures (Loopamp RNA amplification kit (Eiken Chemical Co. Ltd., Japan) and Isothermal Master Mix (IMM kit) (Optigene Ltd., England)) were used. In order to optimize the RT-LAMP mixtures, different concentrations of dNTPs and primers were tested as follows: dNTPs from 0.2 to 1.4 mM, FIP and BIP (inner primers) from 20 to 40 pmole, and F3 and B3 (outer primers) from 5 to 10 pmole.
RT-LAMP amplification was spectrophotometrically monitored by recording the optical density at 400 nm with a real-time turbidimeter (Eiken Co. Ltd., Tokyo, Japan).
RT-LAMP amplification products were visualized by adding 1 μl of fluorescent detection reagent (Eiken Co. Ltd., Tokyo, Japan) to the reaction mixture before incubation. The products were examined under natural light and under UV light.
Using the Maxime™ RT-PCR PreMix Kit (iNtRON Biotechnology, Korea), an one-step RT-PCR reaction was conducted. The mixture was incubated at 45°C for 30 min and denatured at 94°C for 5 min, followed by 30 cycles of 94°C for 30 sec, 50.7°C for 30 sec and 72°C for 30 sec, with a final extension of 72°C for 5 min. RT-PCR products (5 μl) were analyzed by electrophoresis on a 1.5% agarose gel.
In order to examine the specificity of six primer sets, three replicates of the experiments were conducted. When RT-LAMP assays were conducted using the RT-LAMP mixture, primer sets A, C, and F showed ladder-like bands on the agarose gel only for the PVX RNA containing samples (Fig. S1). By repeating the experiments with primer sets A, C, and F, we confirmed true positive reactions for primer sets A and F. To evaluate the sensitivity of the two primer sets, a dilution series of PVX RNA was used for RT-LAMP. The detection limits of primer sets A and F were observed at 0.0002 ng and 0.02 μg, respectively (Fig. 1A and 1B). In addition, the detection limit of RT-PCR was 0.2 ng (Fig. 1C). In comparison, RT-LAMP conducted with primer set A (Fig. 1A) showed higher sensitivity (about 1000 times) than conventional RT-PCR (Fig 1C). Finally, primer set A was selected and used for RT-LAMP PCR. The RT-LAMP products of RNA from leaves infected by PVX showed ladder-like bands on the agarose gel, but not rom RT-LAMP products of RNAs from healthy plant leaves and PLRV-infected plant leaves, indicating that primer set A is specific for PVX (Fig. S2).
To determine the optimal RT-LAMP reaction, various parameters including amplification temperature and the concentration of dNTPs and outer (F3 and B3) and inner (FIP and BIP) primers were tested. RT-LAMP reactions were carried out using gradient PCR setting the temperature range from 56°C to 70°C. Fig. 3A shows that all temperatures were capable of producing RT-LAMP amplicons. A similar amount of product was also generated at temperatures ranging from 59 to 69.1°C. However, temperatures under 56°C or above 70°C showed decreased yield. Fig. 3B shows that the concentration of dNTPs affected the amount of RT-LAMP products. A minimum concentration of 0.4 mM dNTPs was needed to acquire a positive RT-LAMP result. The greatest amount of product was for both 1.0 and 1.2 mM suggesting that the optimum concentration of dNTPs was either 1.0 or 1.2 mM. The concentrations of primers also affected the amount of RT-LAMP products, but the effect of primer concentration depended on the types of primers (Fig. 3C). A greater amount of amplification products were generated from reactions at both 30 and 40 pmole (Fig. 3C1-4) of inner primers compared to 20 pmole of inner primer. Using the same inner primers (FIP and BIP), a similar amount of RT-LAMP products was obtained regardless of the concentrations of outer primers (F3 and B3); thus, showing that the concentrations of FIP and BIP were more effective than those of F3 and B3.
RT-LAMP reactions were performed using primer set A with or without loop primers to examine the effect of loop primers on shortening the reaction time according to the turbidity of reaction mixtures with or without loop primers. The results showed that the time required for the initiation of RT-LAMP amplification was 13 or 26 min with or without loop primers, respectively (Fig. 4). Evidently, the use of loop primers accelerated the amplification of PVX, reducing the reaction time to almost half.
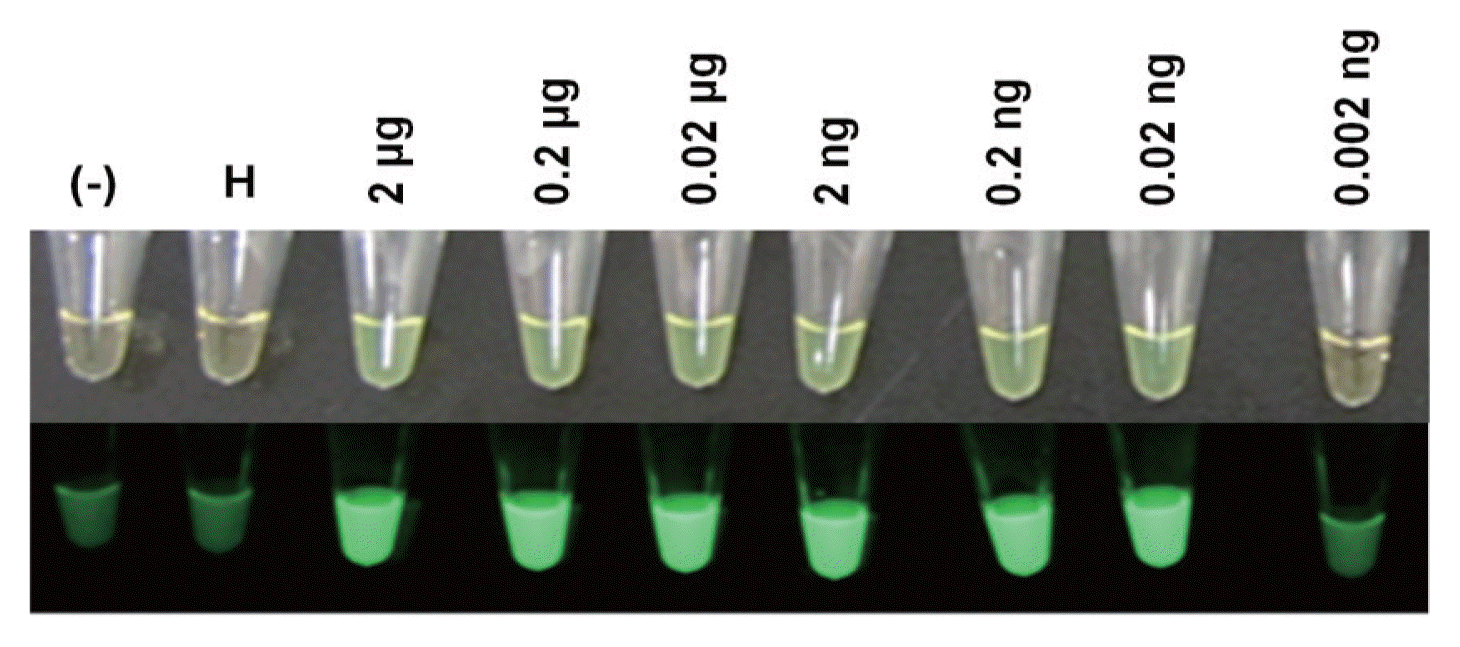
The RT-LAMP amplification products in the reaction tubes were visualized using fluorescent dyes under ambient light or UV light, which also allowed for determination of the detection limit of PVX RNA. RT-LAMP products showing positive results had a light green color under ambient light, whereas the negative control was light yellow-orange (Fig. 5). Under UV light, positive tubes emitted bright fluorescent light, but negative samples showed dim or no fluorescent light (lane 1 and 2). In addition, RT-LAMP amplification products could be visualized when 2 μg to 0.02 ng of PVX RNA was used as the template, whereas no products were visualized when 0.002 ng of PVX RNA was used (lane 9), indicating that 0.02 ng of PVX RNA is the detection limit in this study.
Plant diseases have been increasing due to globalization of trade, increased human mobility, global warming, pathogen’s evolution, and improper crop management (Anderson et al., 2004; Garrett et al., 2006). Therefore, it is essential to diagnose disease agents for proper disease control strategies. Specifically, accurate, rapid, and efficient diagnostic tools are crucial to control viral diseases because agro-chemicals to cure plant viral diseases have not been commercialized.
Many techniques have been developed and used for the diagnosis of potato viral diseases including PVX (El-Araby et al., 2009; Khan et al., 2003). ELISA has been accepted as the most common and reliable detection method for viruses including PVX because it has long history of use and it is also rapid and inexpensive (Clark and Adams, 1977; Makkouk and Kumari, 2006). In general, ELISA methods have drawbacks such as long reaction time, less specificity caused by cross reactivity, fewer numbers of antibodies available, and less sensitivity (El-Araby et al., 2009). Since molecular-based assays are known to have higher sensitivity, RT-PCR may reduce shortcomings caused by less sensitivity. RT-PCR-based assays have also been shown to remedy additional defects of ELISA (Agindotan et al., 2007; El-Araby et al., 2009). However, one of the limitations of RT-PCR is that the assay needs sophisticated and expensive instruments because it depends on thermal cycling for denaturation of double stranded DNA into single stranded DNA and enzymatic replication of the DNA (Saiki et al., 1988).
LAMP PCR is relatively new and does not require thermal cycling because it is an assay based on isothermal amplification (Notomi et al., 2000). RT-LAMP PCR, one of the variants of LAMP PCR, has been used for the diagnosis of plant RNA viruses (Ju, 2011; Nie, 2005). The specificity of LAMP is generally high because the RT-LAMP PCR assay uses four primers that perceive six regions on the target gene. However, primer design seems to be an important limiting factor for the general use of RT-LAMP PCR in PVX diagnosis, as previous studies reported that more non-specific reactions among primers are often found in LAMP PCR compared to conventional PCR (Boubourakas et al., 2009; Notomi et al., 2000; Wei et al., 2012). In order to determine the RT-LAMP primer sets that could be used for detection of PVX, six sets of primers were designed. Only one primer set (set A) was selected based on its specificity (Fig. S1 and S2) and sensitivity (Fig. 2) for highly successful detection of PVX.
Although the sensitivity for detecting Classical swine fever virus (Yin et al., 2010) is slightly lower for RT-PCR compared to RT-LAMP, most RT-LAMP assays are more sensitive than regular RT-PCR or nested PCR. The RT-LAMP PCR for PVX that was developed in this study showed 1,000-fold greater sensitivity than conventional RT-PCR assays, similar to most previous reports (Fig. 2) (Ju, 2011; Kuan et al., 2010; Parida et al., 2005; Venkatesan et al., 2012).
The optimum temperature for different RT-LAMP assays might be different because of different primer sets, for example 62.5 and 65°C for Peach latent mosaic viroid and 61 to 67°C for Squash leaf curl virus (Boubourakas et al., 2009; Kuan et al., 2010). In Fig. 3A, the optimum temperature of the RT-LAMP to detect PVX seemed to range from 59 to 69.1°C. As shown in many studies, increasing or decreasing the reaction temperature beyond this temperature range results in decreased yield (Fukuda et al., 2004; Wei et al., 2012). This might be because of inactivation of enzymes or reaction instability caused by too high or low temperatures.
Different target genes may require different concentrations of dNTPs when those were amplified by LAMP-based methods. This study showed that amplification products were obtained using a concentration of dNTPs ranging from 0.4 to 1.4 mM, but no reaction products at 0.2 mM. The concentration of dNTPs required to detect Roundup Ready soybeans by LAMP ranges from 0.6 to 3.2 mM (Wang et al., 2013).
In general, most RT-PCR amplification takes a couple of hours, including 30 min of an additional reverse transcription step. However, RT-LAMP amplification takes less than 60 min, even with the reverse transcription step (Fukuda et al., 2004). Many studies have revealed a reaction time of less than 30 min for RT-LAMP (Kuan et al., 2010; Soliman and El-Matbouli, 2006). In addition, virus can be detected within 20 min by real-time RT-LAMP when two loop primers are applied (Fukuta et al., 2004; Ju, 2010; Parida et al., 2005). This study showed similar results in that the RT-LAMP assay included two loop primers and took only 15 min for detection of PVX.
In conclusion, the RT-LAMP assay developed to diagnose PVX in this study is rapid, cost-effective, specific, and sensitive for the detection of PVX. Moreover, this assay can be extended many other applications for diagnostic purposes.
Acknowledgments
This paper was supported by Rural Development Administration (RDA) fund PJ01004202, Republic Korea.
Fig. 1
The locations and sequences of PVX CP primers used for RT-LAMP PCR. Lines with the same color indicate a primer set. Primer Explorer V4 was used to design primers sets A, B, and C, and Lamp Designer was used to design D, E, and F.
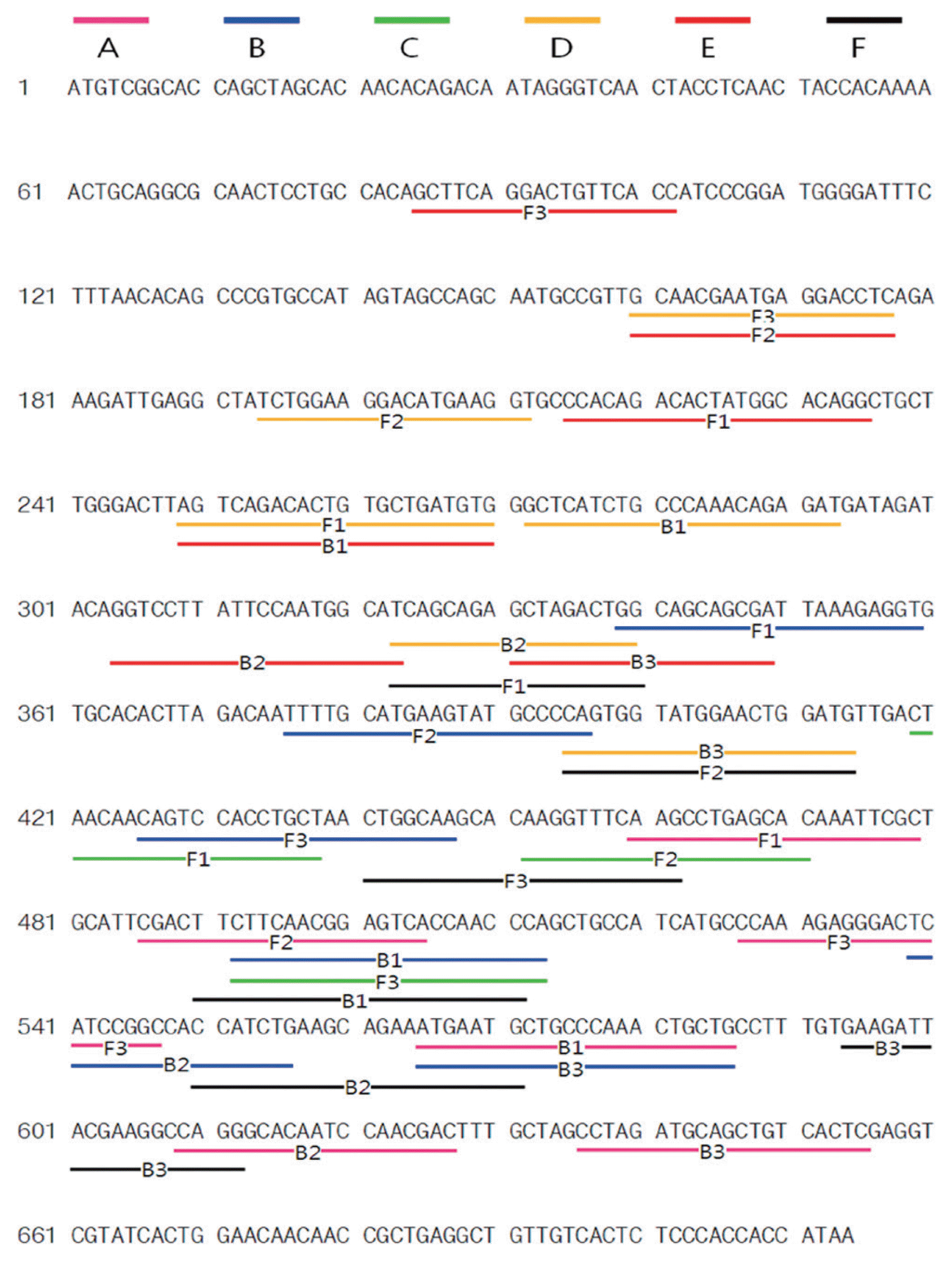
Fig. 2
Comparative sensitivity of RT-LAMP (A and B) versus RT-PCR (C) for detection of the PVX CP gene. Reactions were conducted with primer sets A (A and C) and F (B) and observed by agarose gel electrophoresis. M, molecular size marker; (−), without PVX RNA; 3-10, PVX RNA (2, 0.2, 0.02 μg; 2, 0.2, 0.02 ng, 2; and 0.2 pg).
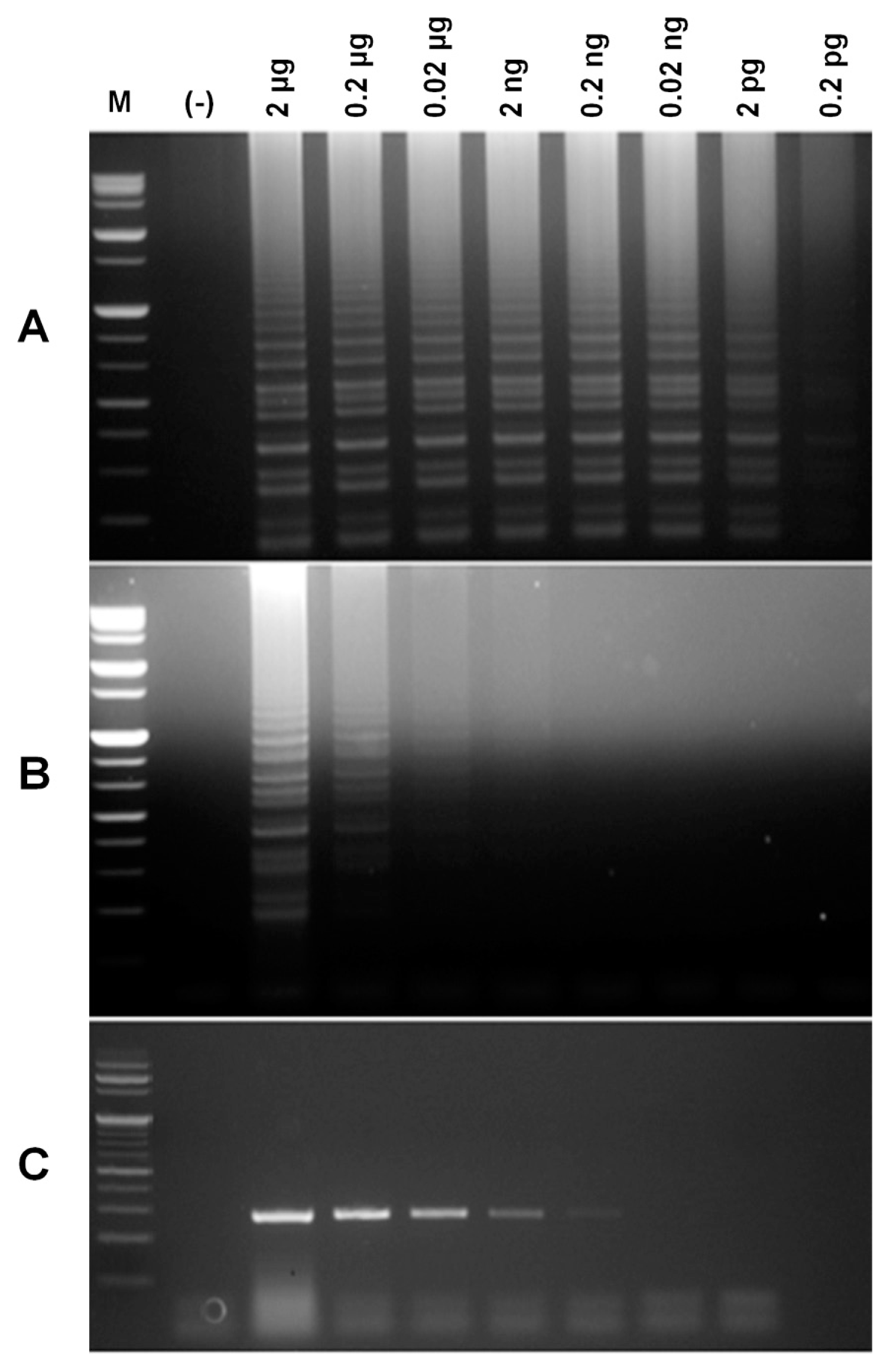
Fig. 3
Optimization of RT-LAMP for PVX detection. (A) RT-LAMP products by gradient PCR. M, molecular size marker; (−), without RNA. (B) Optimization of dNTP concentrations for RT-LAMP. (C) Electrophoresis results of the effects of different primer concentrations on RT-LAMP amplification products. 1, FIP, BIP/F3, B3=40/10 pmole; 2, FIP, BIP/F3, B3=40/5 pmole; 3, FIP, BIP/F3, B3=30/10 pmole; 4, FIP, BIP/F3, B3=30/5 pmole; 5, FIP, BIP/F3, B3=20/10 pmole; 6, FIP, BIP/F3, B3=20/5 pmole.
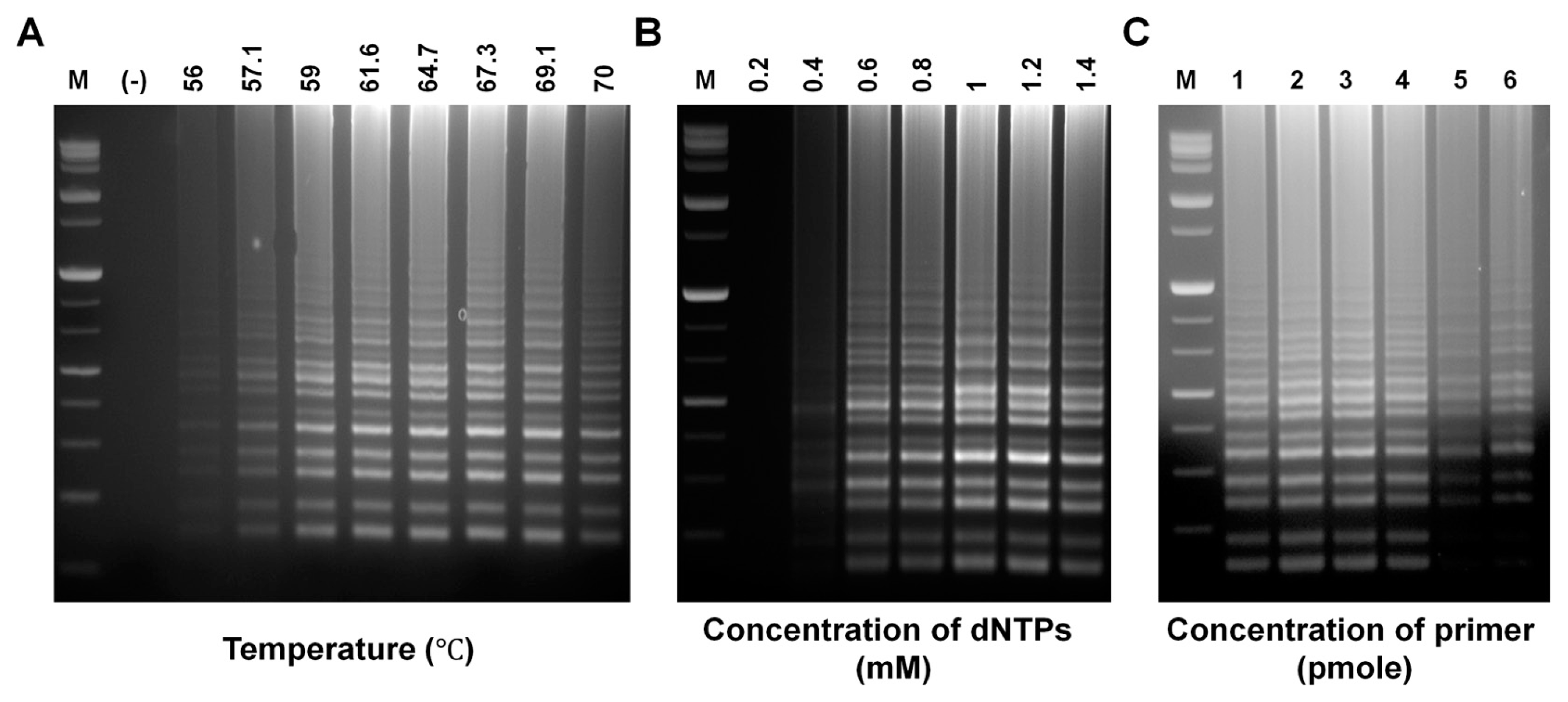
Fig. 4
Effect of loop primers on RT-LAMP amplification of the PVX CP gene. Real-time measurement of optical density with a real-time turbidimeter was used to monitor RT-LAMP amplification with or without loop primers.
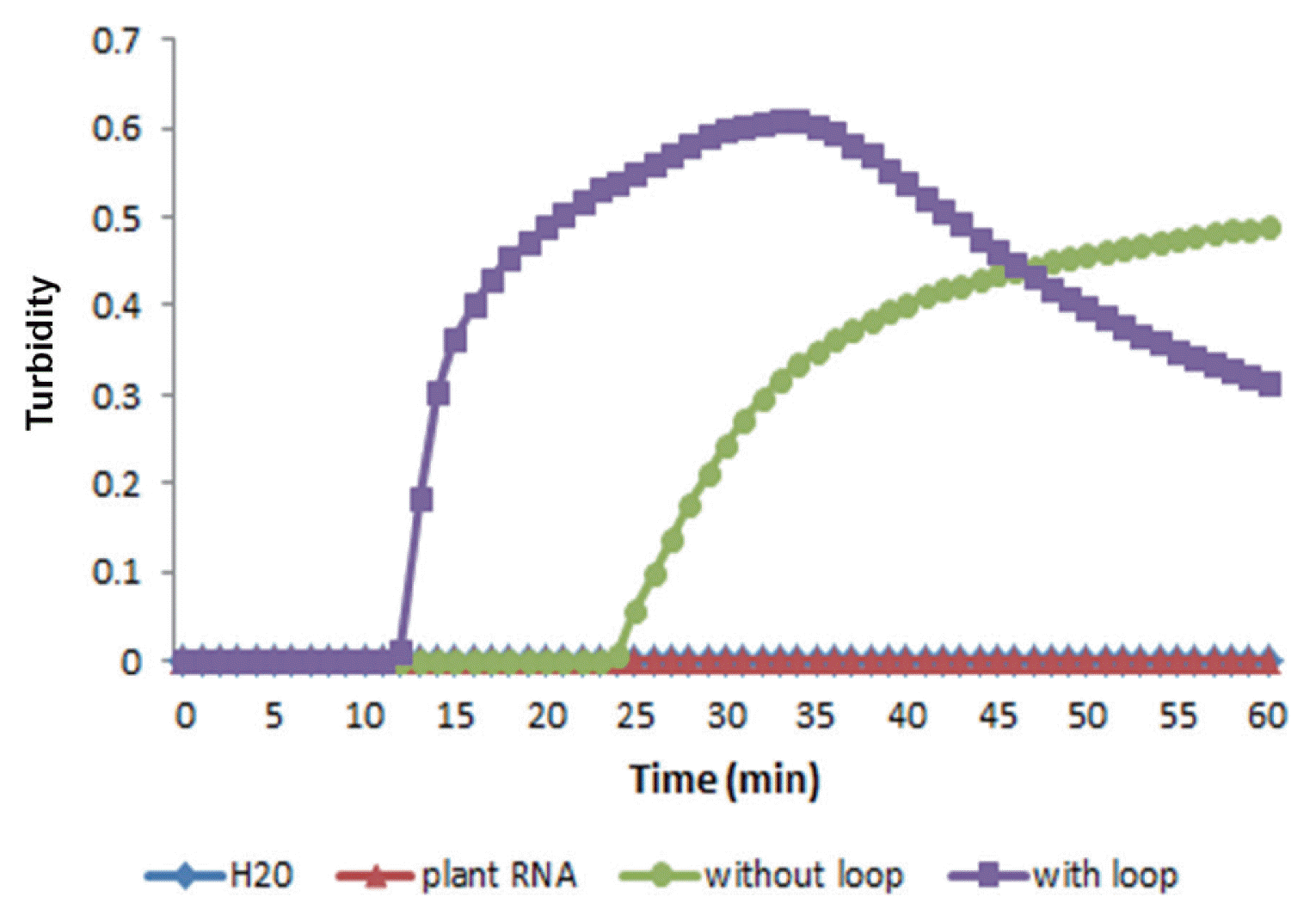
Fig. 5
Fluorescent dye-mediated monitoring of PVX amplification by RT-LAMP. (Top) Naked-eye inspection under normal light. (Bottom) Visual observation of green fluorescence under UV light. (−), without RNA; H, RNA from leaves of healthy Nicotiana benthamiana; lanes 3-9, RNA from leaves of PVX-infected N. benthamiana.
References
Aboul-Ata, AE, Mazyad, H, El-Attar, AK, Soliman, AM, Anfoka, G, Zeidaen, M, Gorovits, R, Sobol, I and Czosnek, H 2011. Diagnosis and control of cereal viruses in the Middle East. Adv Virus Res. 81:33-61.


Adkar-Purushothama, CR, Maheshwar, PK, Sano, T and Janardhana, GR 2011. A sensitive and reliable RT-nested PCR assay for detection of Citrus tristeza Virus from naturally infected Citrus plants. Curr Microbiol. 62:1455-1459.



Agindotan, BO, Shiel, PJ and Berger, PH 2007. Simultaneous detection of potato viruses, PLRV, PVA, PVX and PVY from dormant potato tubers by TaqMan real-time RT-PCR. J Virol Methods. 142:1-9.


Anderson, PK, Cunningham, AA, Patel, NG, Morales, FJ, Epstein, PR and Daszak, P 2004. Emerging infectious diseases of plants: pathogen pollution, climate change and agrotechnological drivers. Trends Ecol Evol. 19:535-544.

Boubourakas, IN, Fukuta, S and Kyriakopoulou, PE 2009. Sensitive and rapid detection of peach latent mosaic viroid by the reverse transcription loop-mediated isothermal amplification. J Virol Methods. 160:63-68.


Cardoso, TC, Ferrari, HF, Bregano, LC, Silva-Frade, C, Rosa, AC and Andrade, AL 2010. Visual detection of turkey coronavirus RNA in tissues and feces by reverse-transcription loop-mediated isothermal amplification (RT-LAMP) with hydroxynaphthol blue dye. Mol Cell Probes. 24:415-417.



Clark, MF and Adams, A (1977):Characteristics of the microplate method of enzyme linked immunosorbent assay for the detection of plant viruses. J Gen Virol. 34:475-483.


El-Araby, WS, Ibrahim, IA, Hemeida, AA, Mahmoud, A, Soliman, AK, El-Attar, AK and Mazyad, HM 2009. Biological, Serological and molecular diagnosis of three major potato viruses in Egypt. Internat J Virol. 5:77-88.

English, JJ, Davenport, GF, Elmayan, T, Vaucheret, H and Baulcombe, DC 1997. Requirement of sense transcription for homology-dependent virus resistance and trans-inactivation. Plant J. 12:597-603.


Fukuta, S, Ohishi, K, Yoshida, K, Mizukami, Y, Ishida, A and Kanbe, M 2004. Development of immunocapture reverse transcription loop-mediated isothermal amplification for the detection of Tomato spotted wilt virus from chrysanthemum. J Virol Methods. 121:49-55.


Garrett, KA, Dendy, SP, Frank, EE, Rouse, MN and Travers, SE 2006. Climate change effects on plant disease: genomes and ecosystems. Annu Rev Phytopathol. 44:489-509.


Helguera, PR, Taborda, R, Docampo, DM and Ducasse, DA 2001. Immunocapture reverse transcription-polymerase chain reaction combined with nested PCR greatly increases the detection of Prunus necrotic ring spot virus in the peach. J Virol Methods. 95:93-100.


Helguera, PR, Docampo, DM, Nome, SF and Ducasse, DA 2002. Enhanced detection of Prune dwarf virus in peach leaves by immunocapture-reverse transcription-polymerase chain reaction with nested polymerase chain Reaction (IC-RT-PCR Nested PCR). J Phytopathol. 150:94-96.

Ju, H-J 2011. Simple and rapid detection of Potato leafroll virus (PLRV) by reverse transcription loop-mediated isothermal amplification (RT-LAMP). Plant Pathol J. 27:1-4.
Jones, RAC 1985. Further studies on resistance -breaking strains of Potato virus X. Plant Pathol. 34:182-189.

Khan, MS, Hoque, MI, Sarker, RH and Muehlbach, H-P 2003. Detection of important plant viruses in In vitro regenerated potato plants by Double antibody sandwich method of ELISA. Plant Tissue Cult. 13:21-29.
Kuan, CP, Wu, MT, Lu, YL and Huang, HC 2010. Rapid detection of squash leaf curl virus by loop-mediated isothermal amplification. J Virol Methods. 169:61-65.


Makkouk, KM and Kumari, SG 2006. Molecular diagnosis of plant viruses. Arab J Plant Protect. 24:135-138.
Moreno, A, Bertolini, E, Olmos, A, Cambra, M and Fereres, A 2007. Estimation of vector propensity for Lettuce mosaic virus based on viral detection in single aphids. Spanish J Agric Res. 5:376-384.

Mortimer-Jones, MS, Jones, MG, Jones, RA, Thomson, G and Dwyer, GI 2009. A single tube, quantitative real-time RT-PCR assay that detects four potato viruses simultaneously. J Virol Methods. 161:289-296.


Nagamine, K, Hase, T and Notomi, T 2002. Accelerated reaction by loop-mediated isothermal amplification using loop primers. Mol Cell Probes. 16:223-229.


Nie, X 2005. Reverse transcription loop-mediated isothermal amplification of DNA for detection of Potato virus Y. Plant Dis. 89:605-610.


Notomi, T, Okayama, H, Masubuchi, H, Yonekawa, T, Watanabe, K, Amino, N and Hase, T 2000. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 28:e63



Olmos, A, Cambra, M, Esteban, O, Gorris, MT and Terrada, E 1999. New device and method for capture, reverse transcription, and nested PCR in a single closed-tube. Nucleic Acid Res. 27:1564-1565.



Parida, M, Horioke, K, Ishida, H, Dash, PK, Saxena, P, Jana, AM, Islam, MA, Inoue, S, Hosaka, N and Morita, K 2005. Rapid detection and differentiation of Dengue virus serotypes by a real-time reverse transcription-loop-mediated isothermal amplification assay. J Clin Microbiol. 43:2895-2903.




Parida, M, Sannarangaiah, S, Dash, PK, Rao, PV and Morita, K 2008. Loop mediated isothermal amplification (LAMP); a new generation of innovative gene amplification technique; perspectives in clinical diagnosis of infectious diseases. Rev Med Virol. 18:407-421.



Park, SH and Kim, K-H 2006. Agroinfiltration-based Potato virus X replicons to dissect the requirements of viral infection. Plant Pathol J. 22:386-390.

Saiki, RK, Gelfand, DH, Stoffel, S, Scharf, SJ, Higuchi, R, Horn, GT, Mullis, KB and Erlich, HA 1988. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 239:487-491.


Soliman, H and El-Matbouli, M 2006. Reverse transcription loo-mediated isothermal amplification (RT-LAMP) for rapid detection of viral hemorrhagic septicaemia virus (VHS). Vet Microbiol. 114:205-213.

Tomita, N, Mori, Y, Kanda, H and Notomi, T 2008. Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of product. Nat Protoc. 3:877-882.



Venkatesan, G, Bhanuprakash, V, Balamurugan, V, Singh, RK and Pandey, AB 2012. Development of loop-mediated isothermal amplification assay for specific and rapid detection of camelpox virus in clinical samples. J Virol Methods. 183:34-39.


Wang, B, Ma, Y, Zhang, Z, Wu, Z, Wu, Y, Wang, Q and Li, M 2011. Potato viruses in China. Crop Prot. 30:1117-1123.

Wang, X, Teng, D, Guan, Q, Tian, F and Wang, J 2013. Detection of Roundup ready soybean by loop-mediated isothermal amplification combined with a lateral-flow dipstick. Food Cont. 29:213-220.

Wang, Z, Xia, Y, Yuan, Q, Tan, W and Yin, Y 2005. Detection of mix-infected potato viruses with multiplex RT-PCR. Acta Phytopathol Sinica. 35:109-115.
Webster, CG, Wylie, JS and Jones, MGK 2004. Diagnosis of plant viral pathogens. Curr Science. 86:1604-1607.



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print