Ultrastructure of the Rust Fungus Puccinia miscanthi in the Teliospore Stage Interacting with the Biofuel Plant Miscanthus sinensis
Article information
Abstract
Interaction of the the rust fungus Puccinia miscanthi with the biofuel plant Miscanthus sinensis during the teliospore phase was investigated by light and electron microscopy. P. miscanthi telia were oval-shaped and present on both the adaxial and abaxial leaf surfaces. Teliospores were brown, one-septate (two-celled), and had pedicels attached to one end. Transmission electron microscopy revealed numerous electron-translucent lipid globules in the cytoplasm of teliospores. Extensive cell wall dissolution around hyphae was not observed in the host tissues beneath the telia. Hyphae were found between mesophyll cells in the leaf tissues as well as in host cells. Intracellular hyphae, possibly haustoria, possessed electron-dense fungal cell walls encased by an electron-transparent fibrillar extrahaustorial sheath that had an electron-dense extrahaustorial membrane. The infected host cells appeared to maintain their membrane-bound structures such as nuclei and chloroplasts. These results suggest that the rust fungus maintains its biotrophic phase with most mesophyll cells of M. sinensis. Such a nutritional mode would permit the rust fungus to obtain food reserves for transient growth in the course of host alteration.
Rust fungi cause diseases that are characterized by rust colored powder on a number of economically important plants worldwide. Belonging to a single order Pucciniales (= Uredinales), the fungi comprise approximately 7,000 obligate parasites of vascular plants in the Basidiomycota, and have the most complicated life cycles with multiple forms in fungi (Aime et al., 2006; Littlefield and Heath, 1979). Only certain stages of the life cycles of some rust fungi have been grown in axenic culture on artificial media (Alexopoulos et al., 1996). Rust fungi are phenotypically and genetically plastic organisms with many characteristics including the occurrence of up to six morphologically different spore types, which set them apart from other Basidiomycota species (Aime et al., 2006). Most rust fungi overwinter in the binucleate teliospore stage, but in some species teliospores germinate soon after they are formed (Alexopoulos et al., 1996). In addition, most rust fungi need two taxonomically unrelated host plants to complete their life cycle (heteroecism).
Miscanthus sinensis Andersson, also referred to as eulalia grass, is a perennial plant that is widely distributed in the mountains of eastern Asia. The presence of C4 photosynthetic apparatus provides this herbaceous plant with the unique ability to grow fast even in acidic soils. It has been favorably used as a biofuel plant for ethanol production (Heaton et al., 2008; Weng and Hsu, 2001; Yoshida et al., 2008). The morphological features of the leaf surface of M. sinensis have been investigated by field emission scanning electron microscopy (FESEM) (Kim, 2013). M. sinensis leaf rust occurred in Korea, where the causal agent was identified as the basidiomycetous fungus Puccinia miscanthi Miura (Hiratsuka, 1958; Lee, 2001). While the aeciospores are found on Plantago species (Hiratsuka, 1958), urediniospores and teliospores are present on M. sinensis, rendering the rust fungus heteroecious (Kim, 2015). A recent study (Kim, 2015) revealed the surface details and in situ cutting of P. miscanthi teliospores by FESEM. However, no information is available on the ultrastructure of P. miscanthi in the teliospore stage during interaction with M. sinensis as observed by transmission electron microscopy. Such a microscopic study could offer some insight into the knowledge of plant-microbe interactions at the cellular level.
Approximately 2-m-tall eulalia grasses were grown in the mountainous region of Muju, Korea (Kim, 2015). Mature leaves with typical leaf rust symptoms were collected from the middle part of the grasses in fall. Telia (sing. telium) and teliospores on the naturally infected leaves were observed with a stereomicroscope (EZ4 HD; Leica Microsystems, Heerbrugg, Switzerland) and a light microscope (Axiophot; Carl Zeiss, Oberkochen, Germany), respectively.
Fragments of both uninfected and naturally infected M. sinensis leaves were fixed and processed as previously reported (Kim, 2015; Seo et al., 2014). Briefly, they were immersed in a modified Karnovsky’s fixative in 0.05 M sodium cacodylate buffer at 4°C overnight. They were washed with the same buffer and postfixed with 1% osmium tetroxide in the same buffer at 4°C for 2 h. The postfixed leaves were washed with distilled water, and en bloc stained overnight with 0.5% uranyl acetate at 4°C. The specimens were dehydrated in a graded ethanol series, treated with propylene oxide, and embedded in Spurr’s resin. Ultrathin sections were each stained with 2% uranyl acetate and Reynolds’ lead citrate for 7 min. The sections were observed using a transmission electron microscope (JEM-1010; JEOL, Tokyo, Japan) at 80 kV.
Stereomicroscopy revealed dark brown telia of P. miscanthi on the leaf surface of M. sinensis (Fig. 1A). Telia were oval-shaped and present on both the adaxial (upper) and abaxial (lower) leaf surfaces. Telia possessed a number of teliospores and protruded from the infected leaf surface (Fig. 1B). Light microscopy showed that teliospores were brown and one-septate (two-celled) (Fig. 1C). Teliospores had pedicels attached to one end.
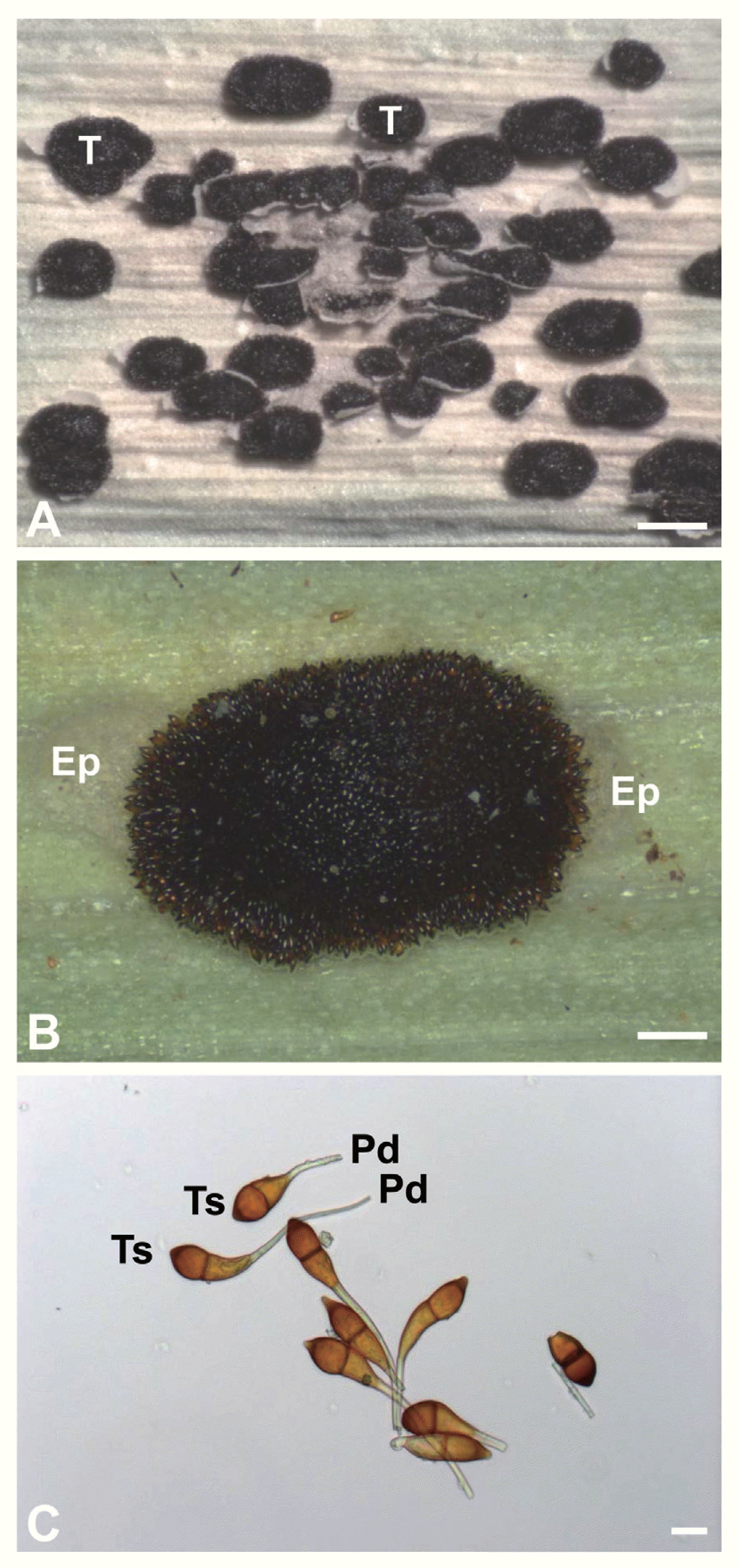
Micrographs of Puccinia miscanthi. (A) Stereomicrograph of P. miscanthi telia on the leaf surface of Miscanthus sinensis. Telia (T) are found on the leaf epidermis. Bar = 1 mm. (B) Stereomicrograph of a P. miscanthi telium on the leaf surface of M. sinensis. The ruptured leaf epidermis (Ep) of M. sinensis. can be discerned around the telium. Bar = 200 μm. (C) Light micrograph of P. miscanthi teliospores. Pedicels (Pd) are attached to one end of teliospores (Ts). Bar = 20 μm.
Transmission electron microscopy revealed the internal features of uninfected M. sinensis leaves (Fig. 2A). Guard cells and substomatal chambers were observed in the cross sections. Mesophyll cells filled with central vacuoles were commonly observed in the leaf tissues (Fig. 2B). Neither typical palisade cells nor spongy cells were found in the tissues (unifacial leaf). On infected leaves, teliospores contained numerous electron-translucent lipid globules in the cytoplasm (Fig. 2C). The fungal cell walls varied in thickness depending on the teliospores and pedicels in the telia. The interface between the pathogen and its host was evident in the telia on the infected leaves (Fig. 2D). The teliospores and their pedicels were often arranged in parallel to the leaf epidermis. A few epidermal cells were necrotized, showing electron-dense material in the cytoplassm.
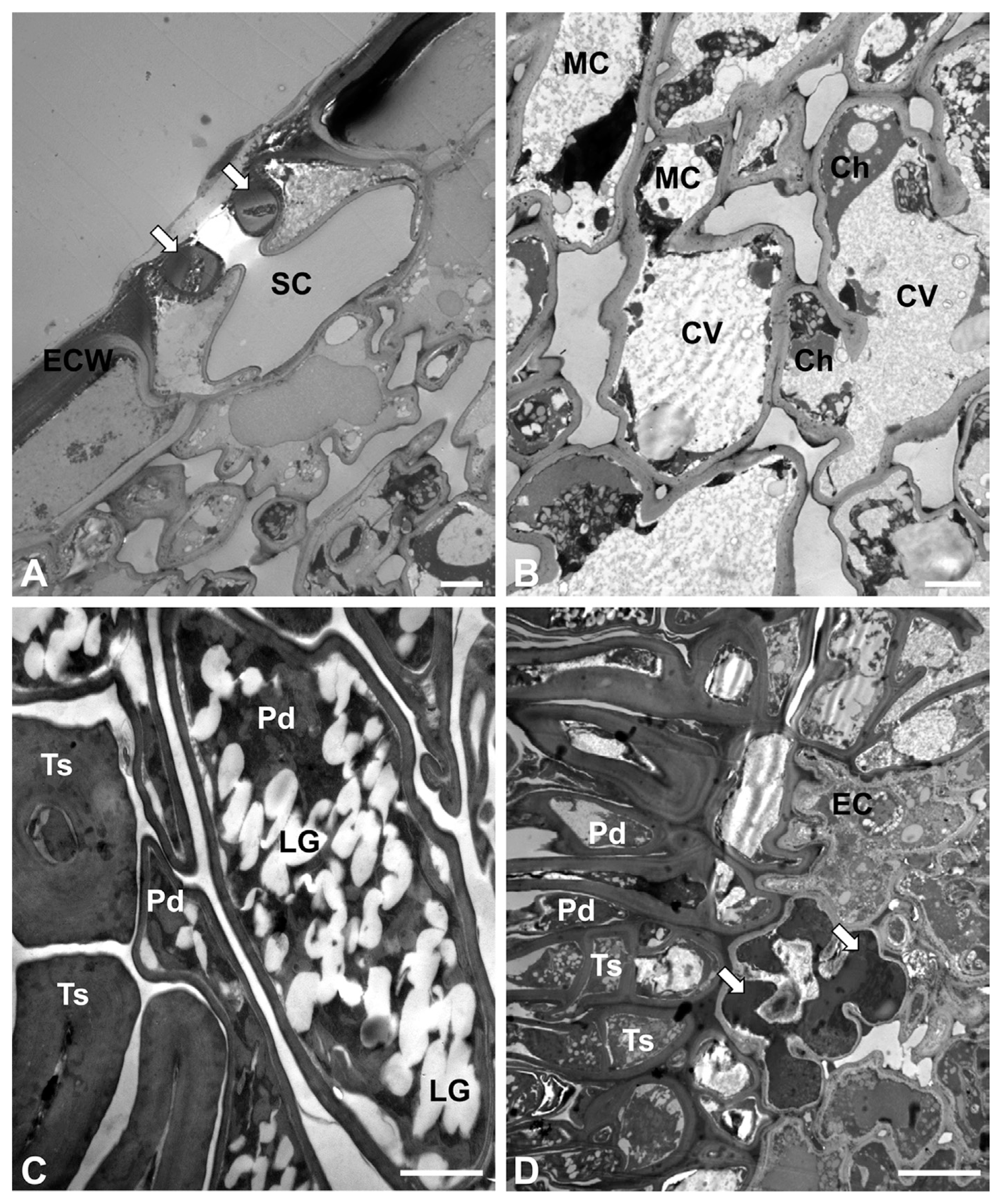
Transmission electron micrographs of uninfected and rust-infected M. sinensis leaves. (A) Stomatal complex of an uninfected leaf. It contains guard cells (arrows) and a substomatal chamber (SC). ECW = epidermal cell wall. Bar = 2 μm. (B) Mesophyll cells (MC) of an uninfected leaf. The cells contain central vacuoles (CV) and chloroplasts (Ch) in the cytoplasm. Bar = 2 μm. (C) Teliospores (Ts) and pedicels (Pd) of P. miscanthi on an infected leaf. They have lipid globules (LG) in the fungal cytoplasm. Bar = 1 μm. (D) Interface between P. miscanthi and M. sinensis. Necrotized host cells (arrows) contain electron-dense material in the cytoplasm. EC = epidermal cell. Bar = 5 μm.
On a few instances, the host cells were observed to be in contact with the teliospores and their pedicels were slightly modified (Fig. 3A). Fragments of wall materials indicated the successive development of teliospores, as shown in Puccinia punctiformis (Baka and Lösel, 1992). Extensive cell wall dissolution around hyphae was not observed in the host tissues beneath the telia (Fig. 3B). The fungal cell walls appeared more electron-dense than the host cell walls did. Hyphae were found between mesophyll cells in the leaf tissues (intercellular hyphae) (Fig. 3C). At higher magnifications, it was clear that the host cell walls contained electron-dense granule-like materials. In addition, hyphae were observed in the host cells (intracellular hyphae) (Fig. 3D). The intracellular hyphae were assumed to be haustoria, and had a fibrillar extrahaustorial sheath outside of the thin electron-dense haustorial cell wall.
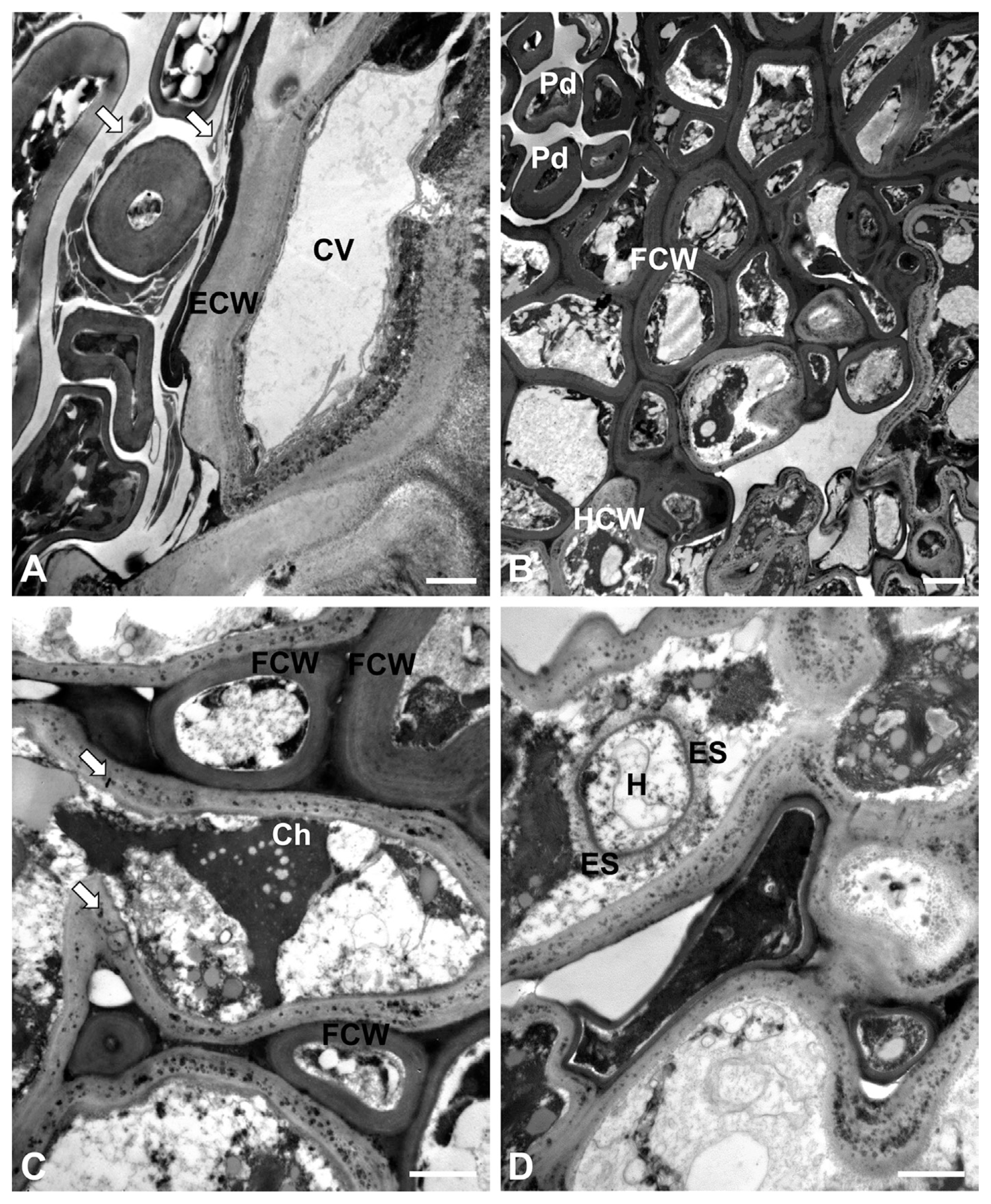
Transmission electron micrographs of P. miscanthi in M. sinensis leaves. (A) Teliospores and pedicels in contact with host cells. Arrows indicate fragments of wall materials. CV = central vacuole. ECW = epidermal cell wall. Bar = 1 μm. (B) Mesophyll cells of an infected leaf. The fungal cell walls (FCW) appear more electron-dense than the host cell walls (HCW) did. Pd = pedicel. Bar = 2 μm. (C) Intercellular hyphae of P. miscanthi. The host cell walls contain electron-dense granule-like materials (arrows). Ch = chloroplast. FCW = fungal cell wall. Bar = 1 μm. (D) Intracellular hypha of P. miscanthi. The hypha, referred to as a haustorium (H), have an extrahaustorial sheath (ES) outside of the thin electron-dense haustorial cell wall. Bar = 1 μm.
Several types of haustoria were observed in the cross sections of host cells. Either amorphous or round-shaped haustoria were found together in host cells (Fig. 4A). The fungal cell walls could be readily identified, but the presence of an extrahaustorial sheath was not always distinct (Fig. 4B). The infected host cells appeared to maintain their membrane-bound structures such as nuclei and chloroplasts (Fig. 4C). No apparent cellular disruption was observed in host tissues. Higher magnifications revealed that the typical round haustoria possessed the electron-dense fungal cell walls encased by an electron-transparent extrahaustorial sheath with the electron-dense extrahaustorial membrane (matrix) (Fig. 4D).
Transmission electron micrographs of P. miscanthi in M. sinensis leaves. (A) Two types of haustoria (H) of P. miscanthi. They are round or amorphous in shape. HCW = host cell wall. Bar = 1 μm. (B) Round haustoria of P. miscanthi. The fibrillar extrahaustrial sheath (arrow) was not always distinct. Ch = chloroplast. Bar = 2 μm. (C) Host cells that have P. miscanthi haustoria. Little cellular disruption are observed. Bar = 2 μm. (D) Higher magnification of P. miscanthi haustoria. Arrows and arrowheads indicate the extrahaustorial sheath and extrahaustorial matrix, respectively. HA = haustorial cell wall. Bar = 500 nm.
This study illustrated the biotrophic nature of P. miscanthi in M. sinensis, as observed by transmission electron microscopy. The reason for the biotrophic nature of the rust fungus could be specifically addressed using the results of transmission electron microscopy. The host cell was in a viable state owing to the presence of intact cellular organelles such as nuclei and chloroplasts. Rust fungi do not kill their host plants, although repeated heavy infections of certain perennial woody hosts may lead to the progressive decline and sometimes death of the plant (Alexopoulos et al., 1996). The rust fungus P. miscanthi formed haustoria that penetrated the host cells and absorbed nutrients and water. Thus, the intercellular hyphae can be designated as haustorial mother cells from which haustoria develop in host tissues. It is likely that the rust fungus maintains its biotrophic phase with most mesophyll cells of M. sinensis. At some sites of infection, however, a few heavily necrotized host cells were observed with electron-dense materials in their cytoplasm. Such a nutritional mode would allow the rust fungus to obtain food reserves for transient growth in the course of host alteration. However, this study does not exclude the possibility that the necrotic cells may be from its final stage of infection, requiring no further peaceful existence with the plant as a biotroph.
In cross sections, several types of haustoria were observed in viable host cells. Apart from nutritional uptake, haustoria are also involved in the suppression of host defenses, and in the reduction or reprogramming of host metabolism (Larous et al., 2008). Based on cytochemical analysis, polysaccharides and proteins were found to be the major constituents of the extrahaustorial sheath or extrahaustorial matrix of Puccinia striiformis (Ma and Shang, 2009). It has been shown that the extrahaustorial membrane lacks adenosine triphosphatase (ATPase) activity, and has no means of counteracting the unidirectional flow of nutrients into the haustorium (Deacon, 1997).
Naturally infected eulalia grasses were collected from the field. The pathogenic reaction of the host with the natural population of P. miscanthi was not addressed in this study. Given the rare occurrence of heavily necrotized host cells that had haustoria or were adjacent to haustorial mother cells, it is possible that the eulalia grasses examined in this study might be susceptible to the pathogen, as inferred in the rust of wheat (Ma and Shang, 2009) and groundnut (Wingfield and Rijkenberg, 1981) collected from the field. The electron-dense granule-like materials in host cell walls are strikingly similar to those observed in the cell wall components of rice leaves (Kim et al., 2002). By using X-ray microanalysis, the granule-like materials have been identified as silicon in rice.
The biofuel plant M. sinensis has been intensively studied, and it has been cultivated on a large scale in the field. The host range and distribution of the rust fungus in the field needs to be investigated to enable a better strategy to be deployed against the pathogen. Elucidation of the association of haustoria with the host endomembrane system will increase our understanding of the biotrophic phase of obligate plant parasites including rust fungi in nature.
Acknowledgments
I am grateful to Jin-Won Kim at the University of Seoul, Korea for providing the plant materials.