Physical Changes in Satsuma Mandarin Leaf after Infection of Elsinoë fawcettii Causing Citrus Scab Disease
Article information
Abstract
Citrus scab disease is one of the destructive diseases that reduce the value of fruit for the fresh market. We analyzed the process of symptom development after infection with scab pathogen Elsinoë fawcettii in the susceptible satsuma mandarin leaves to observe the structural modification against pathogen. The cuticle and epidermal cells along with 3–5 layers of mesophyll tissue were degraded 1–2 days post inoculation. Surrounding peripheral cells of degraded tissues grew rapidly and then enveloped the necrotic area along with the growing conidia. Cross sections through the lesion revealed hyphal colonization in epidermis and mesophyll tissues. In response to the pathogen colonization, host cell walls were lignified, inner cells were rapidly compartmentalized and a semi-circular boundary was formed that separated the infected region from the non-infected region, and finally prevented the intercellular pathogen spread.
Citrus scab disease invades young organs of a variety of citrus plants. Early emerging young plant organs, including leaves, buds, twigs and fruits are vulnerable to the disease and become resistant on maturity (Chung, 2011; Timmer, 2000). Citrus scab is caused by two species viz. Elsinoë fawcettii and E. australis belonging to 8 known pathotypes (Hyun et al., 2009). Scab disease appears as a pale yellow pustule with the corky mass in organs of its infection in maturity (Kim et al., 2004).
Several structural or physical defenses have been noticed during host-pathogen interaction. Among them, the cell wall is a dynamic structure that often determines the outcome of the interactions between plants and pathogens (Bellincampi et al., 2014). Out of such dynamic structural components, lignification is a mechanism for disease resistance in plants (Bhuiyan et al., 2009; Vance et al., 1980). During defense responses, lignin or lignin-like phenolic compound accumulation was observed in plant-microbe interactions. Lignin is the major component of cell wall in vascular plants that is accumulated and developed for initial defense (Vance et al., 1980).
Some susceptible hosts defend after invasion of pathogen for minimal loss of its tissues and organs. They may proceed lignification of cell walls to suberize the adjacent cells in proximity of the pathogen colonization or form specialized protective structures along with the secretion of gums, accumulation of phenolic compounds etc. to avoid the further invasion of the pathogen. Induction of extensive host cell division, cell wall thickening, and deposition of suberin and lignin like structural materials in host tissues are some special features in susceptible host defense (Agrios, 2005; Gabel and Tiffany, 1987). Such a structural alteration of infected host tissues is an indication of typical wound periderm formation (Achor et al., 1991; Simard et al., 2001). The cell walls having alternating lamellation in infected citrus leaves contain suberin, as suberized cell walls are composed of fine alternating lamellae of phenolics and waxes (Kim et al., 2004). Suberization usually involves the deposition of polymeric materials including lignin-like materials in cell walls, providing a protective water-impermeable layer in a variety of plant parts (Kolattukudy, 1984). Consequently, cork layers in wound periderm act as a physical barrier to pathogen growth, block the spread of pathogen-secreted toxic substances and prevent the apoplastic movement of water and nutrients from healthy tissues to infected ones, eventually depriving pathogens of water and nourishment (Achor et al., 1991; Simard et al., 2001).
Information is still lacking on process of structural defense development to limit spread of pathogen in susceptible citrus plants during infection of citrus scab pathogen. Therefore, this study was carried out to observe the physical changes including initial symptom development, cell division, lignin deposition and development of lamellated cell barrier in satsuma mandarin leaves with time course after infection by E. fawcettii.
Materials and Methods
Preparation of inoculum
Typical isolate 16-1 (deposited in Korean Agricultural Culture Collection, KACC45780) of citrus scab pathogen, E. fawcettii belonging to Florida Broad Host Range (FBHR) pathotype (Hyun et al., 2001) was used for the assay. A piece of dried and preserved mycelia in silica gel was cultured on potato dextrose agar (PDA Difco, Sparks, MD, USA) for 15 days at 25ºC. A colony grown on PDA was mashed with a steel spatula on petri dish and the fragments were cultured in 50 ml of sterilized rain water at 180 rpm in a rotary shaking incubator at 25ºC for 24 h for sporulation (Hyun et al., 2014). The conidial suspension was filtered with double layer cheesecloth and quantified on haemocytometer.
Inoculation
Satsuma mandarin seedlings were pruned to stimulate production of uniform flushes of new leaves and maintained at 25°C in growth room. Conidial suspension (106 conidia/ml) was inoculated uniformly by spraying when the leaves were one-fifth of mature size. The plants were covered with plastic bags to keep moist and warm by maintaining at 25°C, in a 65% humid chamber for 16/8 h/day light/dark condition and leaves were harvested for assay everyday until 5 days dpi.
Scanning electron microscopy (SEM) analysis
Scanning election microscopy (S-3500N, Hitachi Corporation, Japan) analyses were carried out to observe initial symptom development, cell division, lignin deposition and development of lamellated cell barrier in satsuma mandarin leaves at an accelerating voltage of 20 kV. Inoculated leaves were harvested, cut approximately into 0.5 cm × 0.5 cm pieces and fixed in Karnovsky’s fixative solution [2% gluteraldehyde (Sigma-Aldrich, Germany) and 2.5% paraformaldehyde (Sigma-Aldrich, Germany) in 0.05 M sodium cacodylate (prepared from cacodylic acid, Sigma-Aldrich, Germany) buffer pH 7.2 to 7.4] for 2 h at room temperature. And then the samples were washed with 0.05 M sodium cacodylate buffer two times for 20 min each and allowed by fixation with second fixative, 1% osmium tetroxide (Fluka Analytical, USA) for 2 h. They were dehydrated using an ethanol series from 10, 20, 30, 40, 50, 60, 70, 80, 90, 95 percent for 15 min each and in 100% ethanol three times for 15 min each. The critical dehydration was finally done by using chemical dehydrant HMDS (hexamethyldisilazane, Merck) or HCP-2 critical point dryer (Hitachi Corporation, Japan) according to instructions of the manufacturer. The specimens were mounted on aluminum stubs. If required, specimens were sputter coated with evaporated platinum for 50 s in vacuum using E-1030 Ion sputter coater unit (Hitachi Corporation, Japan).
Fluorescent microscopy
Some samples fixed with Karnovsky’s fixative were stained in aqueous 0.005% aniline blue (Junsei, Japan) for 30 min and then in 0.02% UVitex2B (Polyscience Inc. Warrington) prepared in diethanol (Sigma-Aldrich, Germany) for 30 min. Samples were washed with 0.05% sodium cacodylate (prepared from cacodylic acid, Sigma-Aldrich, Germany) buffer 3 times and mounted in 50% glycerol (v/v in water) on glass slides for fluorescent microscopy (BX51 Olympus Corporation, Japan) analysis. Rose Bengal (Sigma-Aldrich, USA) was also used to observe fluorescent activities in the assay. Freshly harvested leaf sample was fixed in Karnovsky’s fixative for 2 h and washed in 0.05 M sodium cacodylate buffer three times. The samples crossed sectioned with sharp blade, washed three times with distilled water and stained in 0.05% Rose Bengal (Sigma Aldrich, Gemany) (in aqueous solution) for 20 min. Stained sample was mount in 50% glycerol and observed on fluorescent microscope (BX51 Olympus Corporation, Japan) equipped with exciter and emission filter BFP (390 and 460 nm, respectively).
Lignin staining
Cell wall lignification after infection was analyzed by staining with toluidine blue O (Sigma-Aldrich, Germany), which stains the cell wall constituting lignin deposition as method described by Vargas et al. (2012). Cross sectioned leaf through symptom was fixed by boiling with 95% (v/v) ethanol for for 5 min. The samples were incubated overnight in fresh ethanol (95%) and following day the samples were stained for 1 min with toluidine blue O solution (0.5 mg/l) prepared in 0.1 M phosphate buffer, pH 6.8. The stained tissues were washed with tap water and mounted with 50% glycerol for microscopic observation.
Results
Development of the first symptom and pathogen colonization
The protective cuticle around the conidial extending germ tube was degraded 1–2 days post inoculation on leaves of satsuma mandarin. A few epidermal cells lying below the degraded cuticle had lost their viability and appeared as groove. The adjoining cells of the degraded epidermis started to enlarge and divided repeatedly. It was an initial symptom observed 1–2 dpi of conidia on the leaf under SEM. The surface scanning showed a degraded peel of the outer protective cuticle and resulted necrosis in a group of the epidermal cells lying close or around the germinating conidia (Fig. 1A). Later, the swollen and enlarged mass of cells gradually covered the necrotic area along with the growing conidia that left a furrow or groove shape at the center (Fig. 1B). The infected area was bulged up and formed a first visible lesion (Fig. 1B). Cross sections through the lesion revealed 3–5 mesophyll cell layers beneath the infected epidermis were degraded (Fig. 2). Surface scanning barely visualized the colonized fungi on the surface (Fig. 1B) while the cross sections showed intra and intercelullarly colonized hyphae in the epidermis as well as in the mesophyll (Fig. 2A and E). The colonized hyphae were intermingled; they deformed the host cells and tissues so that they were hardly differentiated (Figs. 2C and D).
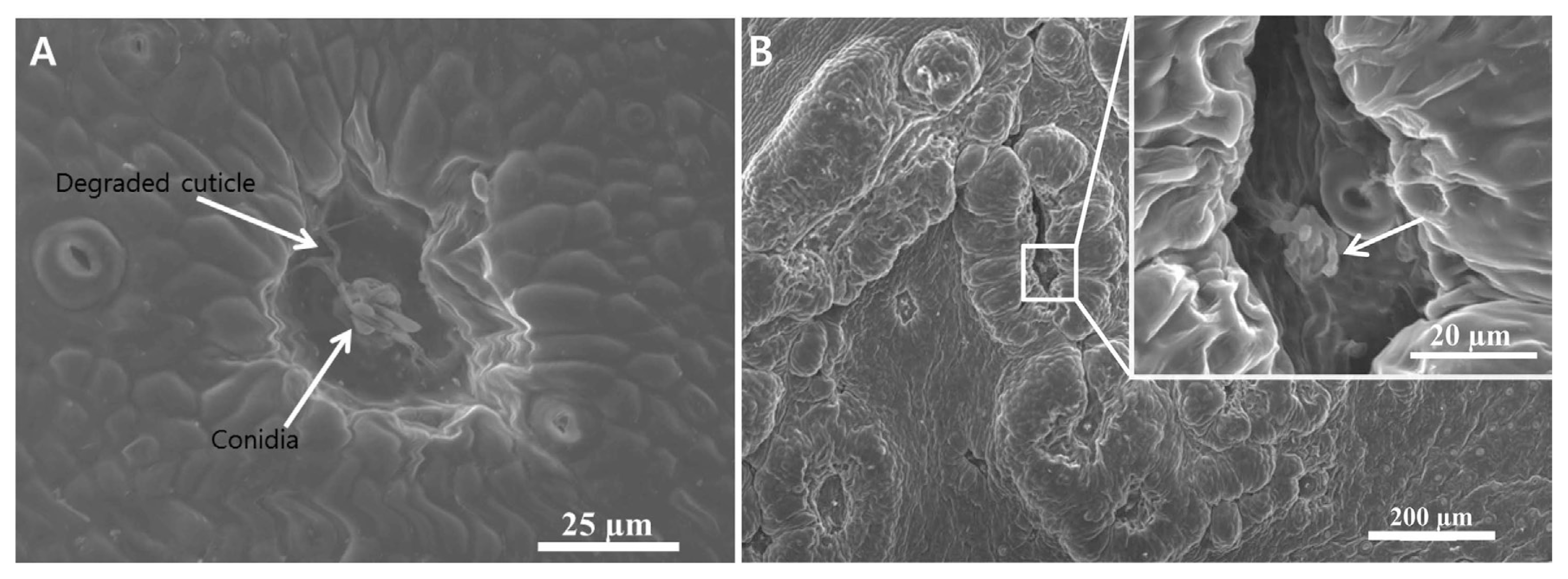
Scanning electron micrographs of symptom on satsuma mandarin leaf infected with Elsinoë fawcettii. (A) Degradation of cuticle and epidermis 2 days post inoculation (dpi); (B) Initial symptoms of citrus scab induced 3 dpi. Upper right corner is an enlarged view of the lesion with germinating conidia. Arrow in the inset of B indicates a germinating conidium.

Cross sections through the lesion formed by Elsinoë fawcettii infection on leaves of satsuma mandarin. (A) Toluidine blue O stained light microscopic image of the lesion showing fungal colonized and lignified tissues 5 dpi. (B) SEM image showing the central grooved shape at the initial infection site. It also shows the fungal colonized and lignified tissues along with the arc of lamellated cell barrier 5 dpi. (C) SEM image showing patches of the fungal colonized and lignified tissues with the arc of lamellated cell barrier in mesophyll 7 dpi. (D) Extent of affected mesophyll layers 7 dpi. (E) Fungal colonized tissue with hyphae. (F) Rose Bengal stained fluorescent microscopic image showing fungal colonized tissue and extent of lignification in the lesion 8 dpi [dpi: days post inoculation; FCT: Fungal colonized tissue; LB: Lamellated cell barrier; LT: Lignified tissue; SEM: scanning electron microscopy].
Structural defense development
The thickness of the cell wall in mesophyll cells in the infected lesion was increased due to lignification (Fig. 2). Lignification was found more dense in adjoining peripheral cells of the initial symptom in the region of pathogen colonization within 5 dpi (Fig. 2A). The lignified cells were sometimes filled with solid materials which made them inconspicuous (Figs. 2B, C and D). They were bright fluorescent and lie between fungal colonized zone and the lamellated barrier in lesions stained in Rose Bengal (Fig. 2F). No fungal colonies were observed at lignified portion of the tissue. Also, the lignified cells usually lay outward to the rapidly grown or lamellated cell border.
Longitudinal as well as transverse cell division or lamellation was observed in the cells from 5 dpi (Fig. 2B) and was continued (Figs. 2C and D). The colonized fungal tissue had been observed in patches in close proximity of the lignified cells (Fig. 2E). Cross section through the lesion also showed mashed cell masses without clear differentiation of fungal zone with plant tissue (Fig. 2E). A rapid cell division in the cells lying beneath the infection lesion pushed the infected lesion further outward and it also altered the morphology of the leaf surface. Multiple divisions also produced the crossed walled lamella that partitioned and separated the infected tissue from the non-infected tissue finally that was visualized as a semi-circular boundary (Fig. 2B and C and Fig. 3). The lamellated or barrier cells were initially grown and enlarged by swelling prior to the cell division. Repeated division formed the multi-layered semi-circular boundary of boarder tissue that separated the infected tissue to the healthy tissue. In 2 weeks post-inoculation, it showed a lamellated mass of tissues which differentiated the infected tissue from the non-infected tissue and bordered as a semi-circular arc of cells (Fig. 3A). The semi-circular boundary of cells separated the infected tissues from the healthy one. Cells located outward to the arc were thick walled due to lignification, whereas the inward cells, including the arc cells were thin and devoid of lignification. Initial grooved shape at the core part of the first symptom was extruded outward and clearly visualized the fungal zone in infection lesion.

Photomicrographs of cross sections through the lesions formed by Elsinoë fawcettii on the leaf of satsuma mandarin. (A) SEM image showing colonized fungal tissue, lignified tissue and lamellated cell barrier 15 dpi, enlarged view (right). (B) Rose Bengal stained section under fluorescent microscope, the lignified tissues were bright fluorescent between fungal colonized tissue and lamellated cell barrier (30 dpi). (C) Aniline Blue and UVitex 2B stained tissues under fluorescent and normal light merged condition showing outer fungal colonized tissue and pale green fluorescent lignified tissues at middle 30 dpi. (D) Lignified tissue observed in toluidine blue O staining under light microscopy 30 dpi. Host inner cells lying close to the colonized fungi were lignified. A multilayered barrier cells separated the infected tissue to the healthy tissue. Aniline blue with UVitex 2B staining showed fungal tissue towards periphery of the lesion. Similarly, no fungal colonized tissues were distinctly visualized from Toluidine Blue O staining. [dpi: days post inoculation; FCT: Fungal colonized tissue; LB: Lamellated cell barrier; LT: Lignified tissue; SEM: scanning electron microscope]
The cross section of one month post inoculated rose bengal stained lesion also showed the fungal colonized region lying toward outer peripheral region (Fig. 3B). The region also contained degraded host tissues along with the colonized fungi successively towards outer surface. Next to the fungal colonized tissue a fluorescent bright region was observed in Rose Bengal stained section (Fig. 3B). However, the region showed a faint brightness in aniline blue and UVitex 2B staining (Fig. 3C) and lignification positive with toluidine blue O (Fig. 3D). Lamellated cells as well as fungal colonized tissues showed no fluorescent brightness in the analyses.
Discussion
Host and pathogen interact each other to inactivate the normal growth process of the others through physical or chemical ways. The infection process leads to biochemical secretion through genetic activation (Agrios, 2005). Series of activation and inactivation process lead to pathogen susceptibility or tolerance (Dodds and Rathjen, 2010; Lodha et al., 2013). Although gene expression is involved in the broad perspective of infection and the tolerance of primary or signal transduced system, it is ultimately expressed in the form of physical or chemical barrier in an organism (Agrios, 2005). Tolerant pathogens are able to neutralize or inactivate the host secretion so that they invade, infect and develop symptoms in their susceptible host. Also, the extent of the pathogen invasion, mostly depends on the lifestyle of pathogen and induced systemic barriers (Bellincampi et al., 2014). Types of barrier development probably depend on the types of the pathogen and the host. The pathogen may strategically maximize the infection while host tends to neutralize or minimize the effects of pathogen in its tissue or organs.
Citrus scab pathogen E. fawcettii invades the young leaf, erodes its cuticle and probably induces the death of a few cells in the epidermis as well as 3–5 layers in the mesophyll tissue. Cell necrosis and cell wall degradation are prerequisite to induce infection on the host tissue (Kubicek et al., 2014; Vargas et al., 2012). The process of protective layer degradation and necrosis was accomplished 1–2 dpi. The pathogen induced initial visible symptoms within 3 dpi. Chung (2011) found the induction of visible symptom as early as 4 dpi. However, the present study indicated that it may vary potentially with cultivars or the species of the host plant and also on pathogen pathotypes. Structural modification after E. fawcettii infection in leaves of satsuma mandarin was observed simultaneously in two processes. The first and initial modification was component of cell wall in adjacent periphery of the necrotic region where cells were lignified. Second aspect of the modification was the development of layers of barrier cells to separate infected tissue to the non-infected tissue.
To defend against pathogen attack, satsuma mandarin started lignification on its wall at the mesophyll cells within 5 dpi. Lignification was observed around the cells of hyphal colonization up to several layer depths in mesophyll tissue in the lesion. Hyphal colonization in the cells below the necrotic region is yet to confirm, however, lignification was observed in cell layers below the necrotic area. It may potentially prevent pathogen spread in adjoining cells. Lignification in mesophyll cell wall might be the initial stage of cork cell formation in proximity of the pathogen colonization or it may begin to develop specialized protective structures within 5 dpi to limit pathogen spread in susceptible host. Induction of extensive host cell division, cell wall thickening, and deposition of suberin and lignin like materials in host tissues are some special features in susceptible host defense (Agrios, 2005; Gabel and Tiffany, 1987). Extensive cell division and lamellation delineated and isolated the infected to the non-infected regions in the leaf.
Existence of fluorescent region between fungal colonized tissue and lamellated tissue in Rose Bengal staining indicated lignin deposition. Extent of the lignified tissues during early infection was increased in later days to limit the inter cellular or tissue spread of the pathogen.
Fungal infection induced citrus leaves to form cork layers bordering necrotic areas below the infected sites as an arc of repeatedly divided cell layers. The repeatedly divided cells formed an arc of bordering layers 5 dpi and continued at later days. It indicated the development of barrier cells begun with the colonization of pathogen to limit their further spread in host mesophyll tissue. These defensive structures altered the internal structural organization of the infected leaf. Such a structural alteration of infected host tissues is an indication of typical wound periderm formation (Achor et al., 1991; Simard et al., 2001). The semi-circular arc formed at the border of infected and healthy tissues pushed and expelled the infected tissue outwardly. Therefore, the lesion was raised out of the surface. It is likely that the cell walls having alternating lamellations in infected citrus leaves contain suberin, as suberized cell walls are composed of fine alternating lamellae of phenolics and waxes (Kim et al., 2004). Suberization usually involves the deposition of polymeric materials including lignin-like materials in cell walls, providing a protective water-impermeable layer in a variety of plant parts (Kolattukudy, 1984). Consequently, cork layers in wound periderm act as a physical barrier to pathogen growth, block the spread of pathogen and its toxins, prevents the movement of water and nutrients from healthy tissues to infected ones, and eventually deprive pathogens from water and nourishment (Achor et al., 1991; Simard et al., 2001). The observation in E. fawcettii revealed that confined fungal invasion to cork layers acts as an effective protection barrier, as reported in other studies concerning the wound periderm formation and occurs within 7 dpi (Kim et al., 2004). Therefore, we suppose the restriction of hyphal extension by an arc of repeatedly dividing cells is caused by the wound periderm formation and separated the infected tissue from the healthy tissue.
It is concluded that lignification in mesophyll cells takes place probably to prevent the hyphal penetration in adjoining cells. Additionally, the initially enlarged mesophyll cells rapidly divided and lamellated to form semi-circular boundary of barrier cells that finally prevents the further hyphal spread and raised the infected tissues outward that results in producing a typical symptom during E. fawcettii infection on leaf of satsuma mandarin.
Acknowledgments
This work was carried out with the support of “Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ01047801)” Rural Development Administration, Republic of Korea.