Present Status and Future Management Strategies for Sugarcane Yellow Leaf Virus: A Major Constraint to the Global Sugarcane Production
Article information
Abstract
Sugarcane yellow leaf virus (SCYLV) is a distinct member of the Polerovirus genus of the Luteoviridae family. SCYLV is the major limitation to sugarcane production worldwide and presently occurring in most of the sugarcane growing countries. SCYLV having high genetic diversity within the species and presently ten genotypes are known to occur based on the complete genome sequence information. SCYLV is present in almost all the states of India where sugarcane is grown. Virion comprises of 180 coat protein units and are 24-29 nm in diameter. The genome of SCYLV is a monopartite and comprised of single-stranded (ss) positive-sense (+) linear RNA of about 6 kb in size. Virus genome consists of six open reading frames (ORFs) that are expressed by sub-genomic RNAs. The SCYLV is phloem-limited and transmitted by sugarcane aphid Melanaphis sacchari in a circulative and non-propagative manner. The other aphid species namely, Ceratovacuna lanigera, Rhopalosiphum rufiabdominalis, and R. maidis also been reported to transmit the virus. The virus is not transmitted mechanically, therefore, its transmission by M. sacchari has been studied in different countries. SCYLV has a limited natural host range and mainly infect sugarcane (Sachharum hybrid), grain sorghum (Sorghum bicolor), and Columbus grass (Sorghum almum). Recent insights in the protein-protein interactions of Polerovirus through protein interaction reporter (PIR) technology enable us to understand viral encoded proteins during virus replication, assembly, plant defence mechanism, short and long-distance travel of the virus. This review presents the recent understandings on virus biology, diagnosis, genetic diversity, virus-vector and host-virus interactions and conventional and next generation management approaches.
Sugarcane (genus: Saccharum) is a member of the family Poaceae. Sugarcane (Saccharum interspecific hybrids) is considered as the industrially significant crop since it is rich source of bioenergy and by-products. Sugarcane is a very useful asset for economic developments in different regions of the globe including India. Sugarcane is grown on 26-million-hectare area in more than 90 countries across the globe. However, sugarcane production is more challenging because this crop is affected by several pathogens and other factors (Birchfield, 1984; Ricaud et al., 2012; Rott et al., 2000). Among the biotic stresses, sugarcane is infected by different virus species viz., Sugarcane yellow leaf virus (SCYLV) inducing yellow leaf disease (YLD), Sugarcane streak virus (SSV) responsible for causing streak disease, Sugarcane Fiji disease virus (SFDV) causing infamous Fiji disease, Sugarcane bacilliform virus (SCBV) known to induce fleck leaf disease (Braithwaite et al., 1995) and Sugarcane streak mosaic virus (SCSMV), and Sugarcane mosaic virus (SCMV) (Rott et al., 2000; Viswanathan and Rao, 2011) are associated with mosaic disease. Among the sugarcane infecting viruses, SCYLV causes YLD in sugarcane in more than 25 countries in the world where sugarcane is widely cultivated (Table 1).

Distribution of SCYLV isolates based on the complete and partial genome and other ORFs sequence originating from different sugarcane genotypes from different countries including India
First occurrence of YLD was described in 1988 in Hawaii on sugarcane cv. H 65-7052, which showed severe yellowing throughout the plantation (Schenck, 1990; Schenck et al., 1997). The origin of YLD ways back to 1960s and 1970s, when the disease was defined as yellow leaf syndrome or yellow wilt in Tanzania during 1962 (Ricaud, 1968; Rott et al., 2008). Since then, the disease has spread in all the regions wherever sugarcane is cultivated (Gonçalves et al., 2012). In India, YLD was first recorded in farmer’s field in 1999 by Viswanathan et al. (1999) and since then its widespread incidence was reported throughout the country (Rao et al., 2000, 2001; Viswanathan, 2002). During 1990s, one decade after its first record from Hawaii, the extracted virus was confirmed with the diseased plants and recognized as a tentative species of Polerovirus (Scagliusi and Lockhart, 2000; Vega et al. 1997). Members of the genus are known to infect dicots and some of them are restricted to monocot plant species (Hull, 2002). Currently, more than 17 species are known to occur in this genus including Potato leafroll virus (PLRV) (Lefkowitz et al., 2018).
Economic Impact of SCYLV
The SCYLV has a negative effect on sugarcane yield and yield contributing parameters (Grisham et al., 2001; Izaguirre-Mayoral et al., 2002; Lehrer et al., 2008, 2009; Rassaby et al., 2003; Rott et al., 2008; Smith et al., 2000; Vega et al., 1997; Viswanathan, 2002; Viswanathan and Rao, 2011; Viswanathan et al., 2014, 2016; Yan et al., 2009; Zhou et al., 2006; Zhu et al., 2010). Due to virus infection, yield losses of 15% and up to 50% in sugarcane were reported from United States (Lockhart and Cronje, 2000) and Brazil (Vega et al., 1997), respectively. The 50% was the highest yield loss reported due to SCYLV in ratoon crops (Grisham et al., 2001; Vega et al., 1997). Up to 14% loss in sugar yield was described in Louisiana (Gonçalves et al., 2005; Grisham et al., 2009). In Florida, 11% loss was recorded in sugar yield and stalk weight (Comstock and Miller, 2004), 14% loss in sugar yield (Flynn et al., 2005) and 11% to 27% in sugarcane yield were reported in different experimental fields (Boukari et al., 2019). In Reunion of Island, 11% and 28% losses were documented in sugar content and stalk weight, respectively due to virus infection (Rassaby et al., 2004). Around 30% loss in yield was stated in asymptomatic sugarcane plants in Thailand (Lehrer et al., 2008). Therefore, the major challenge before the researchers is to pin-point the infection and devising management strategies.
SCYLV infection has significantly affected the cane growth, stalk number, cane diameter, leaf area, chlorophyll content, sugar transport, and sucrose accumulation in the susceptible sugarcane varieties and lead to the substantial decline in sucrose content, HR brix and number of millable canes (Arocha et al., 1999; Cronje et al., 1998; Grisham et al., 2001; Izaguirre-Mayoral et al., 2002; Scagliusi and Lockhart, 2000; Viswanathan, 2002; Viswanathan et al., 2006, 2008). Moreover, yield loss was also recorded due to mixed infection of SCYLV and Sugarcane yellows phytoplasma (SCYP) (Aljanabi et al., 2001; Parmessur et al., 2002). Rassaby et al. (2003) reported 28% and 46% decrease in the stalk weight in plant and ratoon crops, respectively with overall 37% drop in the cane yield due to infection of SCYLV. During the past few decades of investigation on SCYLV showed that infection was found to have a significant effect on sugarcane interference metabolism of phloem cells (Gonçalves et al., 2012; Lehrer et al., 2008; Schenck and Lehrer, 2000). Lehrer et al. (2009) compared biomass of virus-free and diseased plants and showed 44% increased stalk numbers in the virus-free plants that led to the 35% rise in sugar yield when both harvested at 11 months crop age but this scenario changed in sugar yield when crops were harvested in 16 to 24 months due to over maturity stage. Viswanathan et al. (2014) studied the negative effect of SCYLV; reduction in different parameters including 24% in photosynthetic rate, 28% in stomatal conductance, 10% in chlorophyll content, 10% in chlorophyll-fluorescence ratio, 10% in length of the internodes, 15% in girth of the stalk, 28% in stalk weight, up to 44% in leaf sheath weight and 39% in juice yield while, increasing the levels of carbohydrates and transpiration rate by 81% and 16%, respectively in virus infected leaves.
Recognition of Physical Properties, Symptomatology, and Diagnosis of SCYLV
Initially, the SCYLV was suggested for its inclusion in the family Luteoviridae (Scagliusi and Lockhart, 2000; Smith et al., 2000; Vega et al., 1997), which later confirmed by Irey et al. (1997). Virions of SCYLV are icosahedral and ranged from 24 to 29 nm in diameter with a buoyant density of 1.30 g/cm3 in Cs2SO4 (Scagliusi and Lockhart, 2000). SCYLV is comprised of positive-sense single-stranded (ss) RNA of about ~6 kb in size. Molecular weight of coat protein (CP) is 27 kDa.
Symptoms of SCYLV on sugarcane were described earlier by various workers (Ahmad et al., 2006a, 2006b, 2007; Bailey et al., 1996; Borth et al., 1994; Comstock et al., 1994, 1998; Fitch et al., 2001; Izaguirre-Mayoral et al., 2002; Lockhart et al., 1996; Schenk et al., 1997; Vega et al., 1997). In India, Viswanathan et al. (1999) and Viswanathan (2002) first time reported that SCYLV is associated with YLD. The YLD affected sugarcane plants exhibited various symptoms including mild to severe yellowing of the midribs (Fig. 1B and C), smaller and clustering of leaves at the crown region of the plant along with the shortened internodes. Midrib yellowing (Fig. 1D and E), necrosis observed from tip to the base of leaves. Subsequently, complete drying of leaf foliage with stunted and poor growth was observed due to severe incidence of the disease under field situations (Fig. 1F). Natural occurrence of SCYLV was recorded on grain sorghum (Sorghum bicolor) cv. Top76-6 by Elsayed et al. (2018) based on the NCBI GenBank accession numbers (KT960997, KT960996, and KT960995). Similarly, from the United States the natural occurrence of SCYLV on S. bicolor and Columbus grass (Sorghum almum) was identified (Espinoza-Delgado et al., 2016; Wei et al., 2016).
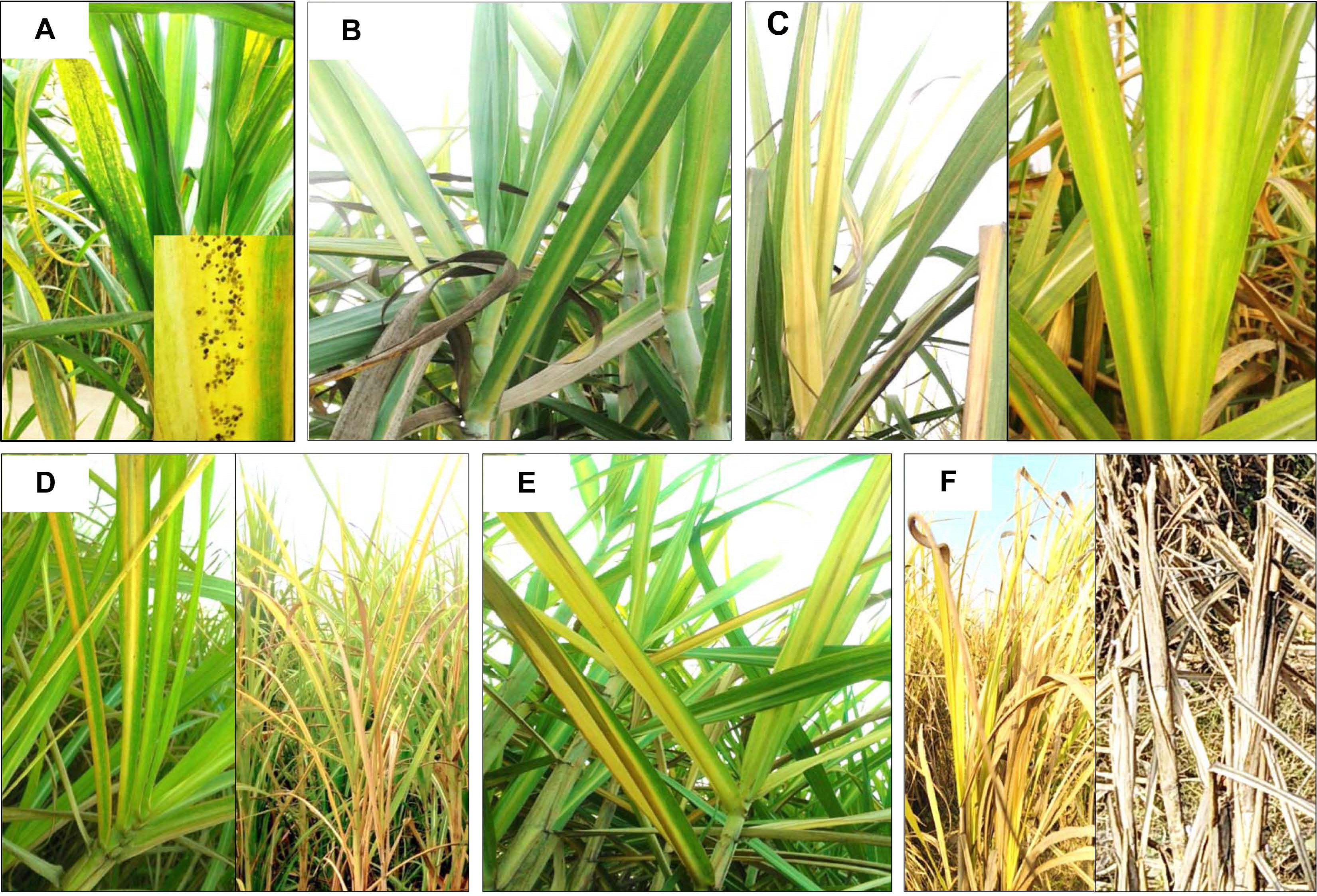
Symptoms of yellow leaf disease in sugarcane and natural occurrence of aphid colonies; aphid (Melanaphis sacchari) infesting sugarcane and reported vector of Sugarcane yellow leaf virus (SCYLV) in India (A); matured leaves with mild yellowing of midrib (B); young leaves showing mild midrib yellowing and matured leaves showing initial discoloration of leaf lamina (C); young leaves showing bright midrib yellowing and matured leaves showing extensive discoloration of lamina with necrosis (D); severe necrosis of leaf area in matured leaves (E) and plant showed extensive stunting with complete drying (F).
Occurrence of virions was observed under electron microscopy (EM) and immunosorbent electron microscopy (ISEM) in moderately cleansed sap of YLD affected sugarcane (Madugula and Gali, 2017, 2018; Moutia and Saumtally, 1999; Scagliusi and Lockhart, 2000). The presence of virus was detected by EM combined with serology. EM studies showed the diameter of isometric virus particles ranged from 24 to 26 nm (Vega et al., 1997). A polyclonal antibody was raised for detection of SCYLV which showed negative reaction against related Luteovirus species viz., Bean leafroll virus (BLRV), Rose spring dwarf-associated virus (RSDaV), Soybean dwarf virus (SbDV), BYDVPAV, BYDV-MAV, BYDV-PAS, BYDV-kerII, BYDV-kerIII by enzyme-linked immunosorbent assay (ELISA) and ISEM (Scagliusi and Lockhart, 2000). In dot immunoblot assays, SCYLV specific antiserum showed cross reaction with BYDV-RPV, due to its biological and serological distinctness from other members of the group (Scagliusi and Lockhart, 2000). Later, tissue blot immuno-assay was developed using the available polyclonal antiserum to detect SCYLV and since then it became a widely used technique for routine virus detection (Chatenet et al., 2001; Comstock and Miller, 2003; Comstock et al., 1998, 1999; Madugula and Gali, 2018; Rassaby et al., 2003; Schenck et al., 1997, Victoria et al., 2005). Moreover, double antibody sandwich (DAS)-ELISA was optimized to detect SCYLV in extract obtained from whole cane, stem and leaf tissues (Madugula and Gali, 2017, 2018; Viswanathan and Balamuralikrishnan, 2004).
Further, reverse transcription–polymerase chain reaction (RT-PCR) and multiplex PCR assays were standardized and detected the presence of SCYLV in the asymptomatic sugarcane plants (Chinnaraja and Viswanathan, 2017; Chinnaraja et al., 2014; Korimbocus et al., 2002; Sharma et al., 2017; Viswanathan et al., 2008, 2010, 2009; Xie et al., 2009; Zhu et al., 2010). Gonçalves et al. (2002) optimized the AmpliDet (RNA) method for SCYLV detection in sugarcane and insect-vector (aphid: Melanaphis sacchari) and accuracy was analyzed with DAS-ELISA, RT-PCR and nucleic acid sequence-based amplification assay combined with northern blotting analysis techniques. In India, identification of SCYLV in both symptomatic and asymptomatic plants have been performed by RT-PCR using the virus specific primers (Singh et al., 2009; Viswanathan et al., 2008, 2010, 2009) (Fig. 2A). Higher sensitivity and specificity of real-time quantitative PCR (RT-qPCR), confirmed the association of SCYLV and its quantification in asymptomatic sugarcane plants. Most of the sugarcane varieties infected by SCYLV do not express symptoms under field conditions (Chinnaraja and Viswanathan, 2017; Chinnaraja et al., 2014; Korimbocus et al., 2002; Sharma et al., 2017; Zhu et al., 2010). Recently, reverse transcription loop-mediated isothermal amplification technique detected presence of SCYLV in sugarcane (Amata et al., 2016; Anandakumar et al., 2018; Hodgetts et al., 2011; Nair et al., 2016; Sharma et al., 2017).
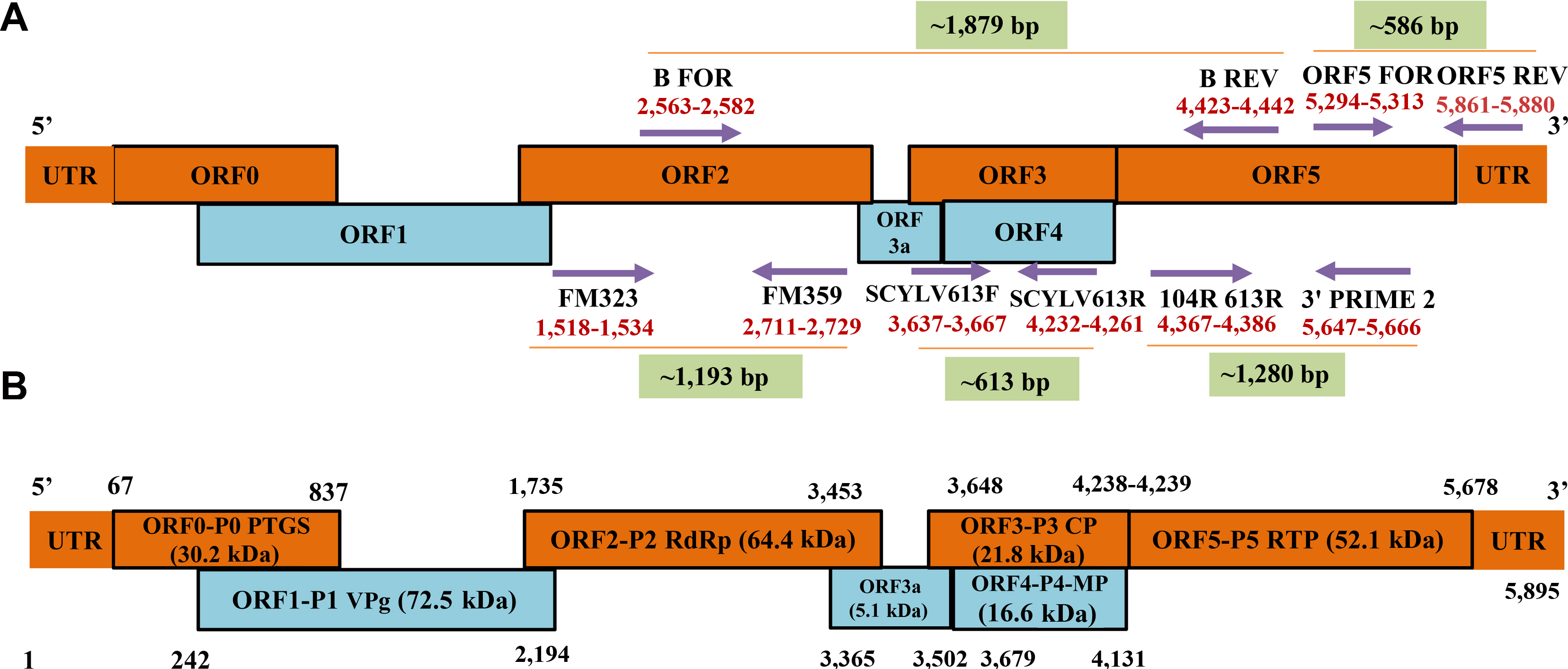
(A) Schematic representation of the primer positions on Sugarcane yellow leaf virus (SCYLV) genome based on Ahmed et al. (2006a), Moonan and Mirkov (2002), Borg et al. (unpublished), and Viswanathan et al. (2008). (B) Schematic representation of the genome organization of Sugarcane yellow leaf virus (SCYLV) with nucleotide positions of different open reading frames which encoding various proteins and their molecular weight based on first evidence given by Smith et al. (2000).
The Genome Organization and Expression Strategies of SCYLV
The SCYLV is a positive-sense, ssRNA virus of about ~6 kb in size (Moonan et al., 2000). SCYLV genome is composed of six open reading frames (ORFs) viz., ORF0, 1, 2, 3, 4, and ORF5 with the three 5′-untranslated regions (Moonan and Mirkov, 2002; Smith et al., 2000). The ORF0 encodes a viral suppressor (P0:30.2 kDa) responsible for RNA silencing mechanism (Ahmad et al., 2006a; Mangwende et al., 2009). ORF1 and ORF2 are translated simultaneously whereas, ORF1 encodes single multifunctional protein (P1:72.5 kDa) while, ORF2 encodes the RNA-dependent RNA polymerase (Smith et al., 2000). ORF3 encodes a viral coat protein P3 (CP; 21.8 kDa), and ORF4 is mainly involved to encode a viral movement protein (16.6 kDa) (Smith et al., 2000). ORF5 is translated by the peptide encoded by ORF3 which act as read-through protein (RTP) by translational read-through process, is known as major component of the virus particle and involved in the virus transmission by aphids (52.1 kDa) (Lockhart et al., 1996; Moonan and Mirkov, 2002; Smith et al., 2000). ORF3 and ORF4 are having distinct characteristics common with several known genotypes (Ahmad et al., 2006a; Zhu et al., 2011). Schematic representation of the genome composition of SCYLV has been shown based on Smith et al. (2000) (Fig. 2B).
The length of SCYLV genome ranged from 5,612-5,899 nucleotide (nts) long in all the genotypes originating from different countries. So far, 36 complete genomes of SCYLV isolates have been characterized, of which 33 were from sugarcane and three from sorghum. Of the total 36 virus isolates, seven were from France (Reunion) (Ahmad et al., 2006b; Lin et al., 2014), six each characterized from India (Chinnaraja et al., 2013; Gaur et al., 2003, unpublished and based on NCBI, Genbank database). Five SCYLV isolates were completely characterized from Mauritius (Joomun et al., 2017, unpublished and based only on NCBI Genbank accessions), three were representing from Hawaii (Elsayed et al., 2011), one each representing from Cuba (Ahmad et al., 2017, unpublished and based only on NCBI GenBank accessions), Colombia (Ahmad et al., 2017, unpublished and based only on NCBI Genbank accessions), and Brazil (Ahmad et al., 2006b). The others were from United States (Elsayed et al., 2015; Moonan et al., 2000; Smith et al., 2000) and China (Ahmad et al., 2006b; Gao et al., 2012; Lin et al., 2014; Wang and Zhou et al., 2010; Wang et al., 2012; Zang et al., 2010; unpublished and based only on NCBI Genbank accessions) (Table 1).
Genome expression strategies of the members of Polerovirus involves four mechanisms viz., leaky scanning, subgenomic (Sg) RNAs, and -1 frameshifting (Mayo and Miller, 1999). Read-through strategy of viral genome expression has been documented for the members of Luteoviridae (King et al., 2011). Polerovirus members comprising six ORFs. The 3′ ORFs are known to be expressed from the Sg RNAs. Translation of ORF 4 is generally followed by leaky scanning strategy from ORF 3 translation initiation (Dinesh-Kumar and Miller, 1993; Tacke et al., 1990). Serine proteinase are found in members of this genus (Spall et al., 1997). The -1 frameshifting strategy studied in Polerovirus type species i.e., PLRV and found the strongly structured regions which were separated by the spacer region frameshift point and pseudoknots (Giedroc and Cornish, 2009).
Geographical Distribution, Genotypes, and Genetic Diversity of SCYLV
Infection of SCYLV in sugarcane is present in around 25 countries i.e., Germany, Guatemala, India, Colombia, Jamaica, Kenya, Martinique, Malaysia, Brazil, Mauritius, Mexico, China, Papua New Guinea, Peru, Australia, Philippines, Reunion of Islands, Senegal, South Africa, Cuba, Sri Lanka, Taiwan, Tunisia, Argentina, and the United States (Table 1, Fig. 3). In India, YLD is present in different states and adversely affected sugarcane varieties viz., CoC 92061, Co 6304, CoA 05323, CoC 86062, CoV 09356, CoA 92081, CoV 06356, Co 86032, CoV 94102, Co 94012, and CoV 92102 (Rao et al., 2000; Viswanathan, 2002; Viswanathan and Rao, 2011; Viswanathan and Rao, 2011, 2017b). Since its first record, the prevalence of YLD was reported from 2012-13 to 2019-20 from different states in India (Fig. 3). The existence of disease in Uttar Pradesh of India in different sugarcane genotypes were documented (Holkar et al., 2015, 2016a). Recently, Madugula et al. (2020) has recorded the status of YLD in Andhra Pradesh and Telangana States of India. So far, ten SCYLV genotypes have been documented based on whole genome characterization and designated them as: BRA (Brazil), CHN1, CHN2 and CHN3 (China), CUB (Cuba), HAW (Hawaii), IND (India), PER (Peru), COL (Colombia), and REU (Reunion Island) (Fig. 4A).
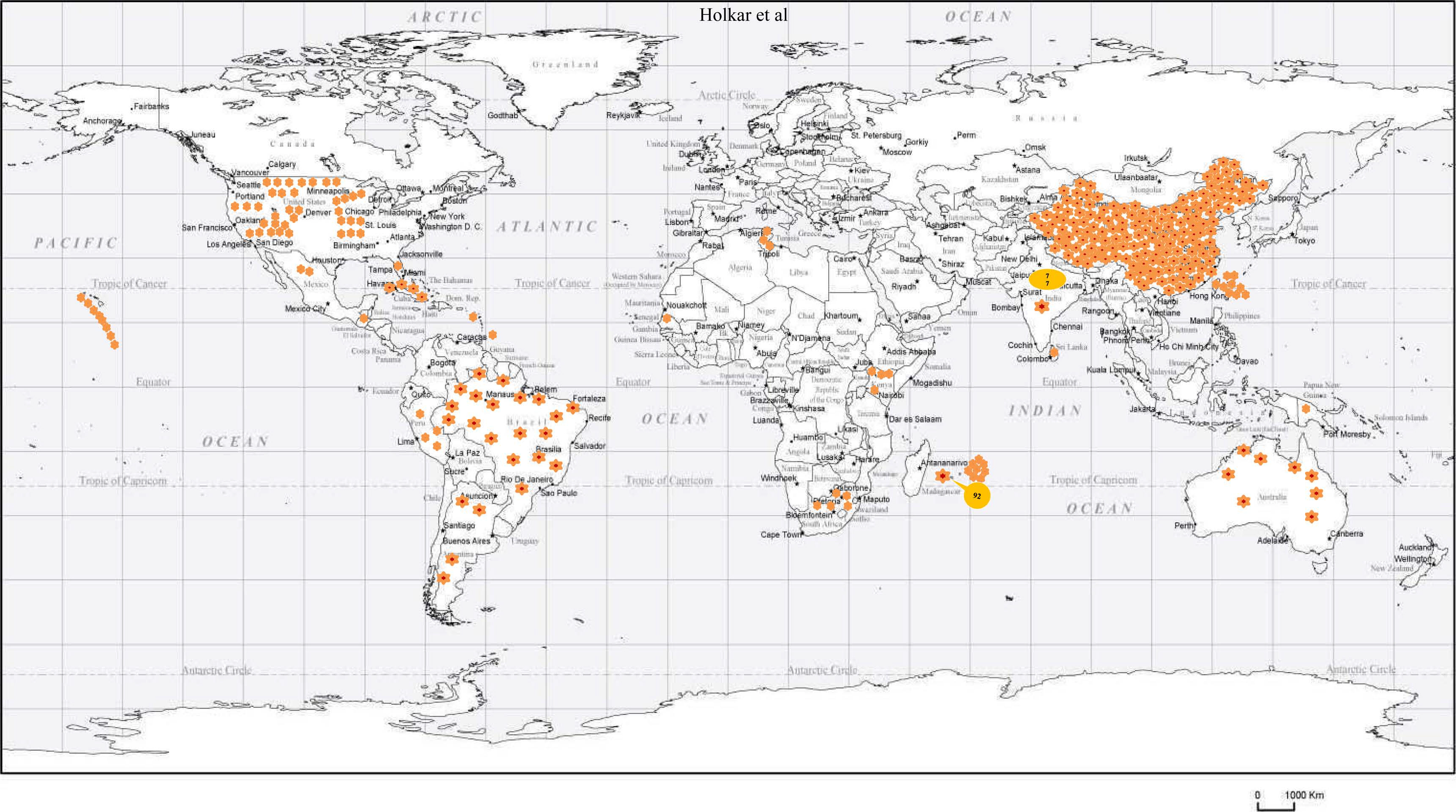
Spread of Sugarcane yellow leaf disease based on geographical distribution of Sugarcane yellow leaf curl virus (SCYLV) in sugarcane. Data presented based on sequence information of SCYLV retrieved from NCBI GenBank https://www.ncbi.nlm.nih.gov/.
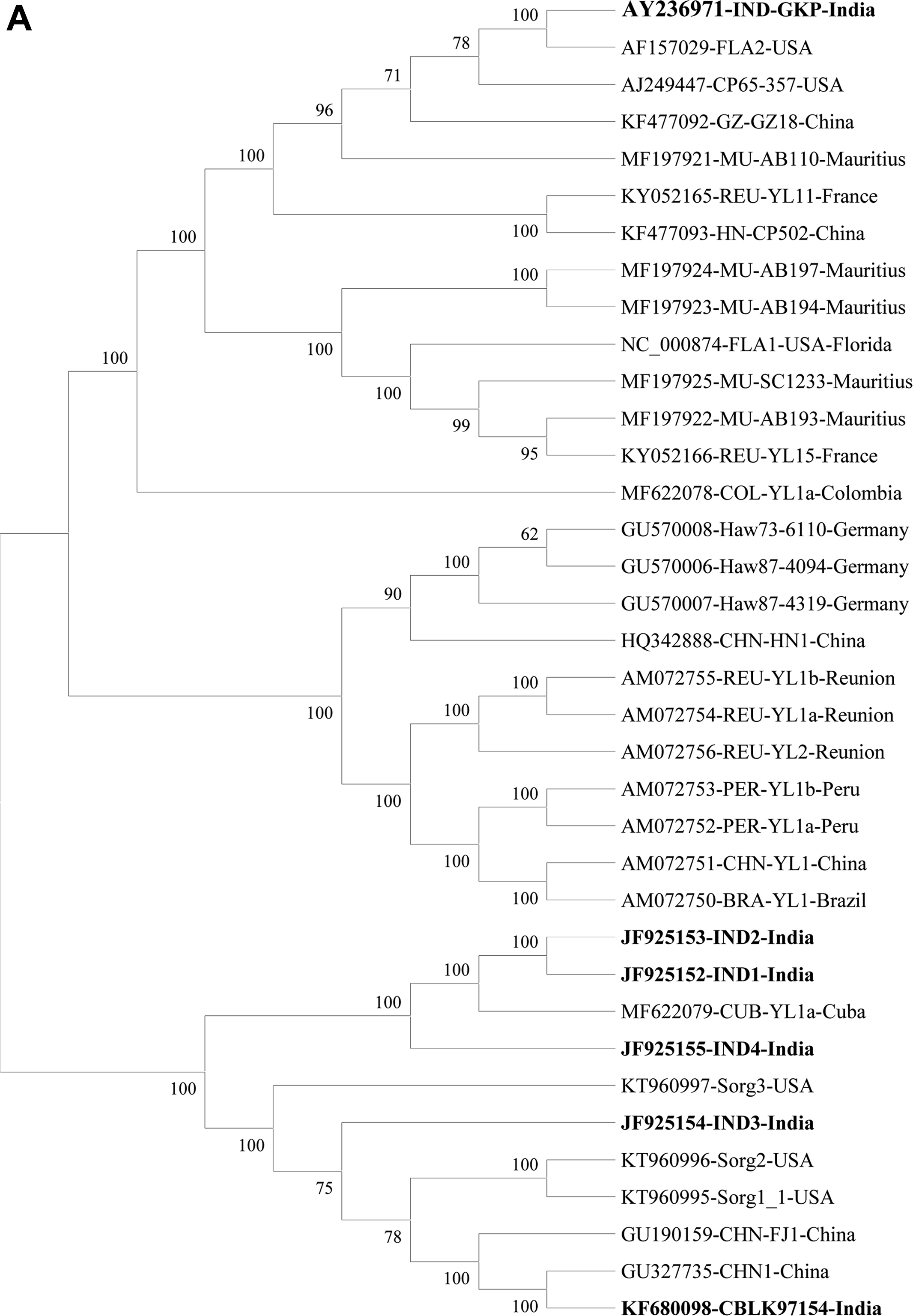
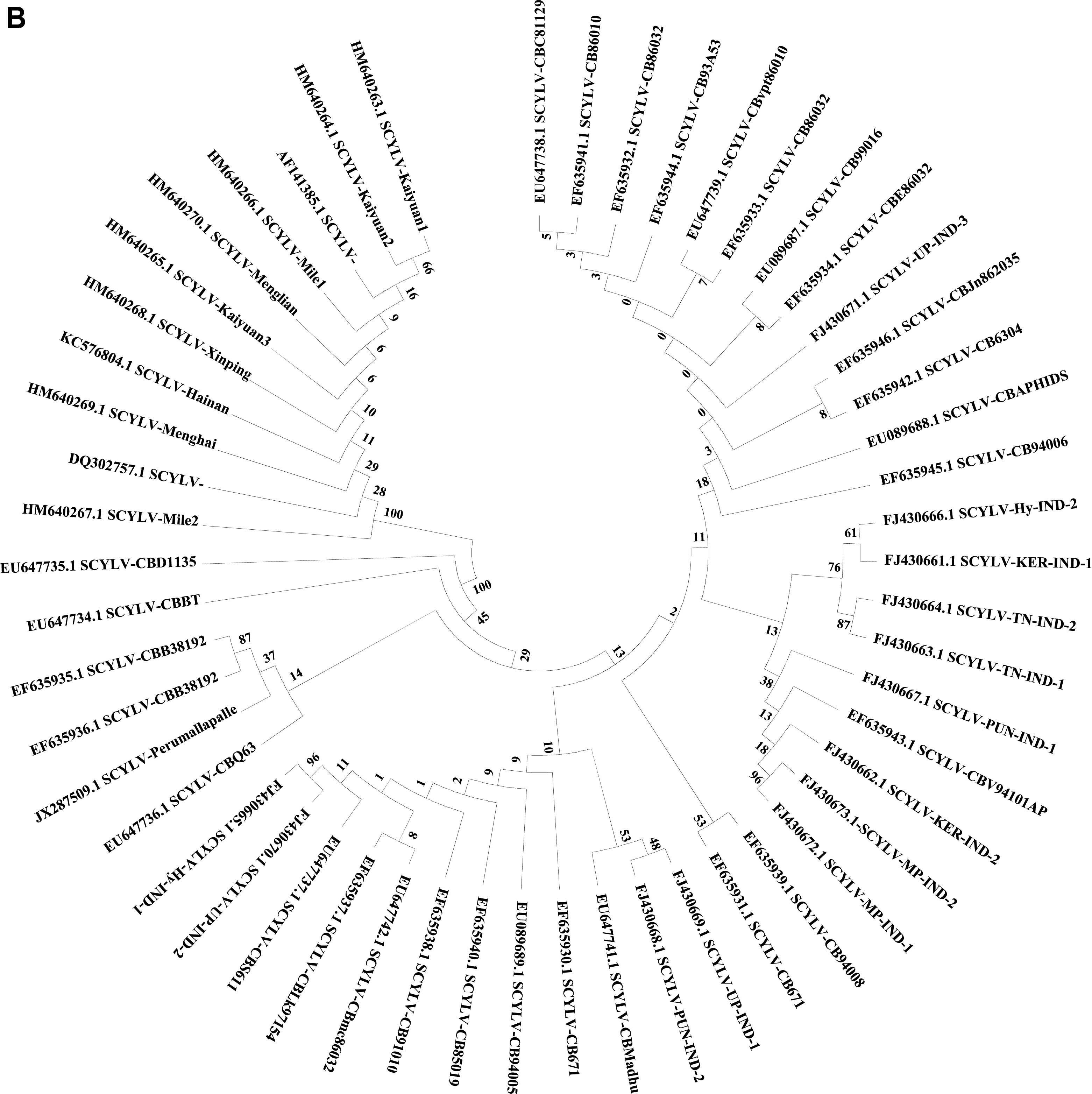
Evolutionary analyses of Sugarcane yellow leaf virus (SCYLV) genotypes originating from different countries from sugarcane (10 genotypes) and sorghum (three isolates) (A) were conducted in MEGA6 (Tamura et al., 2013). The evolutionary history was inferred using the neighbor-joining method (Saitou and Nei, 1987). The optimal tree with the sum of branch length = 1.89755463. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000) are shown next to the branches (Felsenstein, 1985). The evolutionary distances were computed using the Poisson correction method (Zuckerkandl and Pauling, 1965). Total 36 virus isolates with 6,700 positions in the dataset. (B) Evolutionary analyses of the 53 SCYLV isolates originating from different countries from sugarcane based on coat protein were conducted in MEGA6 (Tamura et al., 2013). The evolutionary history was inferred using the neighbor-joining method (Saitou and Nei, 1987). The optimal tree with the sum of branch length = 0.15509519. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000) are shown next to the branches (Felsenstein, 1985). The evolutionary distances were computed using the Poisson correction method (Zuckerkandl and Pauling, 1965) with 591 positions in the dataset. MU, Mauritius; REU, Reunion; HN, Hainan-China; GZ, Guizhou-China; CP, Un-named-USA; IND-GKP, Gorakhpur-India; IND1-4, Coimbatore-India; FLA1-2, Florida-USA; CUB, Cuba; COL, Colombia; CHN-FJ1, Fujian-China; CHN-HN1, Hainan-China; CBLK, Lucknow-India; PER, Peru; BRA, Brazil; HAW, Hawaii-USA; Sorg1-3, Sorghum-USA.
Initially, existence of three SCYLV genotypes including REU, BRA, and PER were reported by Ahmad et al. (2006a) and found distributed among eight virus isolates from different countries. In addition to this, another isolate from Cuba, was partially characterized which showed 77% to 80% homology based on amino acids sequence in ORF1 with virus isolates originating from these three genotypes. Therefore, the Cuban isolate was designated as CUB, a separate genotype (Ahmad et al., 2006b). Based on the phylogenetic relationships, genotypes PER and BRA showed close relationships. Later, both the PER and BRA were pooled and designated them as BRA-PER genotype (Ahmad et al., 2006b).
Subsequently, Viswanathan et al. (2008) characterized four SCYLV isolates from India. These four isolates (SCYLV-IND) showed amino acids sequence differences with those from REU, PER, and BRA in partial ORF0 sequence and therefore the Indian genotype was designated as IND. Phylogenetic analysis showed a separate lineage for IND isolates. Based on sequence information of ORF1 and ORF2, Indian isolates showed that the YLD sugarcane in India is known to be caused by either of the three genotypes, viz., CUB, IND and BRA-PER. Most of the isolates were found infected by CUB genotype followed by IND and BRA-PER genotypes. Subsequently, whole genome of four SCYLV isolates were characterized from India and showed the separate lineage for Indian isolates based on the phylogenetic analyses of the partial ORF0, ORF1 and ORF5 (Chinnaraja et al., 2013).
Genome sequence of 36 virus isolates and its aa level comparisons showed that the IND isolates shared 86.5% to 86.7% sequence identity with the virus isolates belonging to CHN1 genotype. Whereas, 88.6% to 90.4% identity with the Cuba isolates (CUB) and 88.1% to 90.1% with the COL isolates. Whereas, the least sequence identity was obtained with the isolates originating from REU, BRA, and HAW which showed 69.0% to 73.5%, 69.0% to 70.1% and 70.7% to 71.9%, respectively. Moreover, IND1, IND2, IND3, and IND4 shared closest sequence homology with the other Indian isolates which ranged from 92.3% to 93.5% (Supplementary Table 1). Phylogenetic analyses of the 36 virus isolates based on the complete genome information, six Indian isolates showed close relationship and clustering with the CHN, CUB, COL, and FLA (Florida) isolates. The IND3 isolate showed a separate cluster with distinct isolates as compared to other Indian isolates (Fig. 4A). Three virus isolates from sorghum showed separate clustering with the isolates belonging to HAW genotype (Fig. 4A). At present, CP sequence information of 53 virus isolates is available in GenBank, all Indian isolates showed 97%-100% aa sequence identity (data not shown). The phylogenetic analyses of these 53 isolates showed three separate clades of India and China isolates (Fig. 4B).
Mechanism of Plant-Virus Interaction
Plant viruses are obligate organisms and depend upon the host cell protein synthesizing components for their persistence and replication. SCYLV is an aphid borne viral pathogen. Once the virus particles are inoculated by insects in the sugarcane plants, virus proliferates, replicates and moves from one cell to another through plasmodesmata and from one part to another and produce the symptoms on foliage. SCYLV encodes six various proteins, and their interactions ensure transmission by insects and persistence in plant. Presently, least information available on specific site of host-virus protein interaction though such evidences are essential for devising virus management strategies. Some of the host and Polerovirus (PLRV) proteins interactions were studied (Baumberger et al., 2007; Pazhouhandeh et al., 2006; Reinbold et al., 2013; Rodriguez-Medina et al., 2015), however, they do not provide any evidence on direct interactions. Recently, direct protein-protein interactions studies were conducted by application of the protein interactions reporter, a novel technology and demonstrated how PLRV utilize host proteins throughout plant infection (De-Blasio et al., 2016). Further, such studies are required for SCYLV and host interactions and prior to that the efficient SCYLV infectious clone development needs to be worked out.
Recombination, Mutation, and Evolution of SCYLV
Genetic diversity analysis showed that SCYLV evolved through RNA–recombination between the species of three genera i.e., Luteovirus, Polerovirus and Enamovirus (D’Arey and Domier, 2005; Aaziz and Tepfer, 1999; Moonan et al., 2000; Smith et al., 2000). Previous studies on recombination in SCYLV genome (Chinnaraja et al., 2013; ElSayed et al., 2014, 2018; Lin et al., 2014) revealed that recombination is a dominant feature for evolution of SCYLV and is much similar to that of other RNA viruses (Ohshima et al., 2007). Moreover, recombinations among the 36 SCYLV complete genomes were detected using seven different programs executed in recombination (Martin et al., 2015). Selection pressure analysis was carried out based on SCYLV complete genomes using maximum likelihood model in the phylogenetic analysis (Yang, 2007). Four different procedures executed in Datamonkey Adaptive Evolution Server (http://www.datamonkey.org) were also utilized to calculate (dN: Non synonymous) and (dS: Synonymous) ratio of every codon. Recombination hot spots reported throughout the 17 complete genomes of SCYLV and revealed that it is a prominent attribute for evolution, similar observations were also recorded from different countries (Supplementary Table 2) (Chinnaraja et al., 2013; ElSayed et al., 2014, 2018; Lin et al., 2014).
Analysis of selection pressure among 36 virus isolates stated that to a very great degree different codons of complete genomes were under neutral or negative selection except for three codons (255, 249, and 225). The quantitative relationship between (dN: Non synonymous) and (dS: Synonymous) per codon site was calculated by PAML 4.0, which gave value of 0.162, suggesting neutral or negative selection. Further, each individual nucleotide was tested statistically using SLAC (Single-Likelihood Ancestor Counting), REL (Random Effects Likelihood), FEL (Fixed Effects Likelihood), and MEME (Mixed Effects Model of Evolution) methods available from Adaptive Evolution Server and were showed to be negative (P < 0.05), suggesting a very robust purifying selection (data not shown) (Kondrashov, 1988). Based on the mutational deterministic hypothesis (Kondrashov, 1988), mutation is largely deleterious, creating mutational loads and causing existence of isolates that have many slightly deleterious mutations. Twenty-eight recombination events discovered in this study can be explained by this hypothesis; recombination hot spots along with very strong purifying selection may enhance the speed of complete removal of deleterious mutations in the SCYLV genes as described earlier in case of helper component proteinase genes of SCSMV (Bagyalakshmi et al., 2012). The maximum recombination of major and minor parents contributed in the Indian SCYLV isolates (Supplementary Table 2) in the genetic recombination with different SCYLV genotypes suggests an ancestral Indian origin of SCYLV.
Mechanism of Virus-Vector Interaction
SCYLV was believed to be transmitted by three species of aphids viz., Melanaphis sacchari (Zehntner), generally known as sugarcane aphid, Rhopalosiphum maidis (Fitch) known as corn leaf aphid and R. rufiabdominalis known as rice root aphid in Hawaii (Edon-Jock et al., 2007; Schenck and Lehrer, 2000). Later, it was confirmed that only M. sacchari transmits the virus in Hawaii. Initial spread of the virus occurs through infected seed cane (Viswanathan et al., 2006, 2008). Numerous investigations have revealed the secondary transmission capability and effectiveness that M. sacchari is the prominent vector to SCYLV (Ahmad et al., 2007; Lehrer et al., 2007; Rassaby et al., 2004; Scagliusi and Lockhart, 2000). The virus resides in phloem parenchymatous tissues of plants and spread by insect-vectors (aphids) in a persistent, circulative, and nonpropagative means and cannot be transmitted by artificial sap inoculation (Rochow, 1982). Incidence of M. sacchari and its transmissibility was reported from different locations including Mauritius (Behary Paray et al., 2011), Guadeloupe (Francki et al., 1985), and Louisiana (McAllister et al., 2008). In China, natural occurrence and transmission of SCYLV by Ceratovacuna lanigera was studied (Zhou et al., 2006). Among these, M. sacchari is prominent vector transmitting SCYLV in sugarcane worldwide (Rott et al., 2008). Transmissibility of SCYLV is preserved for the complete life of aphids and not even lost during their molting.
Chinnaraja and Viswanathan (2015) studied the virus transmission by inoculating micro-propagated virus-free plantlets of sugarcane cv. Co 86032 with M. sacchari, which harbours SCYLV. Further, virus transmission was confirmed through RT-PCR and RT-qPCR assays. Natural occurrence of sugarcane aphid colony was observed on sugarcane cv. Co 419 in the experimental plot at Indian Institute of Sugarcane Research (IISR), Lucknow, India (Fig. 1A).
To transmit and identify the new hosts under greenhouse conditions numerous investigations were demonstrated on SCYLV transmission by aphids in different crop plants viz., barley (Hordeum vulgare L.), oats (Avena sativa L.), corn (Zea mays L.), wheat (Triticum aestivum L), and rice (Oryza sativa L.). Results revealed that 90% SCYLV transmission was obtained in the inoculated seedlings of wheat, oats and barley, while 10% virus transmission was obtained in the inoculated rice and corn plants by M. sacchari (Schenck and Lehrer, 2000). Moreover, natural transmission on these hosts does not occurs when nearby SCYLV infected sugarcane fields are available (Komor, 2011). Recently, three inherently dissimilar haplotypes of M. sacchhari were reported from United States including haplotype one (H1) infecting Sorghum species and sugarcane whereas, H3 haplotype known to occur on sugarcane, sorghum and Johnsongrass (S. halepense) and H6 haplotype colonizes both sugarcane and Johnsongrass (Nibouche et al., 2018). Moreover, from Florida, lack of efficient transmission of SCYLV from sugarcane and Columbus grass to sugarcane has been shown by M. sacchari and mites (Oligonychus grypus), suggesting the SCYLV vector needs further identification (Boukari et al., 2020).
Polerovirus members transmit the virus by circulative, persistent and non-propagative manner, therefore do not replicate in insect-vectors but circulates into the insect gut, hemolymph and salivary glands for further transmission (Bragard et al., 2013; Pinheiro et al., 2015). Members of this genus are phloem-limited, therefore insect-vectors need extensive feeding for effective transmission from one plant to another (Gray et al., 2014). Members belonging to the family Luteoviridae follow the transcytotic dissemination pathway (Gutiérrez et al., 2013). After the entry of virions in the aphid mouthparts, are moved through foregut, midgut and hindgut. Initially, virion interactions occur with the epithelial cells of gut having receptors which facilitates the adherence on midgut and or hindgut via endocytosis. Afterwards, with the help of exocytosis phenomenon virus particles travel to the haemocoel, which passes through, subsequently, virions reach to salivary glands for its transmissibility via saliva while probing for the plant sap (Garret et al., 1996; Gildow, 1993; Gray et al., 2014). Transmission and acquisition of the members of family Luteoviridae is very specific and mediated by major and minor CP and RTP (Gray et al., 2014; Peter et al., 2008). CP is essential for the translocation of virions from gut to haemocoel whereas, RTP is prerequisite for interaction and movement by the membranes of salivary glands (Bruyère et al., 1997). A schematic representation of the phenomenon of virus-vector interaction of SCYLV is presented (Fig. 5). Moreover, it has anticipated that the role of endosymbionts in the haemocoel cannot be avoided for virion acquisition and transmission processes, still this needs further studies to pin-point their direct or indirect functions.
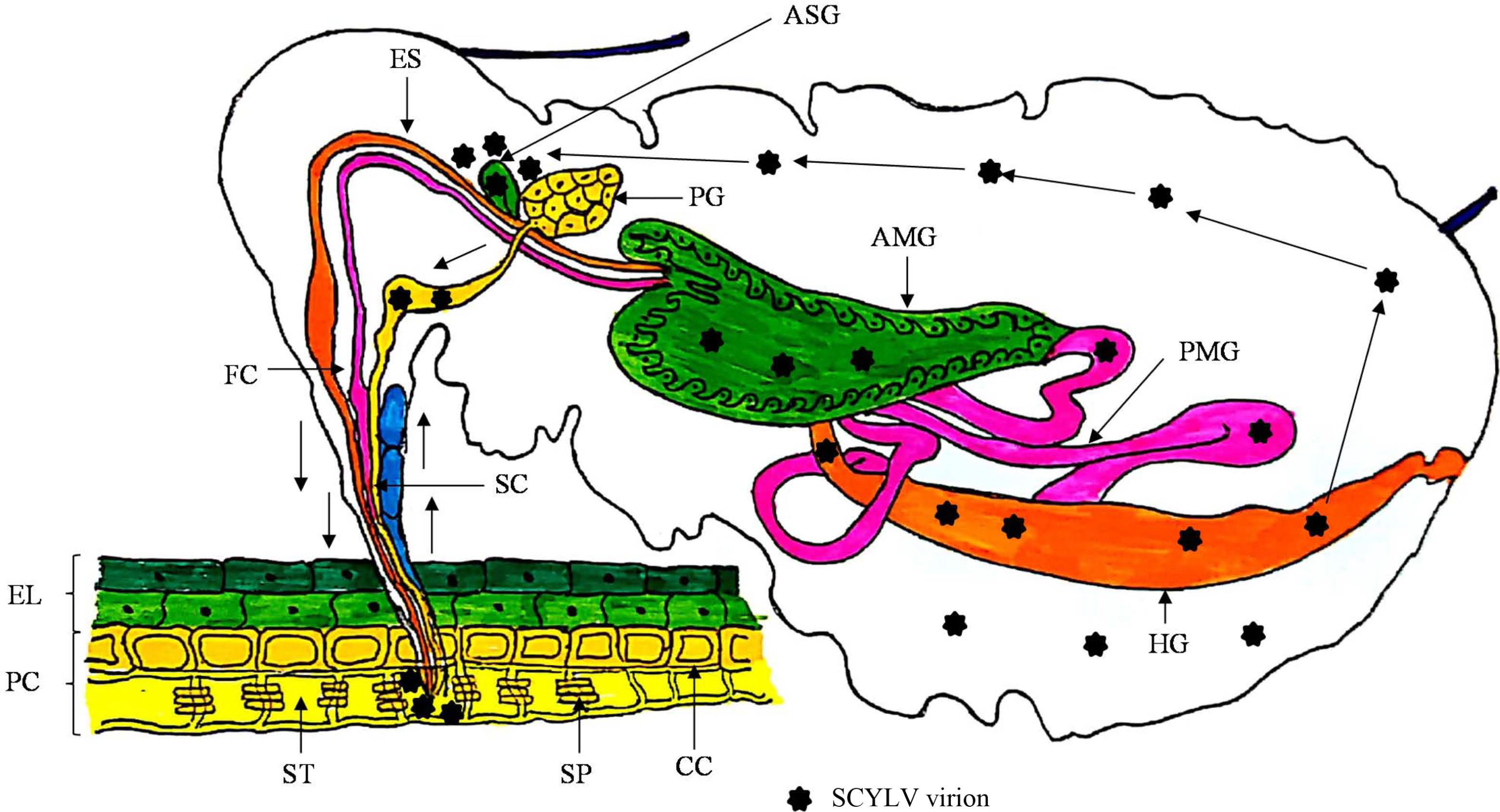
Mechanism of Sugarcane yellow leaf virus (SCYLV) transmission by aphids in a persistent circulative, and non-propagative manner with piercing and sucking type of mouth parts. The general anatomy of the aphids is shown with the alimentary canal, salivary system and host cells with the following labels. AMG, anterior midgut; ASG, accessory salivary glands; CC, companion cells; EL, epidermal layer of the host; ES, esophagus; FC, food canal; HG, hindgut; PC, parenchyma cells; PG, principal salivary glands; PMG, posterior midgut; SC, salivary canal; SP, sieve plate; ST, sieve tube cell.
Strategies for Management of SCYLV
Managing SCYLV is difficult due to its vector-borne nature and transmission through infected seed cane. Disease severity varies from different varieties cultivated in different agro-climatic conditions worldwide. Nevertheless, integrated management strategies including cultural, chemical, biological, and other conventional strategies including identification of sources of resistance and breeding for disease resistance and non-conventional approaches including pathogen derived resistance, RNA silencing, miRNA and CRISPR/Cas (Clustered Regularly Interspaced Palindromic Repeats) needs to be adopted.
Conventional Management Strategies
Healthy seed cane production by three-tier system must be emphasized for enhanced sugarcane productivity in India (Singh and Singh, 2015). Wide-row spacing and early planting can alleviate the impact of YLD (Palaniswami et al., 2014; Viswanathan et al., 2017a). Multiple vegetative propagation of single seed should not be practiced. Moreover, varietal purity needs to be maintained to avoid any admixture of other YLD susceptible varieties. Disease surveillance through remote sensing technique has been recommended for the monitoring and identification of YLD affected sugarcane fields (Palaniswami et al., 2014; Viswanathan et al., 2017a). Yellow sticky traps can also be found effective against management of sugarcane aphids (Satyagopal et al., 2014).
Biological control of aphid vectors could possibly reduce the widespread occurrence and spread of YLD in sugarcane. It has been demonstrated that 45% reduction in aphids was achieved due to the practise of application of grey fungus Verticillium lecanii (Hall, 1987). Moreover, some predators have been showed very efficient bio-control agent for M. sacchari infesting sugarcane including-Olla v-nigrum (Mulsant), Allograpta exotica (Wiedemann), Coleomegilla maculate fuscilabris (Mulsant), Hippodamia convergens (Guerin), Diomus terminates (Say), Lysiphle bustestaceipes (Cresson), Micromus subanticus (Walker), Chrysoperla externa (Hagan), and Cycloneda sanguinea (L.) (Hall, 1987, 1988; White et al., 2001). Application of 0.05% solution of dimethoate 30 EC was found to be effective against M. sacchari with the reduction up to 83.3% of sugarcane aphid population. Whereas, application of endosulfan 35 EC at 0.07%, monocrotophos 36 WSC at 0.04% or chlorpyriphos 20 EC at 0.05% can efficiently control aphid population up to 80.2%, 79.7%, and 77.5%, respectively (Balikai, 2004; Viswanathan et al., 2017a). However, application of insecticide sprays to manage aphids is not feasible when crop in field is more than five to six months old, for which automatic aerial sprays are helpful.
In order to develop virus resistant genotypes, identification of source of resistance is the prerequisite, therefore, Lehrer and Komor (2008), developed 0-6 disease rating scale for evaluation of sugarcane genotypes against YLD under field conditions. Later, Chinnaraja and Viswanathan (2015) developed a rating scale 0-5 based on varying disease symptoms under field studies to find out the sources of resistance against YLD. Subsequently, Viswanathan et al. (2016) screened large number of genotypes (more than 4,000) and recognized 463 SCYLV resilient genotypes from different clones while 773 from the Saccharum spp. Recently, Kumar et al. (2020) screened 189 sugarcane genotypes from tropical and sub-tropical regions of India using a similar 0-5 rating scale. Among the screened genotypes, 95 genotypes showed moderately susceptible to susceptible to YLD while, 94 genotypes showed resistant against YLD. Thus, these findings laid foundation for the improvement of YLD resistant sugarcane progenies in India.
Apical meristem tip culture, auxiliary bud culture and leaf roll callus culture techniques have been found effective in the management of YLD in commercial and noble sugarcane cultivars (Chatenet et al., 2001; Fitch et al., 2001; Parmessur et al., 2002). Meristem tip culture and auxiliary bud culture are the most advantageous and common methods for virus elimination from all susceptible varieties, due to an advantage of the fact that, meristematic tissue remains free from virus (Fitch et al., 2001; Parmessur et al., 2002). Fitch et al. (2001) described that plantlets developed from meristematic tips, where the 1-2 mm explants formed healthy plants and that remained SCYLV free for four years, despite the fact that they were grown in isolated fields or in the glasshouse condition. Meristem tip culture technique using 0.3 mm meristem tip are useful to develop 64% of the disease-free plantlets (Fitch et al., 2001). Leaf rolls callus culture is also most efficient technique which can be used for elimination of virus. Callus resultant from early growing leaf-rolls found 100% removal of SCYLV without any abnormal growth in any of the plantlets (Chatenet et al., 2001; Guiderdoni and Demarly, 1988; Parmessur et al., 2002).
Viswanathan et al. (2018) has efficiently validated impact of virus-free planting materials of sugarcane variety, Co 86032 through meristem tip culture technique. Thus, use of improved diagnostic techniques and production of virus-free plants through meristem tip culture have been found as a feasible strategy to manage YLD and it facilitates continuous supply of healthy seedlings to the sugarcane growers for enhanced and sustained sugarcane productivity in India. Production of disease-free sugarcane seedlings is the prerequisite for enhanced production and productivity. Therefore, tissue culture through meristem tip culture technique provides a best platform for the raising of virus-free and genetically uniform plantlets.
Currently, nested PCR and RT-PCR techniques have been routinely followed for of phytoplasma detection and virus-indexing, respectively infecting sugarcane. Tissue culture producing commercial ventures in India are routinely being followed the virus-indexing and genetic fidelity services from accredited test laboratories (ATLs) established under the national certification system for tissue culture-raised plants system of Department of Biotechnology, Govt. of India, in collaboration with Biotech Consortium India Limited (BCIL), New Delhi for the production and distribution of virus-free and true-to-type seedlings to the growers in India. For its successful implementation DBT has identified two referral centres and five ATLs which certify the tissue culture produced plants based on results of virus indexing and genetic fidelity testing in sugarcane and other crops. One of such ATL’s at ICAR-IISR, Lucknow and ICAR-Sugarcane Breeding Institute (SBI), Coimbatore displayed a key role in the qualification of tissue culture-raised sugarcane plantlets which were produced by different production units (Holkar et al., 2016b; Kumar et al., 2017; Viswanathan et al., 2017a).
Next Generation Strategies for Management of SCYLV
Vegetative propagation of sugarcane favours accumulation of SCYLV and becomes difficult to manage by conventional approaches. Further, no genes have been identified within the Saccharum gene pool that confers resistance to SCYLV. To develop virus-resistant transgenic plants, RNA and protein mediated resistance have been the fundamental rule. These approaches have originated from the impression of pathogen derived resistance (PDR) (Sanford and Johnston, 1985). In case of PDR, RNA or pathogen-coded proteins are utilized for preventing important stages in the infection phase of the pathogen. Transgenic resistance based on RNA interference (RNAi) has already proved in sugarcane based on truncated CP gene of SrMV (Ingelbrecht et al., 1999) and SCYLV (Zhu et al., 2011).
Insufficient SCYLV resistance sources limit the conventional resistance breeding program. Gilbert et al. (2009) conducted a field study in Belle Glade, Florida for the evaluation of level of SCYLV resistance in transgenic lines viz., 6-1, 6-2 and also for their agronomic performance in comparison with the parent genotype CP 92-1,666 (SCYLV susceptible). Further, characterized the genetic changes in the 6-1 and 6-2 lines in comparison with the parental cultivar using simple sequence repeat (SSR) genotyping. Therefore, the study was considered to be the first successful gene transfer for SCYLV resistance in sugarcane and also first to report variation in microsatellite markers amalgamated with regeneration from embryogenic callus. An RNAi expression vector harbouring CP gene fragment of SCYLV was constructed based on the complete genome sequence of SCYLV Hainan isolate (GenBank accession no. HQ342888). The RNAi vector p2300-CP-F-R containing a hairpin structure was confirmed and transformed into 12 tobacco lines by Agrobacterium-mediated transformation system. The CP gene was integrated into the genome of tobacco (Zhang et al., 2011). This work constructed the base for breeding of plant mediated RNAi technology in sugarcane against disease.
Similarly, efforts have been made on the use of nucleotide binding sites-resistance gene analogue (NBS-RGA) markers and kinase analogues from soybean and wheat to amplify DNA from sugarcane. Sugarcane cultivar US01-1158 was recognized as resistant against SCYLV and moderately resistant against rust pathogen (Puccinia melanocephala) (Glynn et al., 2008). Moreover, certain studies have explored the genetic variability of resistance against SCYLV in sugarcane germplasm, Saccharum spontaneum and S. barberi, and other genera viz., Miscanthus spp. and Erianthus spp. showed the least disease incidence (Comstock et al., 2001; Komor, 2011).
The tolerant and resistant phenotypes are generally governed by several genes and are expressed as quantitative trait loci (QTLs). QTL mapping studies in Saccharum hybrids identified is more difficult than other crops due to the complex genome of sugarcane because of its ploidy level, large size genome and interspecific origin. Therefore, it is a challenge for genetic studies. First such major quantitative major allele was tagged for resistance against SCYLV known as Ryl1 relied on QTL by bi-parental progeny of a resistant clone and a susceptible cultivar (Costet et al., 2012). Izquierdo et al. (2013) generated genetic map of sugarcane to detect molecular markers linked with resistance to SCYLV. Total 148 progenies were obtained from the cross between CC 84-75× RD 75-11, SCYLV susceptible and resistant respectively, and were used for mapping. A genome wide association study was also carried out to detect resistant markers (polymorphic diversity arrays technology [DArT] and amplified fragment length polymorphism [AFLP]) in sugarcane against SCYLV in different sugarcane germplasm (Debibakas et al., 2014; Yang et al., 2019). A total of 1465 polymorphic bands were revealed using microsatellite, AFLP and DArT markers. The identification of SCYLV was assessed using tissue-blot and RT-PCR. The integrated genetic map of the two cultivars had 122 linkage groups and coverage of 8.560 cM. The genetic linkage analyses allowed the confirmation of a putative resistant gene to the SCYLV at 16.3 cM of one AFLP marker (Debibakas et al., 2014). Further, SCYLV resistance studies were demonstrated using routine molecular markers including DArT, SSR, restriction fragment length polymorphism, and AFLP (Costet et al., 2012; Debibakas et al., 2014). For the characterization genetic base of resistance against SCYLV in sugarcane, Yang et al. (2017) constructed a genetic map having high-density consisting of 4,607 markers based on genotyping by sequencing of a segregating F1 progeny (parents: CP95-1039 and CP88-1762) and this population also evaluated for SCYLV reaction. Similarly, genomic maps were generated using GBS based markers for CP95-1039 and CP88-1762 by following a pseudo-testcross method. In this study, two QTLs were identified for resistance against SCYLV, among them QTL qSCYLR79 associated with marker 3PAV3154 showed to be specific for SCYLV resistance (Islam et al., 2018). Recently, You et al. (2019) developed an Axiom Sugarcane 100K single nucleotide polymorphism display and magnificently employed to develop the genetic map of sugarcane and to recognize the QTL linked with SCYLV resistance. This arrangement was used to genotype 469 sugarcane clones, having one F1 (parents: Green German and IND81-146), one selfing populations obtained from CP80-1827 and 11 different sugarcane stocks as controls. The genotyping revealed 18 QTLs controlling SCYLV resistance segregating in the two mapping populations, harbouring 27 genes for disease resistance.
Conclusions and Perspectives
An unusual occurrence of SCYLV on sugarcane during 1960s in the form of “yellow wilt” in Tanzania was followed by its further reemergence and widespread distribution in Hawaii, Brazil and subsequently in 25 sugarcane cultivating countries due to its primary and secondary transmission by seed cane and aphid vectors, respectively. Due to severe occurrence of YLD on many sugarcane varieties led up to 50% crop loss in Brazil followed by 37% in Reunion Island, 30% in Thailand and 15% in United States. In India, many sugarcane genotypes have been found degenerated due to its widespread occurrence. SCYLV has been mainly infecting sugarcane, grain sorghum and Columbus grass. Worldwide, during the last twenty years notable research has been performed on diagnosis using recent molecular techniques, genome characterization, genetic diversity, and management by producing healthy seed cane through meristem tip culture and three-tier seed production programme. To address the severe loss in yield due to SCYLV, disease rating scales 0-6 and 0-5 are available and therefore, identification of the sources of resistant genotypes from tropical and sub-tropical condition have been worked out. The systematic studies on virus-vector transmission, presence of virus in asymptomatic plants have been achieved. IDM approaches for management of the disease need to follow to reduce the crop loss.
In the recent years, our understanding of SCYLV has enhanced substantially, nevertheless many queries remain unaddressed on plant-virus interactions, epidemiology, identification of resistant genes and management using CRISPR/Cas technology. The rapid and widespread global occurrence of SCYLV underlines the importance for developing more effective management measures, with considering the possible changes in the virus genome by recombination and evolution. Much efforts are required in identification of resistant genes by mining different genotypes and their subsequent use in developing total resistance against SCYLV. Early detection of the virus in seed cane or in the nursery seedling is the first phase towards devising effective virus management steps, and the virus transmissibility through seed cane in the plated crop is very difficult and more challenging. Virus spread by aphid vectors is manageable at the early stages of the crop, after six months age of the crop the practical application of insecticide becomes more difficult. Therefore, to address this issue, certain aerial drone-based sprayers must be developed and which would be having advantage of managing other insect-pests and diseases of significance in sugarcane. Nevertheless, systematic studies are required to ascertain the existence of other possible aphid species naturally transmitting SCYLV in sugarcane, sorghum and other members belonging to Poaceae family needs to be explored. Genetic engineering has been proved effective against the virus management but due to policy issues it is limited to the research field. Further, development of virus-vectors and virus management using novel techniques like CRISPR/Cas mediated resistance necessarily to be addressed in near future.
Electronic Supplementary Material
Supplementary materials are available at The Plant Pathology Journal website (http://www.ppjonline.org/).
Acknowledgments
Authors are thankful to Dr. A. D. Pathak, Director, ICARIISR Lucknow and Dr. B. Ram, Director, ICAR-SBI, Coimbatore for their constant support and encouragement for writing this review article. No any financial support was received.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.