 |
 |
| Plant Pathol J > Volume 31(3); 2015 > Article |
Abstract
Certain bacterial species associate with plant roots in soil. The plant growth-promoting rhizobacteria (PGPR) stimulate plant growth and yield in greenhouse and field. Here, we examined whether application of known bacilli PGPR strains stimulated growth and asexual reproduction in the succulent plant Kalanchoe daigremontiana. Four PGPR strains B. amyloliquefaciens IN937a, B. cereus BS107, B. pumilus INR7, and B. subtilis GB03 were applied to young plantlets by soil-drenching, and plant growth and development was monitored for three months. Aerial growth was significantly stimulated in PGPR-inoculated plants, which was observed as increases in plant height, shoot weight, and stem width. The stimulated growth influenced plant development by increasing the total number of leaves per plant. Treatment with bacilli also increased the total root biomass compared with that of control plants, and led to a 2-fold increase in asexual reproduction and plantlet formation on the leaf. Collectively, our results firstly demonstrate that Bacillus spp. promote vegetative development of K. daigremontiana, and the enhanced growth stimulates asexual reproduction and plantlet formation.
The rhizosphere is a zone surrounding plant roots, which supports a large and diverse microbial community (Kloepper et al., 2004; Yang et al., 2009). Some rhizosphere bacteria have been studied because of their beneficial interactions with plants. The plant growth-promoting rhizobacteria (PGPR), especially Pseudomonas spp. and Bacillus spp., colonize roots of monocots and dicots, directly or indirectly promote plant growth, and elicit induced systemic resistance (Kloepper et al., 2004). For example, treatment of cucumber plants with B. pumilus INR7 significantly promoted the growth and numbers of the main runners and leaves of plants grown under field conditions, and reduced the disease severity caused by angular leaf spot and anthracnose after inoculation of plants with P. syringae pv. lachrymans and Colletotrichum orbiculare, respectively (Wei et al., 1996). In addition, plant growth was stimulated and disease severity was reduced in cucumber plants treated with B. amyloliquefaciens IN937a and B. subtilis GB03 (Raupach and Kloepper, 2000). The growth promotion and disease reduction effects elicited by treatments with B. amyloliquefaciens IN937a and B. subtilis GB03 also were observed for Arabidopsis thaliana seedlings grown in culture medium (Ryu et al., 2003). Treatment with B. cereus BS107 increased shoot height but not shoot biomass, and elicited induced systemic resistance to Xanthomonas axonopodis pv. vesicatoria in pepper (Capsicum annuum) (Yang et al., 2009) and Pectobacterium carotovorum subsp. carotovorum in tobacco (Yang et al., 2011). Although extensive research indicates that PGPR influence plant growth, development, and disease resistance in a variety of plant species, any reports have never explored the effects of PGPR on asexual reproduction.
Kalanchoe is a succulent plant that reproduces asexually by producing plantlets along the edge of mature leaves. Kalanchoe is a useful and important model plant for research in three fields. First, it is an important model for investigations of crassulacean acid metabolism (CAM), which plays an important role in photosynthetic carbon assimilation (Garcês and Sinha, 2009). Second, natural compounds extracted from Kalanchoe have important applications due to their anti-tumor, anti-inflammatory, and insecticidal properties (Supratman et al., 2000, 2001). Third, Kalanchoe species are used for studies on asexual reproduction (Batygina et al., 1996; Garcês et al., 2007, 2014). Asexual reproduction generates new individuals by reprogramming resident cells in different organs (Guo et al., 2015). These plantlets possess roots, stems, and leaves, and develop asexually at the margin of plant leaves (Guo et al., 2015). The plantlets are only formed under stress conditions in some species, or are spontaneously produced in others. Kalanchoe daigremontiana is a constitutive plantlet-forming species (Garcês et al., 2007).
In this study, we investigated whether previously identified PGPR affected the vegetative growth and asexual reproduction of K. daigremontiana. We tested four strains of bacilli (B. amyloliquefaciens IN937a, B. cereus BS107, B. pumilus INR7, and B. subtilis GB03) for positive effects on K. daigremontiana growth and reproduction. This could be a useful strategy to stimulate propagation of the mother plants. The results showed that shoot biomass production was strongly promoted by treatments with these bacilli strains. The most significant effect on shoot biomass production was observed in plants inoculated with B. pumilus INR7. Root biomass also increased after treatment with three of the bacilli, but not in plants treated with B. cereus BS107. The highest root weight was measured in plants treated with B. pumilus INR7. The asexual production of plantlets on the leaf margins was stimulated by all four bacilli strains, especially B. pumilus INR7 and B. subtilis GB03. Taken together, our data clearly demonstrate that PGPR bacilli positively affect the growth, development, and asexual reproduction of K. daigremontiana.
To investigate whether previously reported PGPR modulated the plant growth and resistance to pathogens, we collected satellite plantlets from the leaf margins of K. daigremontiana. Using the satellite plants for experiments rather than seeds saved time and money. Before performing the experiments, the plantlets were surface sterilized with 6% sodium hypochlorite (Yuhan Corp., Seoul, South Korea) for 5 min, and subsequently washed with sterile distilled water (SDW) at least five times. The sterilized seedlings were sown in soilless potting medium (Punong Co. Ltd., Gyeongju, South Korea). Plants were grown under natural light conditions, and three different temperatures (21, 24, and 28°C) were tested to optimize growth. Plants grew best at 28°C. To evaluate differences between experimental treatments, analysis of variance was performed for all data using JMP software v 5.0 (SAS Institute, Cary, NC, USA). Significant effects were determined by the magnitude of the F value (P=0.05). When a significant F test was obtained, separation of means was performed by Fisher’s protected least significant difference (LSD) at P=0.05.
The B. amyloliquefaciens IN937a, B. cereus BS107, B. pumilus INR7, and B. subtilis GB03 were grown in tryptic soy agar (TSA) containing rifampicin (100 μg/ml final concentration) overnight and then the plates were scrapped out. The bacterial suspensions of these bacilli were adjusted (1×108 cfu/ml). The K. daigremontiana plantlets were soil-drenched once a week for first 3 weeks with suspensions (10 ml of 1×108 cfu/ml). As a control, some plantlets were treated with 0.33 mM benzothiadiazole (BTH) at the same time as application of bacilli. BTH, salicylic acid (SA), methyl jasmonate, and β-aminobutyric acid (BABA) induce disease resistance in plants (Chen et al., 1993; Davis and Ausubel, 1989; Epple et al., 1997; Vallad and Robert, 2004). More specifically, BTH activated plant defense machinery but had a negative effect on plant growth, such as the significant reduction in yield of BTH-treated wheat under field conditions (Heil et al., 2000). Our previous studies showed that aerial and root biomass of pepper plants were significantly reduced by BTH treatment (Park and Ryu, 2014; Yang et al., 2011). These results suggest that BTH treatment may affect the balance between physiological growth and activation of defense machinery (Heil et al., 2000; Heil and Baldwin, 2002). Consistent with previous results, we observed a significant reduction of K. daigremontiana plantlet growth after BTH treatment (data not shown), and the plantlets were killed after 5 weeks of BTH application (data not shown). By contrast, the initial growth of plantlets inoculated with bacilli was not significantly different from that of control plantlets (data not shown).
We assessed phenotypic changes of K. daigremontiana plantlets treated with the four bacilli. At 90 d after inoculation, plants treated with B. amyloliquefaciens IN937a, B. cereus BS107, B. pumilus INR7, and B. subtilis GB03 were taller and healthier than control plants (Fig. 1). B. pumilus INR7 had the most significant effects on promoting plant growth (Fig. 1C). We investigated the effects of bacilli treatment on plant height, shoot weight, stem width, and total leaf number. Plant height was increased by at least 20% in bacilli-inoculated plants compared with control plants, and was statistically equivalent for all bacilli (Fig. 2A). Shoot weight increased by approximately 2.3-, 4.8-, and 3.1-fold in plants treated with B. amyloliquefaciens IN937a, B. pumilus INR7, and B. subtilis GB03, respectively; B. cereus BS107 treatment did not promote shoot weight relative to control plants (Fig. 2B). Stem width increased by at least 1.3-fold in bacilli-inoculated plants compared with that of control plants (Fig. 2C). The shoot weight and stem width trends were similar, suggesting that stem width was positively correlated with shoot weight. The leaf number increased by at least 20% in bacilli-inoculated plants compared with controls, but did not increase in B. amyloliquefaciens IN937a-inoculated plants (Fig. 2D). Taken together, these results indicated that PGPR bacilli strains promote K. daigremontiana growth and development. The most significant effects were observed in plants treated with B. pumilus INR7 (Fig. 1C and Fig. 2).
We also monitored root architecture at 90 d after the bacilli inoculation. Plants treated with bacilli had longer primary roots and more secondary and lateral roots than control plants (Fig. 3A). Root weight increased by approximately 3.0-, 2.1-, 5.0-, and 3.1-fold after treatment with B. amyloliquefaciens IN937a, B. cereus BS107, B. pumilus INR7, and B. subtilis GB03, respectively (Fig. 3B). These data indicate that the bacilli positively stimulate root biomass production. We conclude that the four bacilli strains promote aerial and root growth of K. daigremontiana plantlets. The most significant effects on growth were observed in plants inoculated with B. pumilus INR7.
The effects on plant growth and development may be mediated by volatile organic compounds (VOCs) emitted by bacilli. Previous research discovered that the VOCs 2,3-butanediol and acetoin emitted by B. subtilis GB03 and B. amyloliquefaciens IN937a promoted Arabidopsis plant growth (Ryu et al., 2003). Additional analyses using diverse signaling mutants of Arabidopsis revealed that the cytokinin pathway is involved in responding to VOCs emitted by B. subtilis GB03 (Ryu et al., 2003). Although there was the difference of the population density of Methylobacterium spp. in bacilli-treated and control plants, Methylobacterium spp. were isolated from inside the leaves of K. daigremontiana plants (data not shown). Methylobacterium spp. are reported to stimulate seed germination, plant development, and phytohormone production (Lidstrom and Christoserdova, 2002). Our results suggest that Methylobacterium spp. population density, or any compounds released from Methylobacterium spp. in response to bacilli VOCs, may positively affect K. daigremontiana growth.
Asexual reproduction occurs in many Kalanchoe species (Batygina et al., 1996; Garcês et al., 2007, 2014). To investigate whether bacilli-mediated promotion of K. daigremontiana growth affects asexual reproduction, we examined asexual generation of plantlets on leaf margins at 120 d after bacilli inoculation. The total number of leaves in bacilli-inoculated plants was at least 1.5 times higher than that in control plants (Fig. 4A). The total number of plantlets produced on each leaf of bacilli-treated plants was 2-fold higher than that in control plants (Fig. 4B). The strongest positive effects on asexual reproduction were observed after treatment with B. pumilus INR7 and B. subtilis GB03. Plantlet formation is regulated by shoot meristemless (STM) and leafy cotyledon1 (LEC1), which are central regulators of organogenesis and embryogenesis (Garcês and Sinha, 2009). Recent work shows that a truncated LEC1 gene (KdLEC1) is required for asexual plantlet production of K. daigremontiana (Garcês et al., 2014). Our results suggest that Bacillus spp. may positively regulate asexual reproduction via KdLEC1.
To investigate whether the soil-drenched bacilli colonized roots of Kalanchoe, we measured total population of each of bacilli in rhizosphere of Kalanchoe plants. Since bacterial suspension was applied onto plants once a week for first 3 weeks, the roots of Kalanchoe were harvested and macerated for counting bacterial population. The bacterial colonies were calculated on selection media (TSA + rifampicin). Unexpectedly, we observed the difference of initial population density at day 0 between the bacilli strains even though we adjusted the solution of each bacilli as OD600=1 (1×108 cfu/ml). At this moment, we could not precisely answer for this phenomenon. The fact that the highest and the lowest densities were shown by INR7 and BS107, respectively (Table 1) led us to speculate that this initial colonization ability of the each Bacillus spp. used in this study may be a key factor for plant growth and asexual reproduction. As showing in Table 1, the bacilli population was observed at three different time points (0, 5, and 10 days after application of the bacilli at third times in a row). These results may support that promoting plant growths and developments are affected by biological stimulation of the bacilli than just fertilization effect.
In conclusion, this study provides new insights into the effects of bacilli PGPR on Kalanchoe growth, development, and asexual reproduction. (1) We optimized conditions for using four Bacillus spp. to promote K. daigremontiana growth. (2) We observed a positive association between aerial and root biomass production stimulated by Bacillus spp. PGPR. (3) The PGPR-induced enhancement of K. daigremontiana growth ultimately promoted asexual reproduction and plantlet formation. (4) Among the tested Bacillus spp., B. pumilus INR7 had the greatest effect on both vegetative growth and asexual reproduction in K. daigremontiana.
Acknowledgments
We wish to thank Mr. Young-Ik Bang and Miss. Yong-Hee Jeon for providing Kalanchoe plants. This research was supported by grants from the BioNano Health-Guard Research Center grant from the Ministry of Science, ICT and Future Planning (MSIP) as Global Frontier Project (grant no: H-GUARD 2013M3A6B2078952), the Next-Generation BioGreen 21 Program (SSAC grant #PJ008524 of Rural Development Administration), and the KRIBB initiative program.
Fig. 1
Representative images of Kalanchoe daigremontiana after inoculation of Bacillus spp. K. daigremontiana seedlings were drenched once a week for the first 3 weeks with suspensions (1 × 108 cfu/ml) of (A) B. amyloliquefaciens IN937a, (B) B. cereus BS107, (C) B. pumilus INR7, and (D) B. subtilis GB03, and grown at 28°C for 90 d. Six biological replicates were used for each application of bacilli (n = 6).
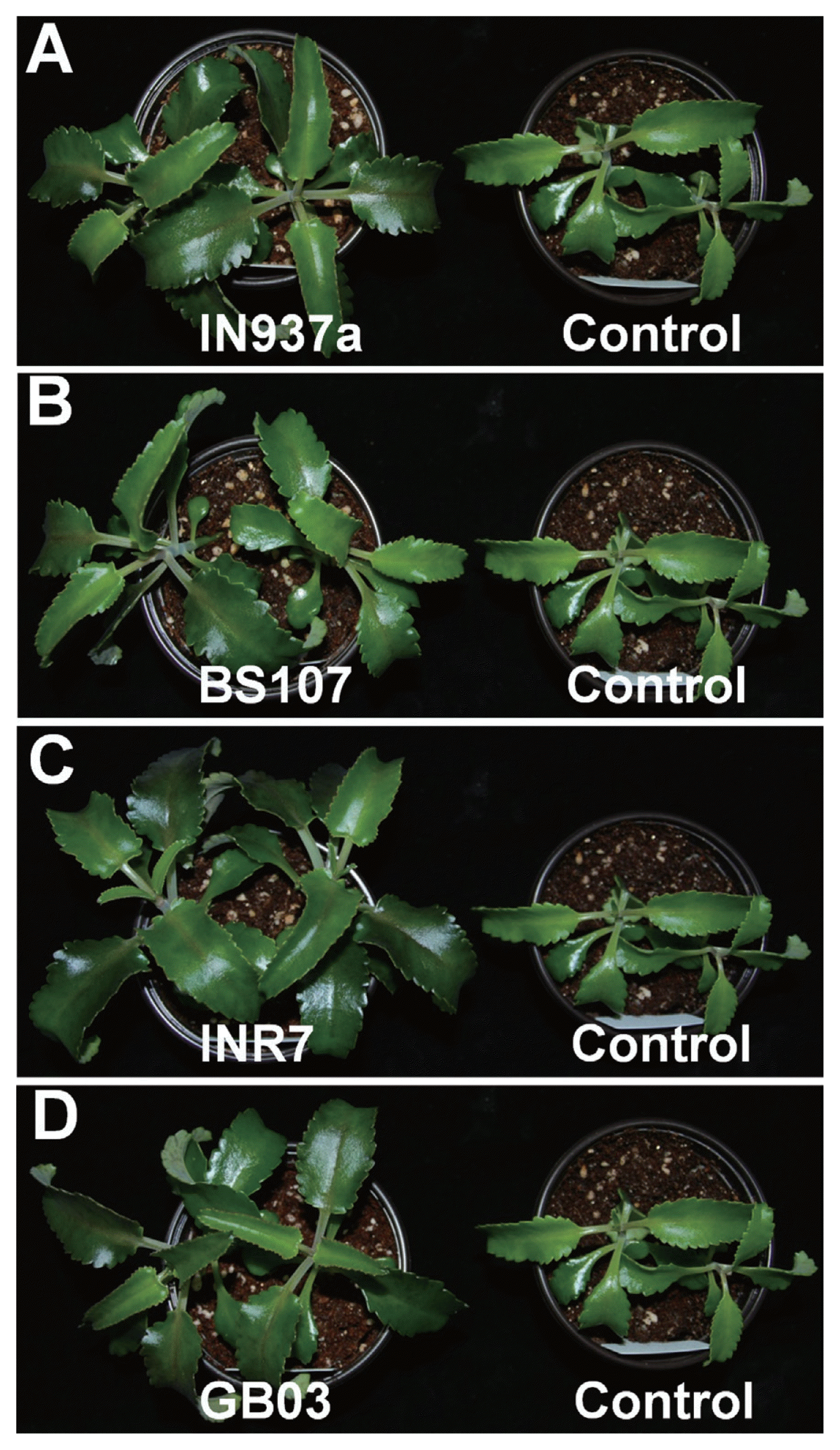
Fig. 2
Kalanchoe daigremontiana growth and development is stimulated by inoculation of Bacillus spp. K. daigremontiana seedlings were inoculated with four Bacillus spp. and grown as shown in Fig. 1. (A) Plant height, (B) shoot weight, (C) stem width, and (D) total leaf number per plant were evaluated at 90 d after the bacilli inoculation. Six biological replicates were used for each treatment (n = 6). Error bars indicate standard error of the mean and different letters indicate significant differences between treatments (P = 0.05).
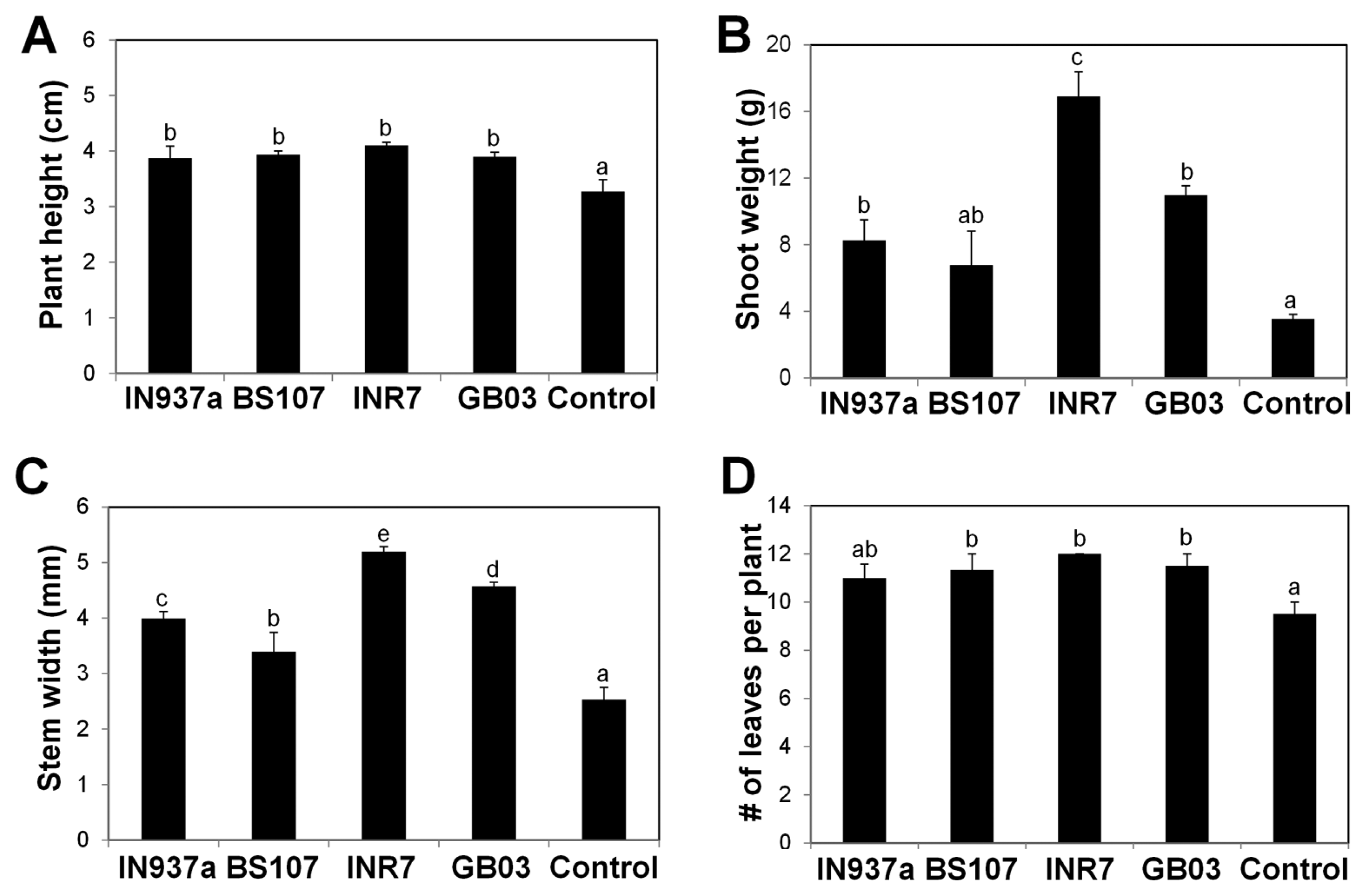
Fig. 3
Root biomass production is stimulated by treatment with Bacillus spp. Kalanchoe daigremontiana seedlings were inoculated with four Bacillus spp. and grown as shown in Fig. 1. (A) Representative images of root growth in bacilli-inoculated and control plants. (B) Total root weight was greater in most of bacilli-inoculated plants than in control plants. Six biological replicates were used for each test (n = 6). Error bars indicate standard error of the mean and different letters indicate significant differences between treatments (P = 0.05).
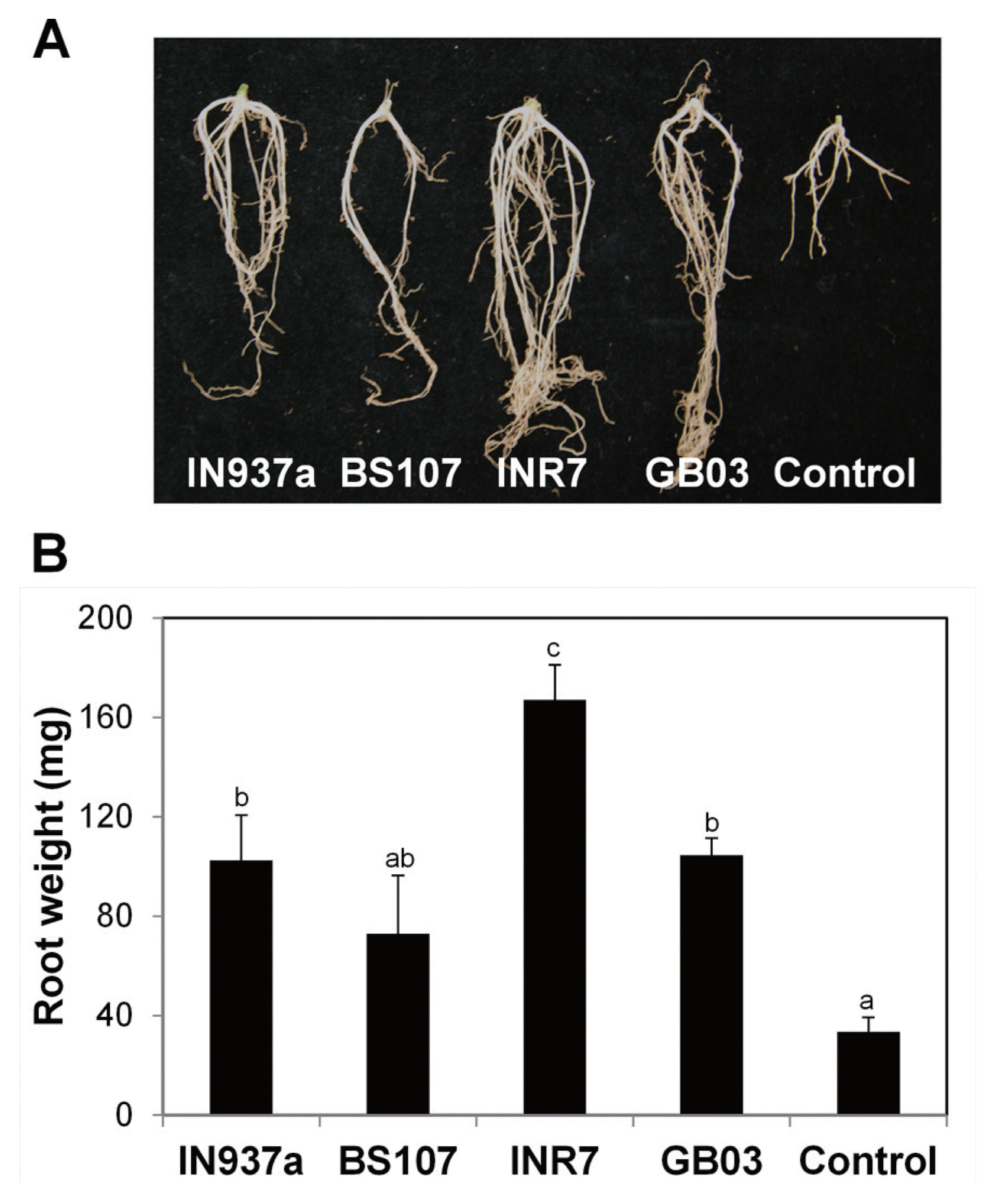
Fig. 4
Asexual reproduction in Kalanchoe plants treated with Bacillus spp. K. daigremontiana seedlings were inoculated with four Bacillus spp. as shown.in Fig. 1 and grown for 120 d. (A) Total number of leaves with plantlet development per plant. (B) Total number of plantlets per leaf. Six biological replicates were used for each treatment (n = 6). Error bars indicate standard error of the mean and different letters indicate significant differences between treatments (P = 0.05).
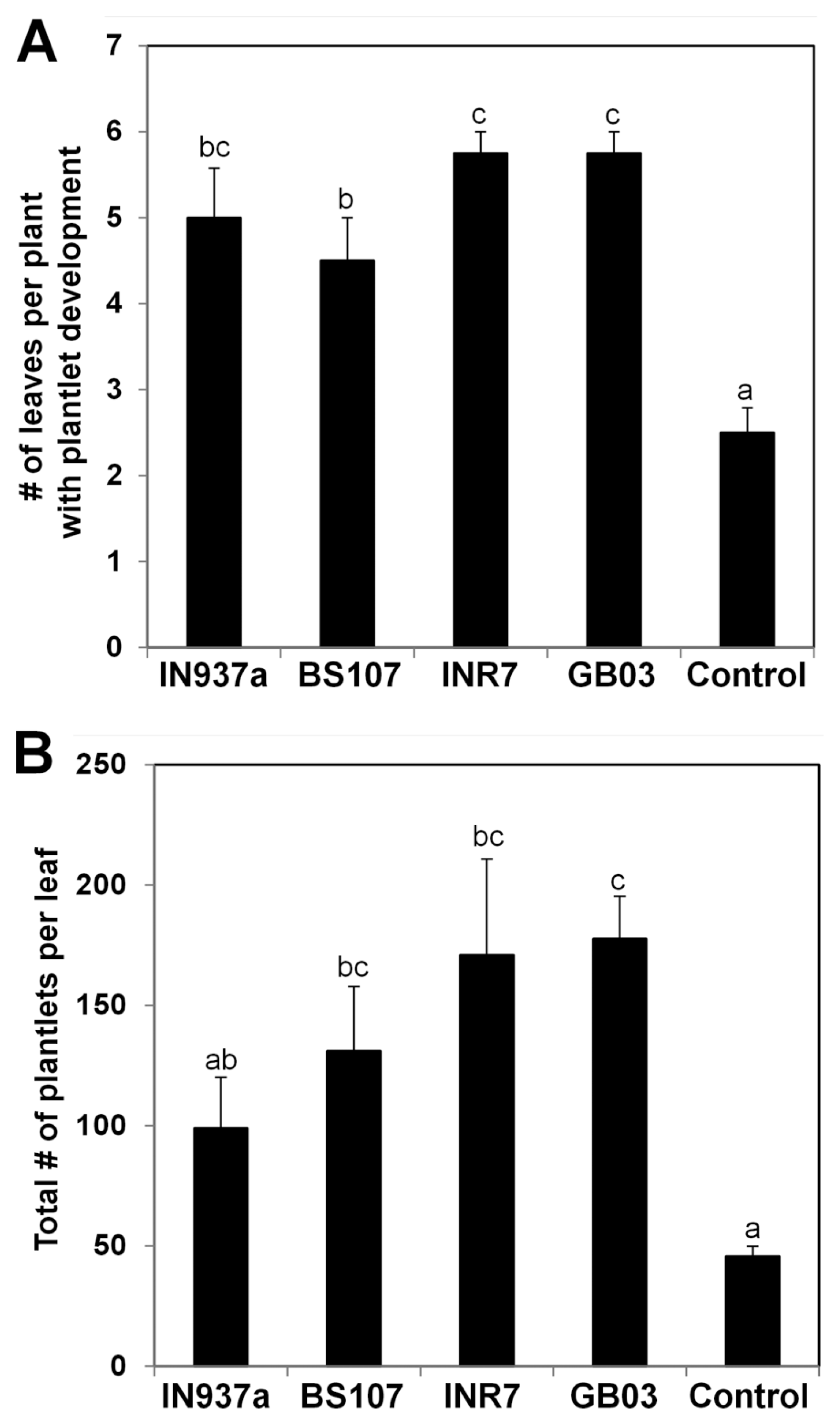
Table 1
Population density of the introduced bacilli on the Kalanchoe roots
Numbers represent the mean of five replications per treatment. Bacterial numbers were counted 0, 5 and 10 days after inoculation of B. amyloliquefaciens IN937a, B. cereus BS107, B. pumilus INR7, and B. subtilis GB03 on the roots. To select targeted bacterial population, an antibiotic 100 μg/ml rifampicin was amended in the tryptic soy broth agar. Different letters indicate significant differences using Fisher’s protected LSD test at P=0.05.
References
Batygina, TB, Bragina, EA and Titova, GE 1996. Morphogenesis of propagules in viviparous species Brylophyllum daigremontiaum and B. calycium. Acta Soc Bot Pol. 65:127-133.


Chen, Z, Silva, H and Klessig, DF 1993. Active oxygen species in the induction of plant systemic acquired resistance by salicylic acid. Science. 262:1883-1886.


Davis, KR and Ausubel, FM 1989. Characterization of elicitor-induced defense responses in suspension-cultured cells of Arabidopsis. Mol Plant-Microbe Interact. 2:363-368.

Epple, P, Apel, K and Bohlmann, H 1997. Overexpression of an endogenous thionin enhances resistance of Arabidopsis against Fusarium oxysporum. Plant Cell. 9:509-520.



Guo, J, Liu, H, He, Y, Cui, X, Du, X and Zhu, J 2015. Origination of asexual plantlets in three species of Crassulaceae. Protoplasma. 252:591-603.



Garcês, HM, Champagne, CE, Townsley, BT, Park, S, Malho, R, Pedroso, MC, Harada, JJ and Sinha, NR 2007. Evolution of asexual reproduction in leaves of the genus Kalanchoe. Proc Natl Acad Sci USA. 104:15578-15583.



Garcês, HM, Koenig, D, Townsley, BT, Kim, M and Sinha, NR 2014. Truncation of leafy cotyledon1 protein is required for asexual reproduction in Kalanchoe daigremontiana. Plant Physiol. 165:196-206.



Garcês, H and Sinha, N 2009. The ‘mother of thousands’ (Kalanchoe daigremontiana): A plant model for asexual reproduction and CAM studies. Cold Spring Harb Protoc. 44:1920-1934.
Heil, M and Baldwin, IT 2002. Fitness costs of induced resistance: emerging experimental support for a slippery concept. Trends Plant Sci. 7:61-67.


Heil, M, Hilpert, A, Kaiser, W and Linsenmair, KE 2000. Reduced growth and seed set following chemical induction of pathogen defence: Does systemic acquired resistance (SAR) incur allocation costs? J Ecol. 88:645-654.

Kloepper, JW, Ryu, C-M and Zhang, S 2004. Induced resistance and promotion of plant growth by Bacillus spp. Phytopathology. 94:1259-1266.


Lidstrom, ME and Christoserdova, L 2002. Plants in the pink: Cytokinin production by Methylobacterium. J Bacteriol. 184:1818




Park, Y-S and Ryu, C-M 2014. Understanding cross-communication between aboveground and belowground tissues via transcriptome analysis of a sucking insect whitefly-infested pepper plants. Biochem Biophys Res Commun. 443:272-277.


Raupach, GS and Kloepper, JW 2000. Biocontrol of cucumber diseases in the field by plant growth-promoting rhizobacteria with and without methyl bromide fumigation. Plant Dis. 84:1073-1075.


Ryu, C-M, Farag, MA, Hu, CH, Reddy, MS, Pare, PW and Kloepper, JW 2003. Bacterial volatiles promote growth in Arabidopsis. Proc Natl Acad Sci USA. 100:4927-4932.



Supratman, U, Fujita, T, Akiyama, K and Hayashi, H 2000. New insecticidal bufadienolide, bryophyllin C, from Kalanchoe pinnata. Biosci Biotechnol Biochem. 64:1310-1312.


Supratman, U, Fujita, T, Akiyama, K, Hayashi, H, Murakami, A, Sakai, H, Koshimizu, K and Ohigashi, H 2001. Anti-tumor promoting activity of bufadienolides from Kalanchoe pinnata and K. daigremontiana × tubiflora. Biosci Biotechnol Biochem. 65:947-949.

Vallad, GEG and Robert, M 2004. Systemic acquired resistance and induced systemic resistance in conventional agriculture. Crop Sci. 44:1920


Wei, G, Kloepper, JW and Tuzan, S 1996. Induced systemic resistance to cucumber diseases and increased plant growth by plant growth-promoting rhizobacteria under field conditions. Phytopathology. 86:221-224.

Yang, JW, Yi, HS, Kim, H, Lee, B, Lee, S, Ghim, SY and Ryu, C-M 2011. Whitefly infestation of pepper plants elicits defence responses against bacterial pathogens in leaves and roots and changes the below-ground microflora. J Ecol. 99:46-56.

- TOOLS



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




