A LysM Domain-Containing Protein LtLysM1 Is Important for Vegetative Growth and Pathogenesis in Woody Plant Pathogen Lasiodiplodia theobromae
Article information
Abstract
Lysin motif (LysM) proteins are reported to be necessary for the virulence and immune response suppression in many herbaceous plant pathogens, while far less is documented in woody plant pathogens. In this study, we preliminarily characterized the molecular function of a LysM protein LtLysM1 in woody plant pathogen Lasiodiplodia theobromae. Transcriptional profiles revealed that LtLysM1 is highly expressed at infectious stages, especially at 36 and 48 hours post inoculation. Amino acid sequence analyses revealed that LtLysM1 was a putative glycoprotein with 10 predicted N-glycosylation sites and one LysM domain. Pathogenicity tests showed that overexpressed transformants of LtLysM1 displayed increased virulence on grapevine shoots in comparison with that of wild type CSS-01s, and RNAi transformants of LtLysM1 exhibited significantly decreased lesion length when compared with that of wild type CSS-01s. Moreover, LtLysM1 was confirmed to be a secreted protein by a yeast signal peptide trap assay. Transient expression in Nicotiana benthamiana together with protein immunoblotting confirmed that LtLysM1 was an N-glycosylated protein. In contrast to previously reported LysM protein Slp1 and OsCEBiP, LtLysM1 molecule did not interact with itself based on yeast two hybrid and co-immunoprecipitation assays. These results indicate that LtLysM1 is a secreted protein and functions as a critical virulence factor during the disease symptom development in woody plants.
During microbe-plant interactions, plants have evolved two tier of defense responses in the face of pathogen attacks. The first layer provides basal responses based on the recognition of pathogen-associated molecular patterns (PAMPs) by plant pattern recognition receptors (PRRs) that activates PAMP-triggered immunity (PTI) (Jones and Dangl, 2006; Macho and Zipfel, 2014; Stergiopoulos and de Wit, 2009). This layer of responses are associated with callose deposition, lignin formation, phenolic compounds deposition, stomata closing, generation of extracellular reactive oxygen species, and a serious of downstream signal transduction events (Han and Kahmann, 2019; Zipfel, 2014). To establish compatible interaction for successful proliferation inside host tissues, microbial pathogens secrete an arsenal of effectors to overcome PTI response. Although pathogens secrete substantial effectors to subvert PTI, plants have evolved a surveilence system to recognize these effector proteins to trigger wellknown effector-triggered immunity, which includes by hypersensitive cell death and defense-gene activation (Han and Kahmann, 2019; Jones and Dangl, 2006; Selin et al., 2016; Thomma et al., 2011). During past decades, many typical PAMPs including bacterial flagellar peptide flg22, EF-Tu peptide, lipopolysaccharide, peptidoglycan, fungal cell wall chitin, and xylanase, and hundreds of effectors such as RxLR, CRN, CFEM, or LysM motif-containing proteins have been identified and characterized (Han and Kahmann, 2019; Liu et al., 2019; Newman et al., 2013; Thomma et al., 2011; Win et al., 2012).
The LysM motif is widely found in various proteins such as chitinases, peptidases, and receptor-like kinases and effector proteins (Akcapinar et al., 2015; Liu et al., 2019). LysM effector proteins were reported to be able to either protect hyphae from being degraded by plant chitinases through binding to fungal cell wall chitin and inhibit the activity of plant chitinase through binding to the chitinases or prevent plant recognizing GlcNAc oligomers to avoid plant defense responses (Kombrink and Thomma, 2013; Rovenich et al., 2016). For example, Slp1 in Magnaporthe oryzae functions as a competitive inhibitor of PRR protein CEBiP and suppresses chitin-induced immune responses in rice (Mentlak et al., 2012). Ecp6 in Cladosporium fulvum was found to be involved in chitin oligosaccharide sequestration through collective actions of LysM1 and LysM3 domains, and also involved in perturbation of host immune receptor dimerization via LysM2 domain (Liu et al., 2012; Sánchez-Vallet et al., 2013). Also, Mg-1LysM and Mg3LysM in Mycosphaerella graminicola were reported to be able to protect fungal hyphae from hydrolysis by plant hydrolytic enzymes (Marshall et al., 2011). Another two LysM proteins ChELP1 and ChELP2 in Colletotrichum higginsianum could also bind to chitin and suppresses chitin-triggered activation of immunerelated plant mitogen-activated protein kinases (Takahara et al., 2016). Another documented chitin-binding protein Vd2LysM in Verticillium dahliae suppresses chitininduced immune responses and protected fungal hyphae against hydrolysis (Kombrink et al., 2017). Moreover, LysM effectors have also been identified to contribute to circumvent plant defense responses and establish arbuscular mycorrhizal symbiosis (Schmitz et al., 2019; Zeng et al., 2020). Furthermore, LysM effectors are involved in mycoparasitism in pathogenic fungi (Romero-Contreras et al., 2019). Even though the biological functions and regulatory mechanisms of LysM proteins have been widely characterized in various fungi, but little is documented in opportunistic plant pathogen Lasiodiplodia theobromae.
Lasiodiplodia theobromae, a member of Botryosphaeriaceae family, can change its lifestyle from endophytic to pathogenic when host defense is weakened by internal and external factors (Chethana et al., 2016; Paolinelli-Alfonso et al., 2016). The fungus is widely distributed in tropical and subtropical regions and is able to infect a wide range of hosts (Correia et al., 2016). Lasiodiplodia theobromae is emerging as one of the most aggressive pathogens that causes severe grapevine canker disease in vineyards, resulting in considerable yield losses to global grape industry (Rodríguez-Gálvez et al. 2015; Úrbez-Torres, 2011; Yan et al., 2013). So far, numerous pathogenicity-related genes have been identified in L. theobromae based on genome sequencing and bioinformatic analyses (Félix et al., 2019; Yan et al., 2018), functional mechanisms of these genes, however, is barely documented.
Based on genome sequence (Yan et al., 2018), we identified six LysM domain-containing proteins in L. theobromae CSS-01s, of which three were predicted to be functional effector proteins. Here, one LysM protein Lt-LysM1 (Lasiodiplodia theobromae LysM domain effector 1) was preliminarily characterized. Relative transcription analyses and pathogenicity tests revealed that LtLysM1 contributes to the virulence of L. theobromae during the infection process. Additionally, LtLysM1 also displayed an effect on the vegetative development of L. theobromae. Moreover, LtLysM1 was confirmed to be a secretory protein by a yeast signal peptide trap system. Surprisingly, LtLysM1 was N-glycosylated when expressed in N. benthamiana. Collectively, these results indicate LtLysM1 functions as a secretory protein and play an important role in pathogenesis and vegetative development of L. theobromae.
Materials and Methods
Fungal and plant materials
The L. theobromae wild type isolate CSS-01s and fungal transformants were cultured on potato dextrose agar (PDA 1 l: 200 g potato, 20 g dextrose, and 20 g agar) and maintained at 28°C at equal light and dark cycles. The fungi and bacteria used in the study were stored in PDA slants at 4°C and 50% glycerol at –80°C, respectively. Mycelium plugs from 2-day-old cultures and healthy green shoots of Vitis vinifera cv. Summar Black from Yanqing, China were used for pathogenicity assays. Mycelia cultured in complete medium (0.6% [w/v] yeast extract, 0.3% [w/v] casein acid hydrolysate, 0.3% [w/v] casein enzymatic hydrolysate, and 1% [w/v] sucrose) were collected for RNA extraction. Bacteria were cultured in Luria-Bertani (1% [w/v] tryptone, 0.5% [w/v] yeast extract, and 1% [w/v] NaCl at pH 7.5). Nicotiana benthamiana used for transient expression was grown in a controlled growth chamber and maintained at 25°C.
Amino acid sequence analyses
The LtLysM1 signal peptide was predicted with SignalP 5.0 Server with server default parameter settings (http://www.cbs.dtu.dk/services/SignalP/). The LysM domain of LtLysM1 was identified through BLASTP program against Pfam database using LtLysM1 amino acid sequence as a query (El-Gebali et al., 2019). The N-glycosylation sites were predicted using the N-GlycoSite tool (https://www.hiv.lanl.gov/content/sequence/GLYCOSITE/glycosite.html).
RNA extractions and quantitative real-time reversetranscription PCR analyses
Wounds of green grapevine shoot were inoculated with mycelium plugs cut with a cork-borer and then maintained under an alternating dark/light cycle in a growth chamber with 70% humidity and 25°C. Inoculated grapevine tissues were harvested at different time points (12, 24, 36, 48, 60, and 72 hours post inoculation [hpi]) for RNA extraction. Fungal mycelia cultured in complete medium broth on a shaking incubator at 160 rpm and 25°C for 36 h were used for RNA extraction. Total RNA was isolated using an EASYspin Plus Complex Plant RNA Kit (Aidlab, Beijing, China) according to the manufacturer’s instructions. Isolated RNA was reverse transcribed into cDNA using the transScript One-Step gDNA Removal and cDNA Synthesis SuperMix kit (TransGen Biotech, Beijing, China) by following the manufacturer’s instructions. The transcriptional profiles of Lt-LysM1 were assessed via quantitative real-time polymerase chain reaction (qRT-PCR). The qRT-PCR assays were conducted using 7500 real-time PCR system (Applied Biosystems, Singapore) with 2× RealStar Green Fast Mixture with ROX II (GenStar, Beijing, China). The qRT-PCR was performed in 16 μl final volumes consist of 1.0 μl cDNA, 0.3 μl primer, 6.4 μl sterile water, and 8 μl RealStar Green Fast Mixture with ROX II. The amplification program was as follows: 2 min for denaturation at 95°C, followed by 40 cycles of 95°C for 15 s, and 60°C for 30 s. The actin gene was used as the internal reference. Relative transcript levels were calculated using the 2-ΔΔCT method (Livak and Schmittgen, 2001). The experiment was repeated at least twice with three independent replicates for each sample. Primers used in the experiments were listed in Table 1.
Pathogenicity tests of overexpressed and RNAi transformants of LtLysM1 gene
The LtLysM1 open reading frame (ORF) was amplified with primer pairs LysM1OEf/LysM1OE-r (Table 1) and then subcloned downstream of the PtrpC promoter in the modified pKSNTP vector. Afterwards, the fusion construct, referred to as pKSNTP:LtLysM1, was transformed into L. theobromae protoplast using polyethylene glycol (PEG)-mediated transformation method as described by Yan et al. (2018). Resultant transformants were screened against neomycin resistance and confirmed by qRT-PCR analysis. Two LtLysM1 overexpressed transformants were selected for subsequent pathogenicity tests on detached Vitis vinifera cv. ‘Summer Black’. Wounds of green grapevine shoots were inoculated with mycelium plugs cut with a cork-borer and then maintained under an alternating dark/light cycle in a growth chamber with 70% humidity and 25°C. Lesion lengths of inoculated shoots were measured at 72 hpi. At least six biological replicates of per LtLysM1 overexpression transformant were performed.
For RNAi transformation, we amplified sense fragment with primer pairs LysM1RNAi-Sf/LysM1RNAi-Sr and antisense fragments with primer pairs LysM1RNAi-ASf/LysM1RNAi-ASr, respectively, and then ligated both fragments into the pRTN vector in the given order. Subsequently, the fusion vector, named as pRTN:LtLysM1, was transformed into the L. theobromae protoplast with methods similar to overexpression vector transformation. The protocols used for pathogenicity tests of RNAi transformants were also similar to that of the overexpressed transformants.
Functional validation of signal peptide of LtLysM1 using a yeast signal peptide sequence trap system
To validate the function of LtLysM1 signal peptide, a yeast signal peptide trap assay was performed (Jacobs et al., 1997; Klein et al., 1996; Lee and Rose, 2012). We engineered a pSUC2:LtLysM1 fusion construct, in which the signal peptide of LtLysM1 was in frame upstream the truncated yeast invertase which lacks its signal peptide. Subsequently, the fusion construct pSUC2:LtLysM1 was introduced into invertase secretion-defective yeast YTK12 using the Yeastmaker Yeast Transformation System 2 kit (TaKaRa, Tokyo, Japan). The resulting transformants were tested for their growth CMD-W (0.67% yeast nitrogen base without amino acids, 0.075% tryptophan dropout supplement, 0.1% glucose, 2% sucrose and 2% agar) and YPRAA (1% yeast extract, 2% peptone, 2% raffinose, 2 μg/ml antimycin A) media, respectively. Transformants expressing pSUC2:Avr1b and pSUC2:Mg87 were used as positive control and negative control, respectively (Fang et al., 2016; Jacobs et al., 1997). All the yeast transformants were incubated at 28°C for 4 days to observe their growth and for then photographed.
Agrobacterium tumefaciens mediated transient expression of LtLysM1 in N. benthamiana
The LtLysM1 ORF was cloned with primers listed in Table 1 and ligated to the transient expression vector p35S-GFP. The fusion vector p35S-GFP:LtLysM1 was transformed into A. tumefaciens strain GV3101 using the freeze-thaw method, respectively (Fang et al., 2016). The recombinant A. tumefaciens strains were cultured in a shaking incubator at 28°C overnight followed by centrifugation at 5,000 rpm for 10 min. The precipitates were washed three times with sterile water and then resuspended in infiltration buffer (10 mM MES, pH 5.7, 10 mM MgCl2, and 150 μM acetosyringone) to get an optical density value OD600 = 0.5. Then, A. tumefaciens transformants were infiltrated into 4-week-old N. benthamiana leaves with a sterile syringe.
Protein extraction and immunoblotting analysis
For protein extraction, N. benthamiana leaves harvested at 48 hours post infiltration with A. tumefaciens were ground into powder in liquid nitrogen and then incubated with RIPA lysis buffer (Beyotime Biotechnology, Nanjing, China) on ice for 10 min followed by centrifugation at 12,000 rpm for 20 min at 4°C. Subsequently, the supernatant solution was mixed with 5× loading buffer and then boiled for 10 min. Next, protein extracts were separated by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and electrophoretically blotted onto a PVDF membrane (Millipore, Billerica, MA, USA). The membrane was blocked with 5% skim milk in TBST buffer (20 mM Tris-HCl, 150 mM NaCl, 0.05% Tween 20) for 1 h at room temperature and further incubated with anti-GFP antibody (1:2,000, Cell Signaling Technology, Danvers, MA, USA) for 1 h at room temperature, followed by three washes (10 min each) with TBST buffer. After rinsing thoroughly, the membranes were incubated with a luminolbased enhanced chemiluminescence horseradish peroxidase substrate (Thermo Fisher Scientific, Waltham, MA, USA) followed by exposure with X-film.
N-glycosylation analyses
LtLysM1 protein was transiently expressed in N. benthamiana via the p35S-GFP vector, respectively, and then extracted at 48 h post infiltration with the methods described above. Total proteins were treated with PNGase F and endoglycosidase H (Endo H; New England Biolabs, Beverly, MA, USA) according to the manufacturer’s directions followed by immunoblotting analyses.
Yeast two hybrid assay
The ORF of LtLysM1 without signal peptide sequence was amplified using the primer pairs listed in Table 1 and subcloned into pGBKT7 as the bait vector pGBKT7:LtLysM1. Additionally, the same fragment was ligated to pGADT7 as the prey vector pGADT7:LtLysM1. Subsequently, both pGBKT7:LtLysM1 and pGADT7:LtLysM1 were cotransformed into yeast strain AH109 using the Yeastmaker Yeast Transformation System 2 kit (TaKaRa). The resulting transformants were tested for their growth on synthetic dropout SD/-Leu/-Trp/-His medium.
Pull down
The LtLysM1 ORF was cloned with primers listed in Table 1 and ligated to the transient expression vectors pGWB414 and p35S-GFP, respectively. The fusion vector pGWB414:LtLysM1 and p35S-GFP:LtLysM1 were transformed into A. tumefaciens strain GV3101 using the freeze-thaw method, respectively (Fang et al., 2016). The recombinant A. tumefaciens strains were infiltrated into N. benthamiana leaves for further protein expression. The A. tumefaciens infiltration and protein extraction were performed using the methods described above. Subsequently, the isolated proteins were incubated with GFP-Trap MA beads for 2 h at 4°C. Subsequently, the GFP-Trap MA beads were magnetically separated and repeatedly washed twice with washing buffer (10 mM Tris-HCl pH 7.5, 150 mM NaCl, 0.5 mM EDTA). Next, the GFP-Trap MA beads were resuspended in 80 μl 2× SDS-sample buffer and then boiled for 10 min at 95°C to dissociate immunocomplex from GFP-Trap MA beads. The beads were magnetically separated and immunoblotting analyses were performed with the supernatant according to the methods described above.
Results
Structural features of LtLysM1
Based on SignalP 5.0 program, LtLysM1 was predicted to contain a signal peptide with 21 amino acids, suggesting that LtLysM1 may be a secreted protein. BLASTP search against Pfam database reveals that LtLysM1 contains a typical LysM domain (PF01476, amino acid residues 111-156). Using N-GlycoSite tool (https://www.hiv.lanl.gov/content/sequence/GLYCOSITE/glycosite.html), LtLysM1 was annotated to contain ten putative N-glycosylation sites, located at Asn25 (NTT), Asn30 (NTT), Asn50 (NAT), Asn89 (NET), Asn98 (NAT), Asn140 (NET), Asn197 (NYS), Asn216 (NCT), and Asn223 (NIT), suggesting that LtLysM1 may be a glycoprotein (Fig. 1A). Moreover, phylogenetic analyses of LtLysM1 with other reported LysM proteins including Slp1 and Slp2 in M. oryzae, Ecp6 in C. fulvum), ChELP1 and ChELP2 in C. higginsianum, and Mg1LysM, Mg3LysM, and MgxLysM in M. graminicola, were carried out to reveal their relatedness to each other (Fig. 1B). The phylogenetic tree shows LtLysM1 shares certain homology with other LysM proteins and LysM proteins are distributed widely across fungal pathogens.

Structural and phylogenic analyses of LtLysM1 in Lasiodiplodia theobromae. (A) Amino acid sequence analyses using different programs. Signal peptide prediction was performed with SignalP 5.0 Server (http://www.cbs.dtu.dk/services/SignalP/). Amino acids with black box indicate the putative LtLysM1 signal peptide. N-glycosylation sites were predicted using the N-GlycoSite tool. Amino acids in green color denote predicted N-glycosylation sites. Amino acids underlined mark the putative LysM domain. (B) The LysM proteins are distributed widely across fungal pathogen. The amino acid sequences of LysM proteins from different fungi species were sourced from NCBI database and then used to generate the phylogenetic tree through MEGA7 with the neighbor-joining method, 1,000 replicates. Bootstrap percentage support for each branch is indicated at the nodes. The number and location of LysM domain were predicted using BLASTP analyses against Pfam database with each amino acid sequence as the query.
Expression profile of LtLysM1 during L. theobromae infection
As LtLysM1 is predicted to be a secreted protein based on bioinformatics analyses, we attempt to examine the relative transcript level of LtLysM1 at different infection stages by qRT-PCR assays. Results showed that expression levels of LtLysM1were highly induced at 36 and 48 hpi, equal to about thirty and forty times of that at mycelial stage (Fig. 2), indicating LtLysM1 may be involved in the virulence of L. theobromae during the infection process.
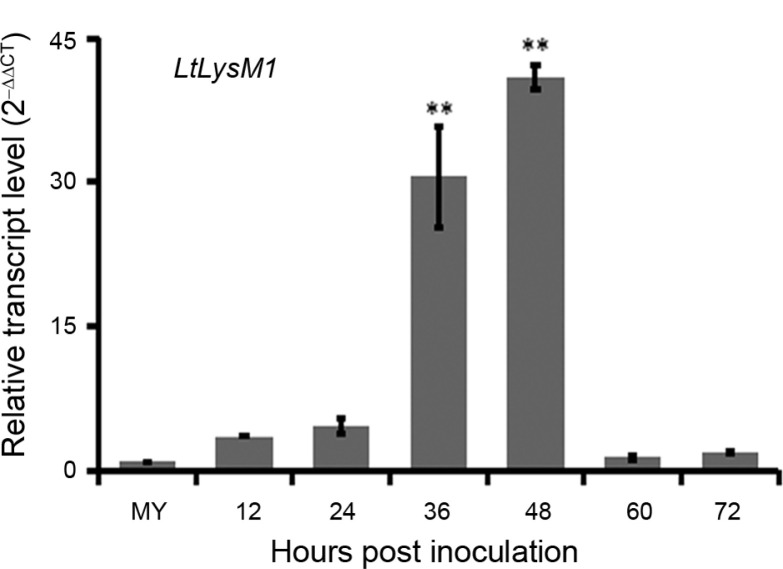
Relative transcript levels of LtLysM1 at differently infectious stages. The mycelia (MY) of wild type CSS-01s cultured in liquid complete medium for 36 h and grapevine tissues infected by wild type CSS-01s were harvested at 12, 24, 36, 48, 60, and 72 hours post inoculation (hpi) for RNA extractions. The isolated RNA was reverse transcribed into cDNA for LtLysM1 gene expression analyses. Relative transcript levels of LtLysM1 at different time points post inoculation were normalized by actin gene and calibrated against that of mycelia. Relative transcript level of LtLysM1 was calculated using the 2-ΔΔCT method. The assays were performed with three independent biological repetitions and three replicates each. A representative set of data are presented. Data are means ± standard error. Asterisks represent significant difference (LSD test, P < 0.01).
The importance of LtLysM1 for the virulence of L. theobromae
To characterize the contribution of LtLysM1 to the virulence of L. theobromae, LtLysM1 gene was overexpressed and silenced in vivo via PEG-mediated protoplast transformation, respectively. The resultant transformants were screened for neomycin resistance and confirmed by qRT-PCR analysis. Two of each overexpressed and RNAi transformants of LtLysM1 were selected for further phenotypic analyses. Colony diameter comparisons between overexpressed transformants (LtLysM1-OE1 and LtLysM1-OE2), RNAi transformants (LtLysM1-RNAi1 and LtLysM1-RNAi2), and wild type CSS-01s showed that overexpression of LtLysM1 did not affect the vegetative hyphae growth of L. theobromae on PDA media, and RNAi of LtLysM1, however, has shown a significantly effect on the hyphal growth (Fig. 3A), suggesting that a certain expression level of LtLysM1 is necessary for the vegetative hyphae growth of L. theobromae.
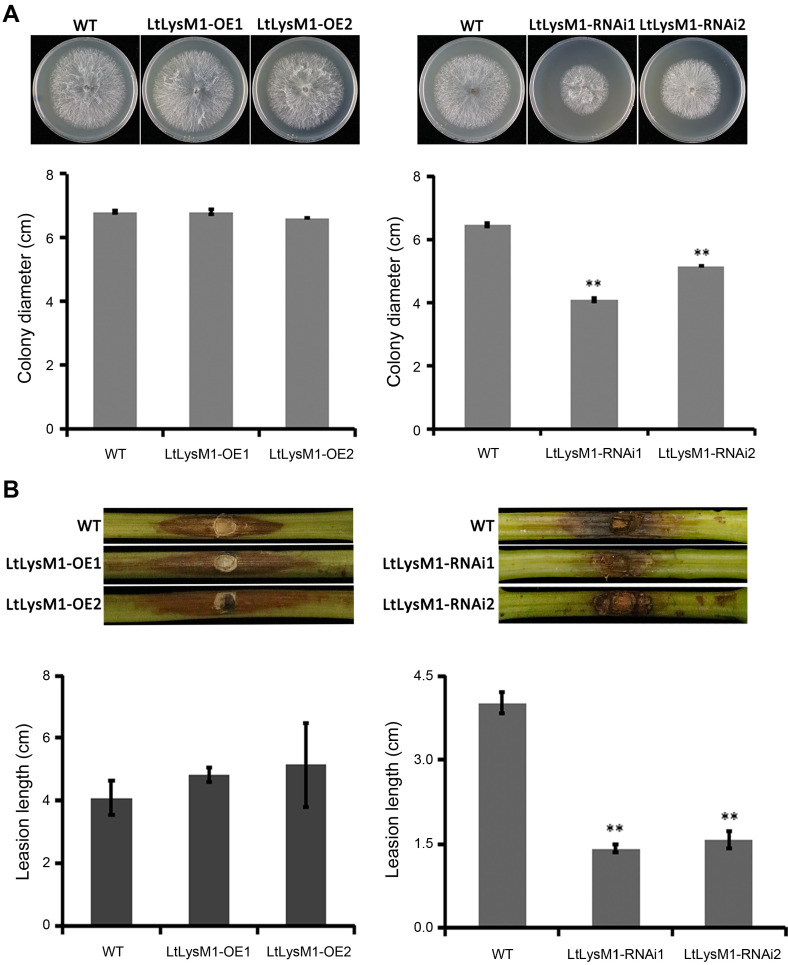
LtLysM1 is important for the virulence and vegetative growth of Lasiodiplodia theobromae. (A) Colonial morphology and diameter comparison of wild type CSS-01s, overexpressed transformants (LtLysM1-OE1 and LtLysM1-OE2), and RNAi transformants (LtLysM1-RNAi1 and LtLysM1-RNAi2) on potato dextrose agar media. Colony diameter was measured and Images were photographed at 36 h post inoculation. Asterisks represent statistically significant difference (least significant difference [LSD] test, **P < 0.01). (B) Pathogenicity assays of the strains mentioned in A. One-year-old grapevine shoots were inoculated with mycelial plugs (5 mm in diameter), and then contained in a chamber at 28ºC. The inoculated shoots were photographed at 3 days post inoculation. At least six biological replicates of per LtLysM1 transformant were performed. Error bars represent standard error of six replicates. Asterisks denote statistically significant difference (LSD test, **P < 0.01).
Furthermore, pathogenicity tests showed that lesion lengths caused by overexpressed transformants LtLysM1-OE1 and LtLysM1-OE2 on the susceptible grape cultivar Vitis vinifera cv. ‘Summer Black’ were increased when compared with that caused by wild type CSS-01s and RNAi transformants LtLysM1-RNAi1 and LtLysM1-RNAi2 exhibited obviously smaller lesion lengths compared to wild type CSS-01s (Fig. 3B). These results demonstrate that LtLysM1 is important for the virulence of L. theobromae.
Funtional validation of LtLysM1 signal peptide
To verify the function of LtLysM1 signal peptide, a yeast signal sequence trap system was adopted. We constructed a pSUC2:LtLysM1 fusion vector, in which the signal peptide of LtLysM1 was in frame to yeast invertase which lacks its own signal peptide. Subsequently, the fusion vector pSUC2:LtLysM1 was transformed into yeast YTK12 strain which was defective in invertase secretion. Yeast transformants expressing N-terminal sequences of Phytophthora sojae Avr1b and M. oryzae Mg87 were used as positive and negative controls, respectively. Yeast transformants carrying pSUC2 vector grew on CMD-W media. However, only transformants expressing pSUC2:LtLysM1 or the positive control grew on YPRAA medium with raffinose as the sole carbon source (Fig. 4). These results indicate that LtLysM1 signal peptide guides the raffinose secretion in yeast cells and that LtLysM1 is a secreted protein with a functional signal peptide.
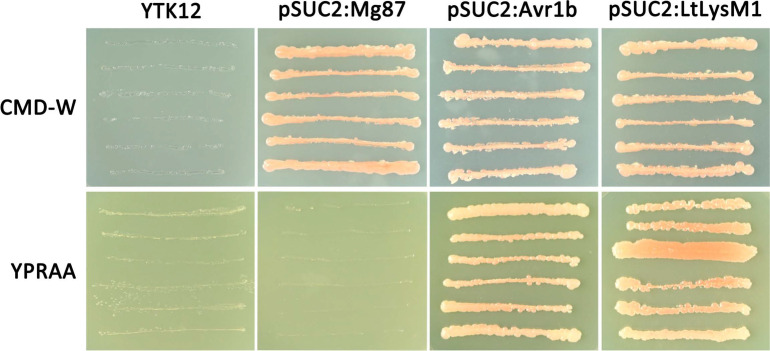
Functional validation of the predicted signal peptide of LtLysM1. Yeast YTK12 transformed with pSUC2:LtLysM1 fusion vector were plated on CMD-W and YPRAA media, respectively. The signal peptide of LtLysM1 was able to guide the secretion of yeast invertase and therefore yeast transformants expressing pSUC2:LtLysM1 grew on YPRAA plate with raffinose as sole carbon source. The untransformed strain YTK12 grew neither on CMD-W medium nor on YPRAA medium. Transformants expressing pSUC2:Avr1b and pSUC2:Mg87 were used as positive and negative controls, respectively.
N-glycosylation analyses of LtLysM1
In previous studies, many secreted pathogenic effector proteins were reported to be N-glycosylated in vivo (Chen et al., 2014; Fang et al., 2016). Therefore, we set out to investigate whether LtLysM1 shared similar molecular feature. During the preliminary studies, we found that LtLysM1 contains ten putative N-glycosylation sites based on NGlycoSite prediction. To substantiate the posttranslational modifications, proteins transiently expressed in N. benthamiana were initially digested by PNGase F, an amidase that cleaves between the innermost GlcNAc and asparagine residues of high mannose and complex oligosaccharides from N-linked glycoproteins, and Endo H, a recombinant glycosidase which cleaves within the chitobiose core of high mannose and some hybrid oligosaccharides from N-linked glycoproteins (Chen et al., 2014), respectively. Subsequently, each digestion was further examined by immunoblotting analyses with anti-GFP antibody. Results showed that proteins digested with PNGase F and Endo H displayed smaller band size compared to that of untreated mature LtLysM1 protein (Fig. 5), indicating that LtLysM1 was an N-glycosylated protein in plant cell.

LtLysM1 is an N-glycosylated protein in plant cells. LtLysM1 was transiently expressed in Nicotiana benthamiana leaves and then extracted for deglycosylation analyses. Total protein extractions were digested with or without PNGase F or endoglycosidase H (Endo H) at 37ºC for 1 h and then examined by immunoblotting analyses with an anti-GFP antibody.
Self-interaction analyses of LtLysM1 protein
The LysM proteins including Slp1 in M. oryzae (Mentlak et al., 2012) and OsCEBiP in rice were characterized to be able to form dimer complexes for their molecular functions (Shimizu et al., 2010). To investigate whether the dimerization occurs in LtLysM1 molecules, we constructed the prey vector pGADT7:LtLysM1 and bait vector pGBKT7:LtLysM1, in which the encoding sequences of LtLysM1 signal peptide were removed, and then transformed both the vectors into yeast strain AH109 simultaneously. Resultant yeast transformants were detected for their growth on synthetic dropout media. Results showed that yeast transformants expressing LtLysM1 did not grow on SD/-Leu/-Trp/-His media (Fig. 6A), suggesting that LtLysM1 molecule did not have the capacity to interact with itself.
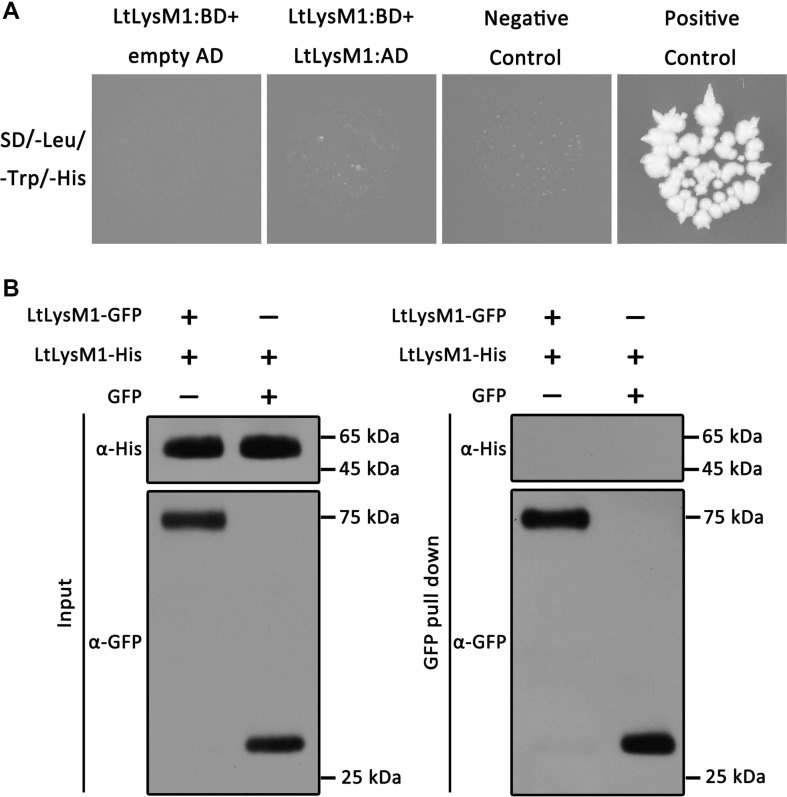
LtLysM1 molecule does not interact with itself. (A) Yeast cells expressing prey vector LtLysM1:AD and bait construct LtLysM1:BD were tested for their growth on SD/-Leu/-Trp/-His media. In both vectors, the encoding sequence of LtLysM1 signal peptide were removed off. Yeast transformant carrying empty prey vector pGADT7 with empty bait vector pGBKT7 was used as negative control. Yeast transformant carrying pGADT7-T and pGBKT7-53 vectors was used as positive control. Plates were photographed at 3 days post inoculation. (B) LtLysM1 did not interact with itself via GFP pull down experiments. Recombinant proteins LtLysM1-GFP and LtLysM1-His from Nicotiana benthamiana were subjected to GFP pull down analyses. Interactions between LtLysM1 molecules were detected through immunoblotting tests with anti-GFP and anti-His antibodies.
To supply more cogent evidences, further GFP pull down experiments were conducted. The LtLysM1-GFP proteins isolated from N. benthamiana leaves were incubated with LtLysM1-His proteins extracted from N. benthamiana leaves. Protein immunoblotting analyses showed that LtLysM1 was not pulled down by itself (Fig. 6B), which was consistent with results demonstrated above. These results strongly supported that LtLysM1 molecule did not interact with itself physically and homodimerization complexes were not formed among LtLysM1 molecules.
Discussion
Lasiodiplodia theobromae, a woody plant pathogen belonging to Botryosphaeriaceae family, has received much attention recently for the considerable damage caused by this fungus in fruiter’s industry. Research on this fungus have made great progress during the past two decades including strain isolation, diseases symptoms characterization, enzymatic activity analyses, and genome and transcription analyses (Cao et al., 2020; Félix et al., 2019; Gonçalves et al., 2019; Paolinelli-Alfonso et al., 2016; Úrbez-Torres et al., 2008; Yan et al., 2018). However, investigation on the pathogenic mechanism are still at a preliminary stage and remain stagnant because of the multinuclear cell structure of this fungus, which seriously constrains the genetic manipulations in molecular studies. Here, we set out to expand the molecular research on this fungus and perform an investigation on the biological functions and molecular features of a LysM domaincontaining protein LtLysM1 in L. theobromae, and therefore, this study means the first step in molecular research of this fungus.
LysM proteins have been documented in a wide range of organisms including animals, plants, fungi, and bacteria (Buist et al., 2008; Kombrink et al., 2011; Sánchez-Vallet et al., 2013). Some LysM proteins were characterized as virulence factors in plant pathogenic fungi (de Jonge et al., 2010; Kombrink et al., 2017; Marshall et al., 2011; Mentalk et al., 2012; Takahara et al., 2016). Here, we found LtLysM1 was a pathogenicity-related protein during infection process upon qRT-PCR analyses. Because of the lack of effective and efficient gene deletion stratergy on multinuclear protoplast, we performed overexpression and gene-silencing of LtLysM1 gene. The pathogenicity assays revealed that LtLysM1 is contributed to the full virulence of L. theobromae, confirming the results of qRT-PCR tests. Moreover, LtLysM1 was proven to be important for the mycelial growth in RNAi transformants of LtLysM1, but overexpressed transformants of LtLysM1 did not exhibit increased mycelial growth. It is plausible that a certain expression level of LtLysM1 is sufficient for maintaining mycelial growth under normal growth condition. Therefore, LtLysM1 is an important regulator that mediates the mycelial development in L. theobromae. Moreover, LtLysM1 was proven to be a small secreted protein based on the yeast signal peptide trap system which has also been widely used to identify the signal peptide in other fungi (Fang et al., 2016; Gu et al., 2011; Oh et al., 2009). These data indicate that LtLysM1 can be secreted to the outside cellular space during plant-pathogen interaction and plays an important role in disease symptom development.
N-Glycosylation has been reported to be an important feature for the function of effector proteins. For example, the N-glycosylation of Slp1 in M. oryzae was required to suppress host immunity and additionally regulate the chitin-binding ability of Slp1 (Chen et al., 2014). Also, the N-glycosylation of UV_6205 and UV_1423 in Ustilaginoidea virens were involved in regulating host cell death (Fang et al., 2016). In this study, we transiently expressed LtLysM1 protein in N. benthamiana via two expression systems and treated the protein extracts with two enzymes PNGase F and Endo H. Proteins digested with two enzymes exhibited smaller band size when separated on 12% SDS-PAGE, revealing LtLysM1 was an N-glycosylated protein. Additionally, a total of ten N-glycosylation sites were predicted based on our predicted, whereas we did not examine the actual N-glycosylation sites of this protein. More questions such as the biological functions and molecular roles of N-glycosylation in this protein also need further investigation.
Molecular dimerization has been reported in many effector proteins. Slp1 in M. oryzae was reported to be capable of forming protein aggregates based on proteinprotein interaction (Mentlak et al., 2012). Another two LysM receptor molecules OsCERK1 with CEBiP were able to form a hetero-oligomer complex to induce downstream immune responses (Shimizu et al., 2010). Plant pathogenic effectors form complexes to overcome plant immunity to promote disease by interacting with themselves or with other effector proteins (Alcântara et al., 2019). PsCRN63 in P. sojae was capable of suppressing PCD associated with PTI via self-dimerization (Li et al., 2016). However, it was found that LtLysM1 molecule did not interact with itself based on yeast two hybrid assay. This may be due to structural and functional differences in comparison to other LysM proteins.
Many published LysM effectors were characterized with multiple LysM domains. For example, Ecp6 (Sánchez-Vallet et al., 2013), Mg3LysM (Marshall et al., 2011), and ChELP7 (Takahara et al., 2016) were identified to contain three LysM domains. Slp1 (Mentlak et al., 2012), ChELP1, ChELP2 (Takahara et al., 2016), and Vd2LysM (Kombrink et al., 2017) were reported to contain two LysM domains. Moreover, structural analyses of Ecp6 revealed that composite LysM1-LysM3 binding site provides a single binding event with ultra-high affinity for chitin binding, whereas the LysM2 may be involved in suppression of chitin-triggered immunity by preventing the immune receptor dimerization (Sánchez-Vallet et al., 2013). Unlike these effectors, LtLysM1 was predicted to contain a single LysM domain, implying that LtLysM1 may function in a different manner. Thus, further structural analyses will be conducive to reveal the molecular functions of LtLysM1.
Acknowledgments
This work was supported by Outstanding Talents of Beijing grant 2017000020060G119 and National Technology System for Grape Industry CARS-29. We acknowledged Prof. Wenxian Sun at China Agricultural University for supplying the pSUC2 plasmid and yeast strain YTK12. Authors declare no conflicts of interest.

