Field Sanitation and Foliar Application of Streptomyces padanus PMS-702 for the Control of Rice Sheath Blight
Article information
Abstract
Rice sheath blight (ShB), caused by Rhizoctonia solani Kühn AG1-IA, is one of the destructive rice diseases worldwide. The aims of this study were to develop biocontrol strategies focusing on field sanitation and foliar application with a biocontrol agent for ShB management. Streptomyces padanus PMS-702 showed a great antagonistic activity against R. solani. Fungichromin produced by S. padanus PMS-702, at 3.07 mg/l inhibited 50% mycelial growth, caused leakage of cytoplasm, and inhibited the formation of infection structures of R. solani. Fungichromin could reach to 802 mg/l when S. padanus PMS-702 was cultured in MACC broth for 6 days. Addition of 0.5% S. padanus PMS-702 broth into soil decreased the survival rate of the pathogen compared to the control. Soil amended with 0.5% S. padanus broth and 0.5% tea seed pomace resulted in the death of R. solani mycelia in the infested rice straws, and the germination of sclerotia was inhibited 21 days after treatment. Greenhouse trials revealed that S. padanus cultured in soybean meal–glucose (SMGC-2) medium after mixing with different surfactants could enhance its efficacy for inhibiting the pathogen. Of six surfactants tested, the addition of 2% tea saponin was the most effective in suppressing the pathogen. S. padanus broth after being fermented in SMGC-2, mixed with 2% tea saponin, diluted 100 fold, and sprayed onto rice plants significantly reduced ShB disease severity. Thus, S. padanus PMS-702 is an effective biocontrol agent. The efficacy of S. padanus PMS-702 for disease control could be improved through formulation.
Rice (Oryza sativa L.) is the major staple crop in many Asia-Pacific countries (Food and Agriculture Organization of the United Nations, 2020). The Food and Agricultural Organization’s Statistics (2020) reported that 150 million hectares of rice are cultivated and 782 million tonnes of rice are harvested, which constitute 9% of world crop production. China, India, and Indonesia are the top three main producers of paddy rice (Food and Agriculture Organization of the United Nations, 2020). Rice sheath blight caused by Rhizoctonia solani Kühn AG1-IA, is one of the destructive diseases in rice fields worldwide (Lee and Rush, 1983). It could cause 31-43% yield loss (Tsai, 1975). Chang (1986) also reported a correlation between the disease severity of rice and yield, and suggested the disease severity of sheath blight in scales of 9 disease index which was referred to the Standard Evaluation System for rice by International Rice Research Institute would result in 27-48% loss of rice yield (Chang, 1986). The pathogen infects all growing stages of rice, especially in the tillering and grain filling stages (Lee and Rush, 1983). The soilborne sclerotia and mycelia in plant debris are the main survival structures and the primary inocula (Lee and Rush, 1983). Strategies used to manage rice sheath blight consist of cultural manipulations, host resistance, fungicides, and rotation schemes (Lee and Rush, 1983). However, the control efficacy is limited for the field sanitation practice because sclerotia could survive more than 2 years in the temperate rice production fields (Lee and Rush, 1983).
Streptomyces species is the largest genus of Actinobacteria and the type genus of the family Streptomycetaceae (Kämpfer, 2006). They produce over 61% of the clinically and agriculturally essential antibiotics (Waksman et al., 2010). Various studies have been conducted to explore the potential of using Streptomyces species as biocontrol agents for controlling plant diseases or biofertilizers to enhance plant growth and yield (Buzón-Durán et al., 2020; Sharma et al., 2020). The molecular mechanisms of Streptomyces species contributed to plant beneficial roles involving the production of chitinases, glucanases, excretion of antifungal metabolites and plant growth regulators, induction of plant immune responses, acting as a mycoparasite of fungal root pathogens, and modulation of enzymatic and defense pathways (Mun et al., 2020; Sharma et al., 2020). The findings by Araujo et al. (2019) revealed that application of biocontrol Streptomyces strains modulated the wheat root microbiome in R. solani-infested soils by decreasing Paenibacillus and increasing other bacterial and fungal operational taxonomic units. However, there are limited commercial biopesticide products consisting of live Streptomyces species available (Gwynn, 2021; Sharma et al., 2020). The known commercial biopesticides include Mycostop which is composed of Streptomyces griseoviridis strain K61 (Gwynn, 2021; Palaniyandi et al., 2013), and Actinovate, Micro108 or Actino Iron which contain Streptomyces lydicus strain WYEC 108 (Gwynn, 2021; Yuan and Crawford, 1995).
Biocontrols of rice sheath blight by antagonistic microorganisms including Bacillus species (Kakar et al., 2018; Peng et al., 2014; Shrestha et al., 2016), Streptomyces species inclusive of Streptomyces aurantiogriseus VSMGT1014, Streptomyces philanthi RM-1-138, and Streptomyces padanus JAU4234 (Boukaew and Prasertsan, 2014; Harikrishnan et al., 2014; Xiong et al., 2013) and Trichoderma species (Chen et al., 2015) have been reported. Recent study indicated that antifungalmycin N2 produced by Streptomyces sp. N2 induced defense responses in rice seedling against R. solani infection (Zhang et al., 2020). S. padanus PMS-702 originally isolated from soils in Taiwan showed antagonistic activities against several pathogenic fungi, but not bacteria (Shih, 2003). Our previous findings also indicated that S. padanus PMS-702 culture broth in soybean meal-glucose medium suppressed several plant diseases including lettuce brown spot, Chinese cabbage anthracnose, mango anthracnose, orange green mold, peach fruit rot, tomato late blight, and Rhizoctonia damping-off of cabbage (Shih, 2003). The major antibiotic effectively suppressing S. padanus PMS-702 has been identified as fungichromin by nuclear magnetic resonance analysis and mass (fast atom bombardment mass spectrometry) spectral data (Shih, 2003). The potential of S. padanus PMS-702 for the control of rice sheath blight was evaluated and the optimization of culture formula for the enhancement of fungichromin production and control efficacy was investigated in this study.
The utilization of organic amendments for control of soilborne plant diseases has often been considered as a nonchemical pesticide control strategy, and commonly used in agriculture production for their fertility (Bonanomi et al., 2007). Zhou and Everts (2004) reported the suppression of Fusarium wilt of watermelon by soil amendment with hairy vetch. Ascencion et al. (2015) found that soil amendment with dry tissues of Brassica suppressed R. solani dampingoff disease by increasing the Actinomycetes population in soil. Formulated soil amendments with inorganic and organic materials e.g.,: S-H mixture was effective in controlling fusarial wilt of watermelon, radish, and pea, club root of crucifers, Pythium damping-off and Phytophthora blight of cucumber, and bacterial wilt of tomato; SF-21 mixture was able to reduce damping-off of slash pine caused by R. solani, Pythium aphanidermatum and Fusarium moniliforme var. subglutinans, and promoted growth of pine seedlings (Huang and Kuhlman, 1991). Tea seed pomace, a by-product from oil extraction of tea (Camellia oleifera Abel) seeds, contains triterpenoid saponins. Tea seed pomace is commonly used as a molluscide for controlling apple snail in rice fields (Kijprayoon et al., 2014), and has been shown to reduce the disease incidence of cabbage seedling damping-off caused by R. solani AG4 (Kuo et al., 2010; Yang, 2006).
In this study, we aimed to assess the efficacy of biocontrol of rice sheath blight by S. padanus PMS-702, and to optimize the fermentation formula for the control of rice sheath blight by field sanitation and foliar application of S. padanus PMS-702. The soil amendments and surfactants were also evaluated as supplements for the enhancement of biocontrol efficacy by S. padanus PMS-702 fermented broth. The possible mechanisms by which S. padanus PMS-702 displayed an inhibitory effect against R. solani were investigated.
Materials and Methods
Microorganisms and growth conditions
S. padanus PMS-702 was isolated from soil in Taiwan (Shih, 2003) and was routinely cultured on ISP medium No. 4 (ISP4, BD Difco, Franklin Lakes, NJ, USA) at 30°C, 7 days unless otherwise stated. Rhizoctonia solani AG1-IA isolates (RS1-024 and RS1-731) were obtained from Dr. L. C. Chen, Department of Plant Pathology, National Chung Hsing University and was cultivated on potato dextrose agar (PDA, BD Difco) at 25°C, 2 days.
Inoculation methods and pathogenicity assays of rice sheath blight
Rice seedlings was inoculated with R. solani by the method modified from Jia et al. (2007). Briefly, the 3-week-old rice seedlings with three leaves were inoculated with agar discs (9 mm in dia.) of R. solani by tapping the agar discs on the stem bases of the seedlings. The development of symptoms was recorded every 2 days starting at the third day post-inoculation, and the disease index was calculated by the following formula: disease index = the lesion length/the plant height × 9. To make the rice culm as an inoculum of R. solani, the rice culm (3 to 5 cm in length) was soaked in distilled water for 12 h, autoclaved, inoculated with the agar discs (9 mm in dia.) of R. solani isolate RS1-731, and incubated at 25°C, 2 days.
The fermentation formulation for S. padanus PMS-702
To assess the optimal carbon and nitrogen sources for the cultivation of S. padanus, S. padanus was cultured in 2% (w/v) agar (BD Difco) supplemented with 2% (w/v) of each the following carbon or nitrogen sources. Carbon sources included corn starch, potato starch, mung bean meal, malt extract, rice husk, maltodextrin, crab and shrimp shell powder. Nitrogen source included rape seed pomace, peanut pomace, alfalfa seed meal, soybean meal, sesame pomace, tobacco debris, and caster bean pomace. S. padanus was cultured at 30°C, 2 days, and assayed for the antagonistic activity to R. solani isolates. The growth inhibition of R. solani by S. padanus in each medium was calculated by the following formula: inhibition (%) = (growth diameter of the control – growth diameter of the strain PMS-702 treatment)/growth diameter of the control × 100.
To assess the optimal carbon and nitrogen sources for the cultivation of S. padanus, S. padanus was cultured in 2% (w/v) agar (BD Difco) supplemented with 0.5, 1, 1.5, 2, 2.5, or 3% (w/v) of malt extract or alfalfa seed meal at 30°C, 2 days, and were assayed for the antagonistic activity to R. solani.
To optimize the formulation for liquid fermentation of S. padanus, S. padanus was resuspended in 0.05% tween 20 at optical density at 620 nm (OD620) of 1.08 (ca. 4 × 108 cfu/ml), and cultured in broth medium containing 1% (w/v) malt extract, 1% (w/v) alfalfa seed meal and amending with 1% (w/v) each of the plant oils, corn oil, coconut oil, sunflower oil or soybean oil, at 30°C, 120 rpm, 6 days. The rice culms inoculated with R. solani as mentioned above were soaked in the broth culture of S. padanus at 200-fold dilution for 2 h, and assessed the mycelial growth of R. solani on tannic acid differential media (water agar containing 300 ppm tannic acid) (Hsieh et al., 1996). Three rice culms were placed in each broth culture and the experiment was performed with three replications. Additionally, S. padanus was cultured in the broth media containing 1% (w/v) malt extract, 1% (w/v) alfalfa seed meal, and 1% (w/v) corn oil, and supplemented with 0%, 0.05%, 0.1%, 0.15%, 0.2%, 0.25%, and 0.3% (w/v) CaCO3 at 30°C, 120 rpm, 6 days, and the production of fungichromin by S. padanus in each medium was analyzed by high performance liquid chromatography (HPLC).
Quantification of fungichromin by HPLC
The fungichromin in the broth culture of S. padanus was extracted with equal volume of ethyl acetate (5 ml, Sigma, St. Louis, MO, USA). The extraction mixture was sonicated for 15 min, and incubated at 28°C, 100 rpm, 4 h in the dark for liquid-liquid extraction. The ethyl acetate in 1 ml of the supernatant from the extraction mixture was evaporated and the reconstituted in 1 ml of methanol (Merck, Damstadt, Germany) for HPLC analysis. The concentration of fungichromin was quantified by HPLC analysis using the procedure described by Wu et al. (2008). The HPLC system (PU-780, Jasco, Tokyo, Japan) was equipped with a UV/vis detector (UV-970, Jasco) and a C18 reverse phase column (250 mm × 4.6 mm, Phenomenex, Torrance, CA, USA). Separation was performed with a mixture of methanol and water (60:40, v/v) with the flow rate of 0.5 ml/min. The fungichromin standards (Sinon Corp., Taichung, Taiwan) in concentrations of 15, 30, 40, 60, and 75 mg/l were used for constructing the calibration curve for fungichromin quantification.
The effect of fungichromin on the mycelial viability of R. solani
The agar discs (9 mm in dia.) of R. solani RS1-731 cultured on PDA were placed on the sterile glass slides for 2 days. One hundred microliter of 0, 10, 50, and 100 mg/l fungichromin in DMSO was added on the mycelia of R. solani RS1-731 and incubated in the dark for 24 h. Sterile water and 0.1% (v/v) DMSO were used as controls. Then, the treated mycelia were stained using a Live/Dead BacLight Bacterial Viability Kit (L7007, Molecular Probes, Eugene, OR, USA) for 10 min, and observed under a fluorescence microscope equipped with Chroma 31002 - TRITC (Rhodamine) filter. Green and red fluorescent mycelia represented live and dead cells, respectively.
The effect of fungichromin on the electrolyte leakage from hyphae of R. solani RS1-731
Ten agar discs (9 mm in dia.) of R. solani RS1-731 were washed three times with sterile Milli-Q water and respectively put in 50 ml of sterile water, 0.1% (v/v) DMSO, 2 mg/l fungichromin, 5 mg/l fungichromin, or 10 mg/l fungichromin at 30°C, 120 rpm, 3 h. The sterile Milli-Q water without R. solani RS1-731 was used as a control. The conductivity in each treatment was measured using a conductivity meter (Hanna HI8820, Hanna Instruments, Padova, Italy) at a 3-h interval for 21 h. Three agar discs of R. solani RS1-731 in each treatment treated at 21 h post-treatment were washed with sterile water, and mycelial growth of R. solani was assessed on tannic acid differential media (Hsieh et al., 1996).
The effect of fungichromin on the infection structure formation of R. solani RS1-731
Five-day-old rice seedlings were soaked in 0.5% carboxymethyl cellulose salt solution containing 2, 10, 25, or 50 mg/l fungichromin, and 5% carboxymethyl cellulose salt solution was used as a control. Rice seedlings were placed on the glass slides 1 cm apart from the agar discs (9 mm in dia.) of R. solani RS1-731. There were four replications per treatment. After a2-day incubation, rice seedlings were stained with cotton blue and observed using a light microscope for the formation of infection cushions or appressoria by R. solani RS1-731.
Effect of S. padanus PMS-702 fermented broth on the viability of R. solani RS1-731 infested on rice straws
S. padanus was cultured in the malt extract-alfalfa seed meal broth (10 g malt extract, 10 g alfalfa seed meal, 1 g CaCO3 and 10 ml corn oil in 1 l distilled water) at 30°C for 6 days. Infested rice straws were immersed in S. padanus fermented broth at 200-fold or 500-fold dilution for 0 to 48 h, removed, and placed on the tannic acid medium plate. Myceliumgrown out from the rice straws was measured 6 h after incubation. There were three replications per treatment and three infested rice straws were used in each replication. The infested rice straws were immersed in sterile water as a control.
Effect of S. padanus PMS-702 fermented broth on the sclerotia germination of R. solani RS1-731
Sclerotia of R. solani RS1-731 were collected from a 10-day culture on PDA at 25°C. The sclerotia were immersed in S. padanus PMS-702 fermented broth in the malt extract-alfalfa seed meal broth at 200-fold or 500-fold dilution. Ten sclerotia were taken out every day and placed on the tannic acid medium plate and examined for the germination rate of the sclerotia 48 h after incubation. The sclerotia were immersed in sterile water as controls. There were three replications per treatment.
Effect of S. padanus PMS-702 fermented broth in the field soil on the viability of R. solani RS1-731 infested on rice straws
Field soil (1.5 l) in a pot (25 cm × 18 cm × 10 cm) containing 30 g R. solani RS1-731 infested rice straws were mixed with 1.5 l of 0.2 and 0.5% (v/v) S. padanus PMS-702 fermented broth cultured in the malt extractalfalfa seed meal broth at 30°C, 6 days. The sterile water and the malt extract-alfalfa seed meal broth were used as controls. There were three replications per treatment. The pots were incubated at room temperature and the water level was kept in 2 to 3 cm in depth. Ten R. solani RS1-731 infested rice straws were taken out from each treatment every three days in a span of 21 days, and were placed on the tannic acid medium plate for recording the survival of the pathogens at 24 h incubation.
The effect of S. padanus PMS-702 fermented broth amended with tea seed pomace on the mycelial survival of R. solani RS1-731 in the infested rice straws in the field soil
Field soil (1.5 l) with 30 g R. solani RS1-731 infested rice straws were immersed with 0.5% (v/v) S. padanus PMS-702 fermented broth cultured in the malt extract-alfalfa seed meal broth at 30°C, 6 days (1.5 l), and respectively amended with equal volume of 0.2, 0.5, 0.75, and 1% (w/v) tea seed pomace. The sterile water was used as a control. S. padanus PMS-702 was cultured in the malt extract-alfalfa seed meal broth at 30°C, 6 days. Ten infested rice straws were randomly picked from each treatment and placed on the tannic acid medium plate for testing the survival rate of the pathogen at 24 h incubation.
Efficacy of S. padanus PMS-702 fermented broth in SMGC-2 mixed with different types and concentrations of surfactants on the mycelial growth of R. solani RS1-731
S. padanus PMS-702 was cultured in the modified soybean meal–glucose medium (SMGC-2, 11.2 g soybean meal, 11.2 g glucose, 0.46 g CaCO3, and 10 ml coconut oil in 1 l distilled water (Fan, 2017; Fan et al., 2019) at 30°C, 5 days and mixed with equal volume of 4% (v/v) each surfactant including tween 20 (Sigma), tween 80 (Sigma), PAOS detergent (Nice Group, Chiayi, Taiwan), EasyClean detergent (Taiwan Tobacco & Liquor Corp., Taipei, Taiwan), tea saponin (Kingfex Co. Taichung, Taiwan), sapindus extract (Sapberry Biotech. Progress Co., Tainan, Taiwan). SMGC-2 without the addition of surfactant was used as a control. R. solani RS1-731 infested rice straws were immersed in each culture broth at 100-fold dilution for 2 h. Then, the treated rice straws were placed on the tannic acid medium plate for testing the mycelial growth of the pathogen at 16 h incubation. Additionally, S. padanus PMS-702 was cultured in the SMGC-2 with the addition of equal volume of 1%, 2%, 3%, 4%, and 5% (v/v) tea saponin and each culture broth was assessed for the inhibitory ability of R. solani RS1-731 growth from the infested rice straws.
Efficacy of S. padanus PMS-702 in SPT for controlling rice sheath blight
S. padanus PMS-702 was cultured in SMGC-2 and mixed with equal volume of 2% (v/v) tea saponin broth (SPT). The SPT at 100-fold or 200-fold dilution was spray-applied on the rice seedlings with 3 to 4 leaves, then the R. solani RS1-731 was inoculated at the base of the stem as the above described for pathogenicity assay. Alternatively, the SPT at 100-fold dilution was spray-applied on the leaves of rice at tillering-stage for 35 days in the greenhouse once (at day 0), twice (at days 0 and 7), and three times (at days 0, 7, and 14) on rice plants. The disease severity (%) in each treatment was calculated by the following formula (International Rice Research Institute, 2013): Disease severity (%) = [(n0 × 0) + (n1 × 1) + … + (n9 × 9)]/N × 9. Where: n0-n9 is the number of culms in each disease index and N is total number of culms tested. The disease index level: 0 = no lesion, 1 = the appearance of water soaked lesion length less than 20% of the plant height, 3 = 20-30% of the plant height, 5 = 31-45% of the plant height, 7 = 46-65% of the plant height necrosis, 9 = higher than 65% of the plant height.
Statistical analyses
Data were analyzed for significance by analysis of variance (ANOVA), followed by Fisher’s least significant difference (P < 0.05), with the SAS version 6.1 (SAS Institute Inc., Cary, NC, USA).
Results
Pathogenicity assays
Pathogenicity of two R. solani AG1-IA isolates RS1-024 and RS1-731 was evaluated on rice seedlings. The results indicated both isolates showed sheath blight symptoms on the 3rd day of inoculation. Rice seedlings inoculated with isolate RS1-731 had a disease index of 7, which was more virulent than isolate RS1-024 with the disease index ranged from 2 to 3 (data not shown). Thus, R. solani RS1-731 was used for further study.
The fermentation formula for S. padanus PMS-702
Natural substrates including corn starch, potato starch, mung bean meal, malt extract, rice husk, maltodextrin, crab, shrimp shell powder, rape seed pomace, peanut pomace, alfalfa seed meal, soybean meal, sesame pomace, tobacco debris, and caster bean pomace were used to culture S. padanus. The results showed that S. padanus exhibited the best antagonistic activity against R. solani when cultured on agar containing 2% (w/v) malt extract and alfalfa seed meal, showing mycelial growth inhibition of 46-47% and 27-34%, respectively (Table 1). When cultured on agar supplemented with 1% each of malt extract and alfalfa seed meal, S. padanus displayed the highest antagonistic activity against both R. solani isolates (Fig. 1A and B). Additionally, the inhibitory effect by S. padanus PMS-702 was enhanced to 50.6%, 53.1%, and 48.8% when 1% (w/v) corn oil, coconut oil, and sunflower oil, respectively, were amended in the culturing agar plates compared to the control (Fig. 1C). Because corn oil was much cheaper than coconut oil, and sunflower oil, corn oil was selected for further study. HPLC analysis revealed that the concentration of fungichromin produced by S. padanus increased from 194.34 ± 10.66 mg/l to 550.42 ± 11.21 mg/l when S. padanus was grown in malt extract-alfalfa seed meal broth amending with 1% corn oil. Addition of 0.10 % CaCO3 in the malt extract-alfalfa seed meal broth amended with 1% (w/v) corn oil was found to further enhance 26.5% fungichromin production by S. padanus. The concentration of fungichromin produced by S. padanus was 693.64 ± 14.00 and 547.97 ± 5.90 mg/l in the medium with and without 0.10 % CaCO3, respectively (Fig. 1D). Thus, the medium used to culture S. padanus PMS-702 was designated “malt extract-alfalfa seed meal-corn oil-CaCO3 broth, MACC broth”, each liter containing 10 g malt extract, 10 g alfalfa seed meal, 1 g CaCO3 and 10 ml corn oil.

Effect of Streptomyces padanus PMS-702 cultured on different nutrient regimes on the inhibition of mycelial growth of Rhizoctonia solani isolates RS1-024 and RS1-731

Effect of Streptomyces padanus PMS-702 cultured in different compositions of media on the inhibition of mycelial growth of Rhizoctonia solani isolates RS1-024 and RS1-731 and fungichromin production. Strain PMS-702 was cultured in different concentrations of (A) malt extract and (B) alfalfa seed meal which were added in 2% (w/v) agar, and (C) 1% (v/v) malt extract - 1% (v/v) alfalfa seed meal broth amended with 1% (v/v) different plant oils at 200-fold dilution and assessed for their inhibitory ability on the inhibition mycelial growth of R. solani. (D) The production of fungichromin by S. padanus PMS-702 cultured in malt extract-alfalfa seed mealcorn oil broth amended with different concentration of CaCO3 at 30°C for 6 days. Data represented mean and standard deviation of three replicas (error bar) and columns with the same letter are not significantly different (P > 0.05) according to Fisher’s least significant difference test.
Toxicity of fungichromin on the growth inhibition of R. solani
To reveal the putative mechanisms of fungichromin produced by S. padanus PMS-702, experiments were conducted to determine the effect of fungichromin on the mycelial viability of R. solani RS1-731, the electrolyte leakage from hyphae of R. solani RS1-731, and the infection structure formation of R. solani RS1-731 on rice seedlings. Assays for the viability of R. solani using a Live/Dead BacLight Bacterial Viability Kit revealed that the mycelia of R. solani after treating with water (Fig. 2A and B) or DMSO (Fig. 2C and D) showed green fluorescence indicative of live cells. R. solani hyphae treated with 10 mg/l fungichromin treatment showed red fluorescence indicative of dead cells, which contained large vacuoles (Fig. 2E and F).

Effect of fungichromin on the hyphal viability of Rhizoctonia solani RS1-731. Hyphae were treated with distilled water (A, B), 0.1% (v/v) DMSO (C, D), or 10 mg/l fungichromin (E, F) for 24 h. Hyphae in each treatment was stained using a Live/Dead BacLight Bacterial Viability Kit for 10 min, and then observed under fluorescence microscope equipped with Chroma 31002 - TRITC filter. Green and red fluorescent hyphae represented live and dead cells, respectively.
The increase in conductivity of R. solani RS1-731 culture broth with the supplement of 2, 5, and 10 mg/l fungichromin compared to the sterile water and 0.1% (v/v) DMSO was found at 3rd h of treatment, suggesting that fungichromin affected membrane integrity causing electrolyte leakage from hyphae of R. solani RS1-731 (Fig. 3). Notably, fungichromin at the concentration higher than 5 mg/l completely killed R. solani (data not shown).
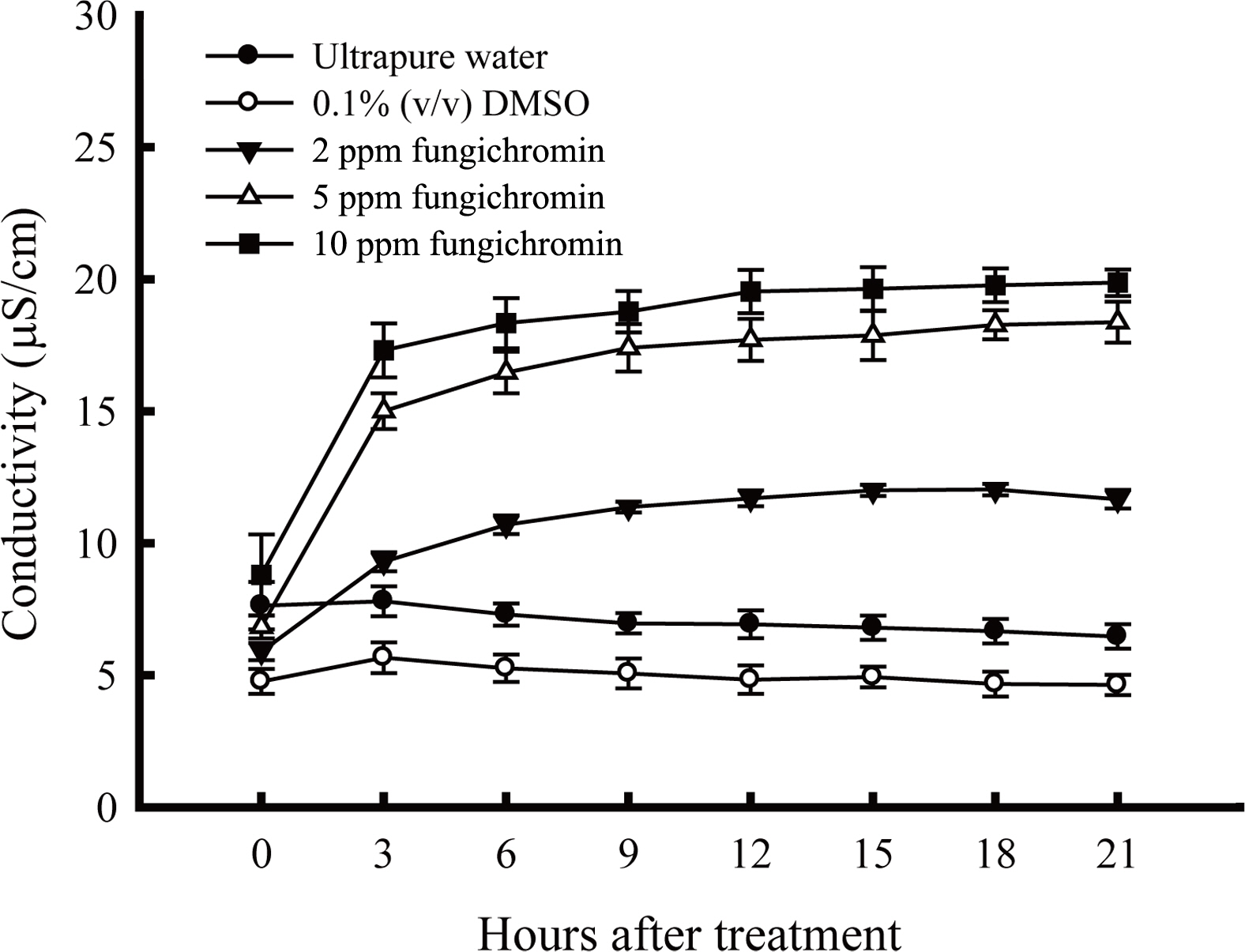
The electrolyte leakage from hyphae of Rhizoctonia solani RS1-731 after being treated with fungichromin. Ten mycelial disks of R. solani RS1-731 were put into each solution and cultured at 30°C, 120 rpm. Data represented mean and standard deviation of three replicas (error bar). The conductivity in each treatment was measured by HANNA HI-8820 conductivity meter every 3 h for a total of 21 h.
When R. solani was co-cultured with rice seedlings treated with distilled water or 0.1% (v/v) DMSO, R. solani formed infection cushions and lobated appressoria (A and B in Fig. 4). In contrast, R. solani after being treated with fungichromin failed to form infection structures (C, D, and E in Fig. 4).

Effect of the fungichromin on the formation of infection structures by Rhizoctonia solani RS1-731. Rice seedlings were treated with distilled water (A), 0.1% (v/v) DMSO (B), 2 mg/l fungichromin (C), 5 mg/l fungichromin (D), or 10 mg/l fungichromin (E). Five-day-old rice seedlings in each treatment and mycelia of R. solani RS1-731 were co-cultured for 2 days, and then observed under dissecting microscope. White arrows indicated the lobated appressoria of the isolate RS1-731 on rice seedlings surface.
Efficacy of S. padanus PMS-702 fermented broth on soil sanitation
Rice straws infested with R. solani sclerotia were immersed in S. padanus PMS-702 fermented broth from the culture in the MACC broth to evaluate the efficacy of S. padanus broth after fermentation as soil sanitation agents. The results indicated that S. padanus broth at 200-fold or 500-fold dilution was effective in inhibiting mycelial growth and sclerotia germination of R. solani (Fig. 5). The inhibitory efficacy was increased with the increase of immersing time and concentrations of the R. solani-infested rice straws and sclerotia in S. padanus PMS-702 fermented broth. The inhibitory rate on the growth of R. solani from infested rice straws reached to 90% after treating with S. padanus broth at 200-fold dilution for 28 h. Less than 10% of R. solani sclerotia germinated 5 and 8 days after treating with S. padanus broth at 200- and 500-fold dilution, respectively.
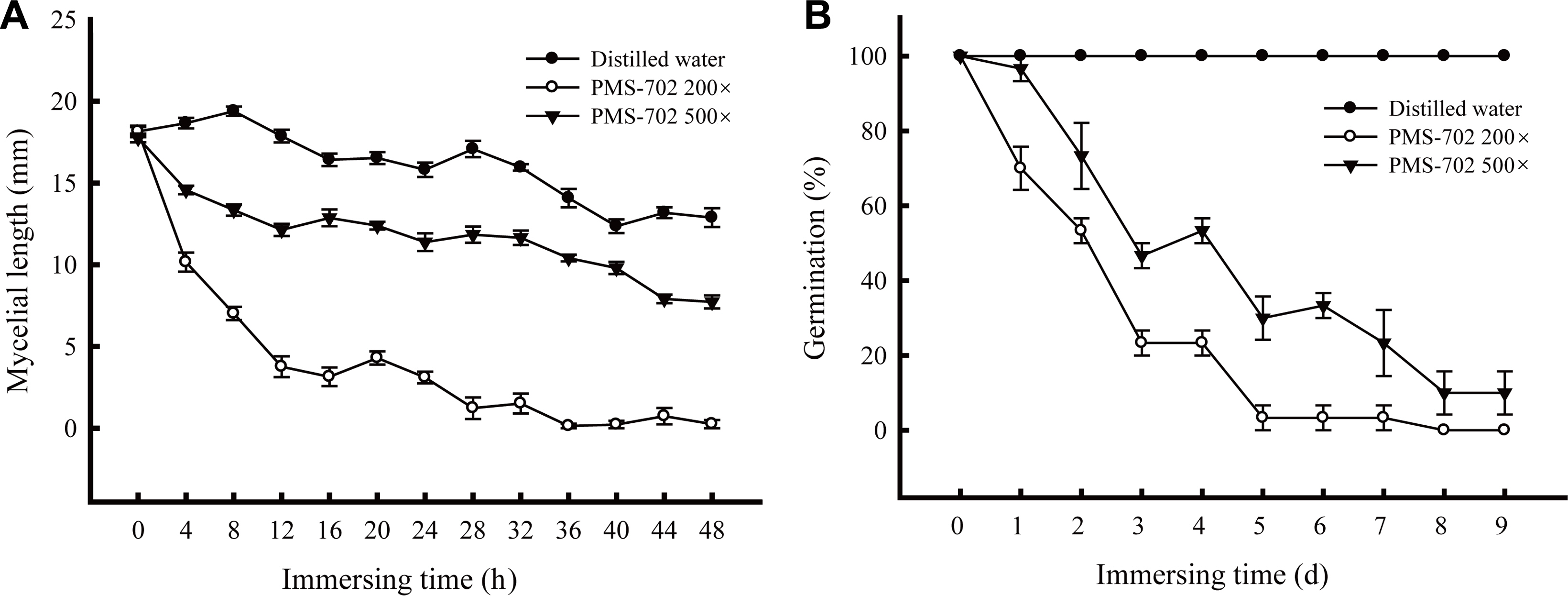
Effect of time period of immersing rice straws infested with Rhizoctonia solani RS1-731 in Streptomyces padanus PMS-702 fermented broth on the viability of the pathogen. (A) Mycelial growth from the rice straws; (B) Germination percentage of sclerotia of R. solani RS1-731 after treatment. S. padanus PMS-702 was cultured in the MACC broth (10 g malt extract, 10 g alfalfa seed meal, 1 g CaCO3 and 10 ml corn oil in 1 l distilled water) at 30°C for 6 days. Infested rice straws were immersed in S. padanus PMS-702 fermented broth at 200-fold or 500-fold dilution for 0-48 h, and then put onto tannic acid medium plate for measuring the mycelial length grew out from the rice straws after 16 h incubation; the immersed sclerotia of R. solani RS1-731 in S. padanus PMS-702 fermented broth from 0 to 9 days on their viability for germination. Data represented mean and standard deviation of three replicas (error bar).
R. solani-infested rice straws mixed with field soil were immersed in 0.2 and 0.5% S. padanus broth cultured in the MACC broth as a model for evaluating the efficacy of S. padanus as soil sanitation agents. The survival rate of R. solani was significantly reduced 6 days after being treated with S. padanus broth compared to the water and media controls (data not shown). R. solani treated with 0.5% S. padanus broth displayed a better inhibition rate of growth than those treated with 0.2% S. padanus broth (data not shown). Thus, 0.5% S. padanus broth was used for further study.
Tests were carried out to determine if tea seed pomace would suppress R. solani in the field soil and if it had a synergistic effect with S. padanus broth. Field soil mixed with R. solani-infested rice straws were immersed in 0.5% (v/v) S. padanus broth and mixed with an equal volume of 0.25%, 0.5%, 0.75%, or 1% tea seed pomace, and the survival rate of the R. solani in each treatment was assessed. The treatment with a combination of 0.5% (v/v) S. padanus broth and tea seed pomace showed a better inhibitory effect on R. solani survival than that with only 0.5% (v/v) S. padanus broth (Fig. 6). No growth from R. solani RS1-731 infested straws was observed 12 days after treating with a combination of 0.5% (v/v) S. padanus broth with 0.5, 0.75, or 1% tea seed pomace. Additionally, in the field soil immersed with 0.5% (v/v) S. padanus broth and 0.5% (w/v) tea seed pomace, the germination rate of sclerotia reduced to 16.66 ± 4.41% compared to 100 ± 0.00% with the water control 21 days after treatment (significance at P < 0.05 according to Fisher’s least significant difference test). Rice seedlings inoculated with R. solani sclerotia developed no sheath blight symptoms after being treating with 0.5% (v/v) S. padanus broth and 0.5% tea seed pomace in a span of 3 weeks. In contrast, 44.44 ± 6.42% of sheath blight incidence was observed in the water control group. The results suggested that a combination of S. padanus broth and tea seed pomace could reduce the sclerotia viability of R. solani, and reduced the disease incidence of rice sheath blight.
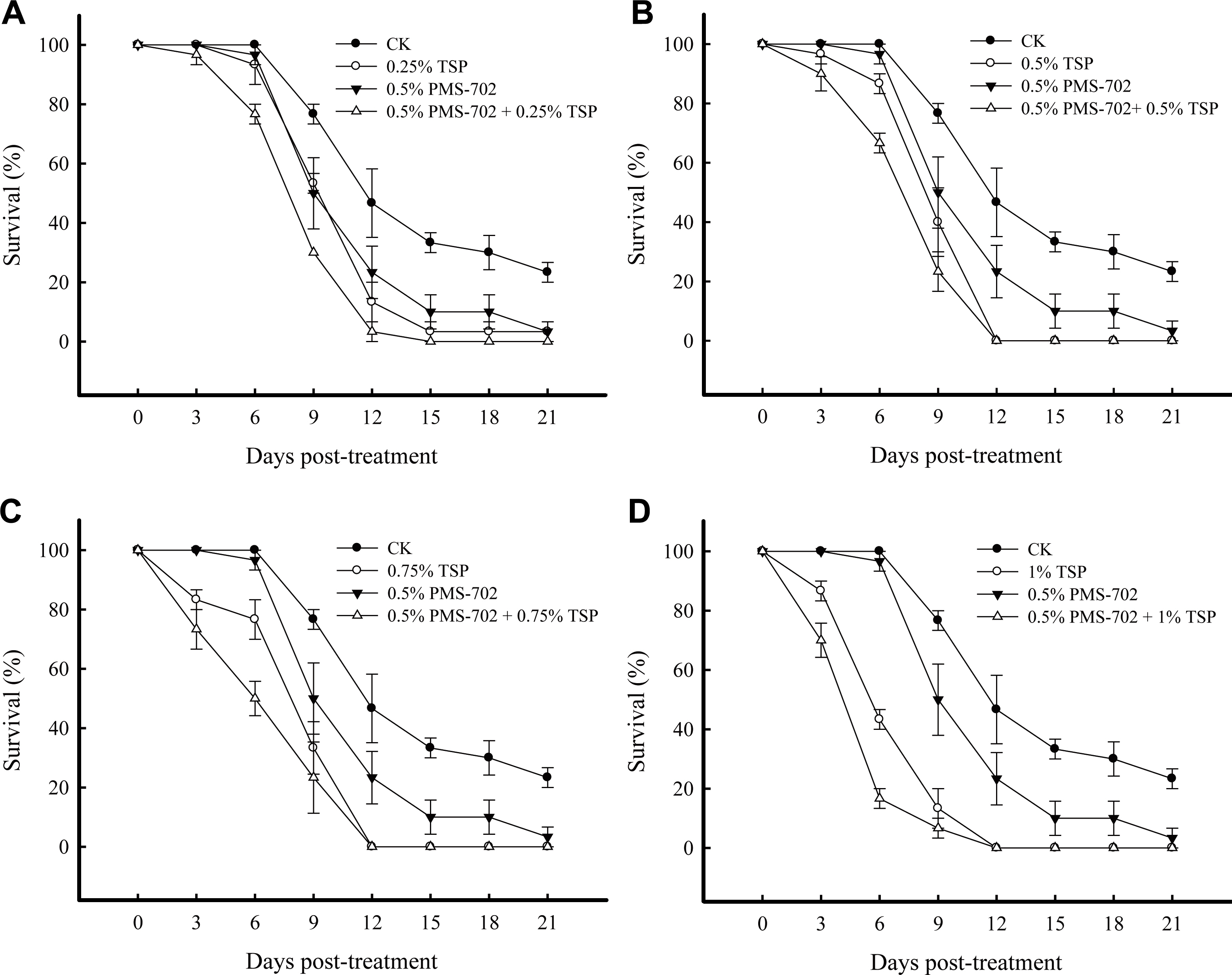
Effect of Streptomyces padanus PMS-702 fermented broth amended with tea seed pomace on the mycelial survival of Rhizoctonia solani RS1-731 from the infested rice straws in the field soil. Field soil with infested rice straws were amended with 0.5% (v/v) PMS-702 fermented broth (PMS-702) and 0.25% (A), 0.5% (B), 0.75% (C), or 1% (w/v) (D) tea seed pomace (TSP). The malt extractalfalfa seed meal broth was used as a control (CK). S. padanus PMS-702 was cultured in the malt extract-alfalfa seed meal broth at 30°C, 6 days. Rice straws infested with R. solani RS1-731 were incorporated into field soil per treatment. Ten infested rice straws were randomly picked from each treatment and placed on the tannic acid medium plate for testing mycelial viability, and then the survival rate of the pathogen was calculated after 24 h incubation. Data represented mean and standard deviation of three replicas (error bar).
Efficacy of foliar application of S. padanus PMS-702 on the control of rice sheath blight
Different types of surfactants were mixed with S. padanus PMS-702 culture broth in the modified soybean meal-glucose medium (SMGC-2) (Fan, 2017) to evaluate their inhibitory effect on mycelia growth of R. solani RS1-731. The results indicated that a combination of S. padanus broth with tween 20, EasyClean detergent, tea saponin, or sapindus extract enhanced the inhibition of R. solani mycelial growth compared to use of only S. padanus culture broth (Fig. 7A). The mixtures of tea saponin and S. padanus culture broth showed the best efficacy in all test surfactants (Fig. 7A), thus were further tested for the optimal concentrations. Results showed that S. padanus cultured in the SMGC-2 at 100-fold dilution with the addition of equal volume of 2, 3, or 4% (v/v) tea saponin exhibited the inhibitory ability to R. solani growth relative to the other treatments (Fig. 7B).
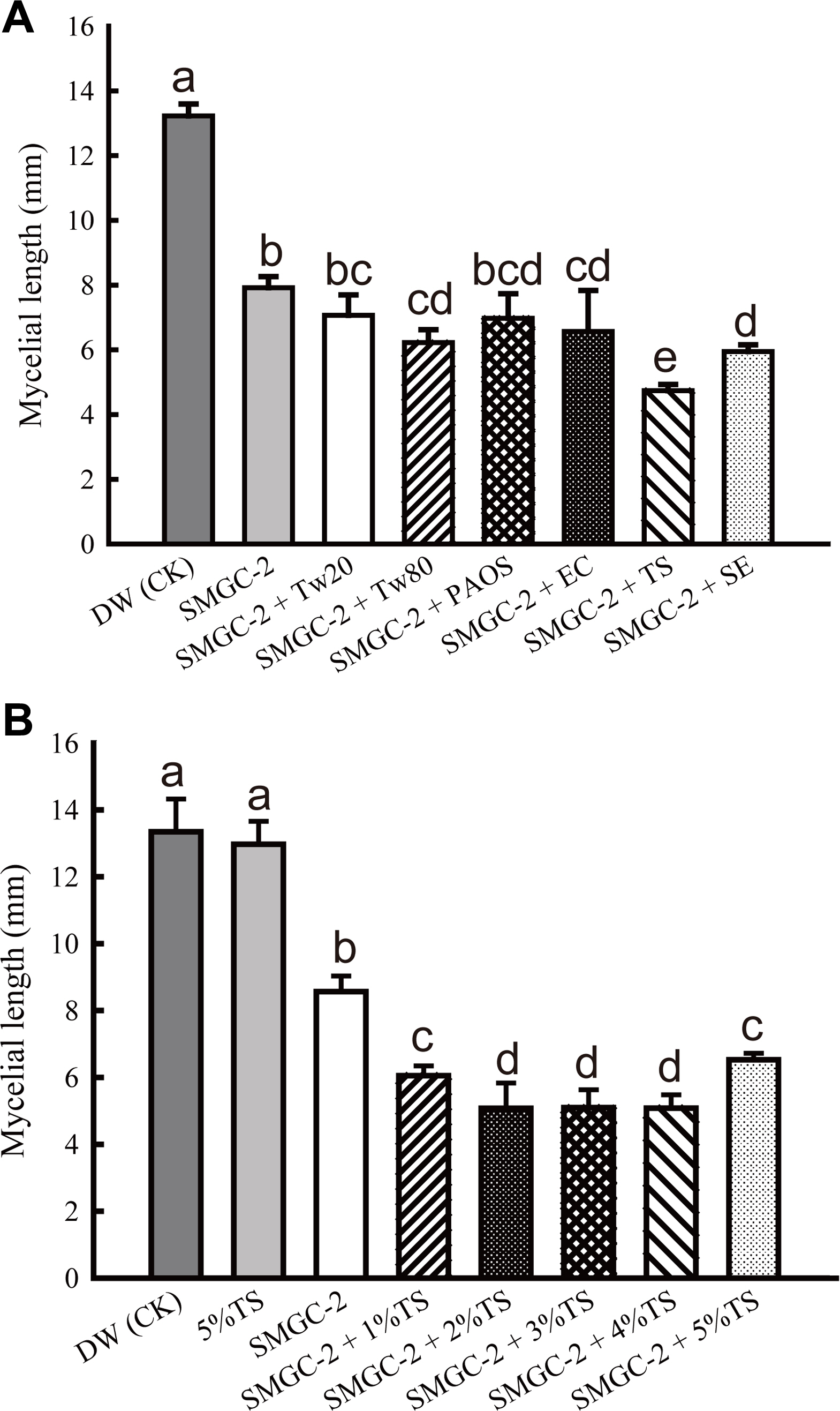
Effect of SMGC-2 fermented broth of Streptomyces padanus PMS-702 mixed with different types and concentrations of surfactants on the mycelial growth of Rhizoctonia solani RS1-731. (A) Effect of S. padanus PMS-702 with 4% (v/v) each surfactant. DW, distilled water was used as a control (CK); SMGC-2, SMGC-2 fermented broth; Tw20, tween 20; Tw80, tween 80; PAOS, PAOS detergent; EC, EasyClean detergent; TS, tea saponin; SE, sapindus extract. (B) Effect of S. padanus PMS-702 with different concentrations (%, v/v) of tea saponin. S. padanus PMS-702 was cultured in the modified soybean meal-glucose medium (SMGC-2, 11.2 g soybean meal, 11.2 g glucose, 0.46 g CaCO3, and 10 ml coconut oil in 1 l distilled water) (Fan, 2017) at 30°C, 5 days. Culture broth with equal volume of each surfactant at 100-fold dilution was used for the assay. Columns with the same letter are not significantly different (P > 0.05) according to Fisher’s least significant difference test.
S. padanus PMS-702 cultured in SMGC-2 at 100-fold dilution and mixed with equal volume of 2% (v/v) tea saponin broth, namely SPT, was foliar spray-applied on the rice seedlings or rice plants at tillering stage inoculated with R. solani RS1-731. The disease indexes of the rice seedlings treated with tea saponin, S. padanus PMS-702 culture broth in SMGC-2, and SPT at 100-fold dilution were significantly reduced to 4.50 ± 0.36, 3.72 ± 0.44, and 2.58 ± 0.21 compared to 5.63 ± 0.44 with the water control (significance at P < 0.05 according to Fisher’s least significant difference test). The disease severity for one, two, and three times applications of SPT on rice plants at tillering stage were reduced to 51.85 ± 3.21%, 41.98 ± 5.35%, and 24.04 ± 6.42%, respectively, from 66.67 ± 5.56% of the water control (Fig. 8), suggesting the control efficacy of rice sheath blight by foliar application of SPT on rice plants.

Effect of application frequency of Streptomyces padanus PMS-702 in SPT for controlling rice sheath blight on rice at tillering-stage (35 days-old rice plants) in the greenhouse. (A) Disease severity (%) in each treatment. Columns with the same letter are not significantly different at P > 0.05 according to Fisher’s least significant difference test. (B) Symptom development of rice sheath blight on rice plants in each treatment. DW, distilled water was used as a control (CK); SPT, S. padanus PMS-702 was cultured in SMGC-2 medium for 5 days at 30°C and mixed with 2% (w/v) tea saponin by 1:1 (v/v). SPT at 100-fold dilution was applied once at 0 day post inoculation (dpi) [SPT(1)]; twice at 0 and 7 dpi [SPT(2)]; and three times at 0, 7, and 14 dpi [SPT(3)] on rice plants. The disease severity (%) was calculated by the following formula (International Rice Research Institute, 2013): Disease severity (%) = [(n0 × 0) + (n1 × 1)+…+(n9 × 9)]/N × 9. Where: n0-n9 is the number of culms in each disease index and N is total number of culms tested. The disease index level: 0 = no lesion, 1 = the appearance of water soaked lesion length less than 20% of the plant height, 3 = 20-30% of the plant height, 5 = 31-45% of the plant height, 7 = 46-65% of the plant height necrosis, 9 = higher than 65% of the plant height.
Discussion
Our results demonstrated that S. padanus PMS-702 is an effective biocontrol agent for the rice sheath blight. Additionally, the fermentation formula “malt extract-alfalfa seed meal-corn oil-CaCO3 (MACC) broth” for S. padanus PMS-702 were optimized and showed an enhancement of fungichromin production, a major active compound formed by S. padanus PMS-702, and in disease control. The soil amemdent for soil sanitation and the surfactant addition for foliar application in a combination use with S. padanus PMS-702 fermentation broth were developed in this study and were shown to further enhance the biocontrol efficacy for the rice sheath blight disease.
The production of antibiotics and enzymes by Streptomyces spp. has been demonstrated to be affected by the compositions of the fermentation media (Singh et al., 2017). Addition of tryptophan in the culture media resulted in an increase in the production of actinomycin V by Streptomyces triostinicus (Singh et al., 2009), and decrease in candicidin production by Streptomyces griseus (Sanchez and Demain, 2002). Choi et al. (1996) found that supplementation of rapeseed oils in the culture media resulted in a 7-fold increase in tyloson production by Streptomyces fradiae (Choi et al., 1996). Zhou et al. (2014) indicated that the metal salts in the media could also affect the production of antibiotics. They found that addition of Na2SO4, MnSO4•H2O, and MgSO4•7H2O in the culture media increases the nosiheptide production by Streptomyces actuosus to 1.5 fold. Our previous study reported that fatty acids and oils could serve as carbon sources or stimulators for the fungichromin by S. padanus PMS-702 (Zang et al., 2011). Here, we showed that addition of 1% corn oils in the malt extract-alfalfa seed meal based media increased the production of fungichromin by S. padanus PMS-702 to 550 mg/l from 194 mg/l in the medium without corn oils addition. Additionally, the fungichromin concentrations produced by S. padanus PMS-702 could further reached to 693 mg/l by supplementing 0.1% CaCO3 in the media.
The known mechanism of action of antibiotics produced by Streptomyces species could be due to the inhibition of DNA replication, RNA synthesis, cell wall synthesis, and protein synthesis, and interference of membrane integrity (de Lima Procópio et al., 2012). Shih (2003) reported that S. padanus PMS-702 produced polyene macrolide antibiotics, fungichromin (Shih, 2003). The investigation by Efimova et al. (2014) suggested that polyene macrolide antibiotics could bind to sterol-containing phospholipid-bilayers of fungi and form ion-permeable nanopores which result in leakage of cell constituents, and eventually cause death of the fungal cells (Efimova et al., 2014). Another polyene macrolide antibiotics, amphotericin B, were demonstrated to induce apoptosis in a medically important fungal pathogen (Phillips et al., 2003). Data from the observation with light microscope and scanning electron microscope indicated that fungichromin could induce plasma agglutination, and cause cell malformation and collapse of sporangia and zoospores of Phytophthora infestans (Shih, 2003). Additionally, it resulted in membrane rupture of Phytophthora zoospores and plasma leakage (Shih, 2003). Our results showed that R. solani hyphae treated with fingichromin resulted in loss of mycelial viability by live and dead staining analysis, caused intercellular vacuolization, and electrolyte leakage. Our other study also indicated that application of S. padanus PMS-702 could induce expression of plant defense related genes PAL (encoding phenylalanine ammonia-lyase), POX (encoding peroxidase), and PR1a (encoding pathogenesis-related protein 1 a) in cucumber seedlings (unpublished data). During the infection process in R. solani, the formation of infection cushions or lobated appressoria on rice plants are the key determinants for tolerance and susceptibility of the rice plants (Basu et al., 2016; Lee and Rush, 1983). Our data indicated that rice seedlings treated with 2 mg/l fungichromin could prevent the formation of infection structure by R. solani, suggesting that application of fungichromin prior to the pathogen infection could protect rice plants. Additionally, we found that disease severity of rice sheath blight was reduced with the increase of application frequencies of S. padanus PMS-702 SPT agents.
To prevent the carbon dioxide emission during the burning of rice straws, burying the rice straws into the rice fields as basal fertilizers become a common practice for the farmers. However, the pathogen infested rice straw could be a reservoir of pathogens. R. solani could survive in the infested rice straws for several months in wet or flooding paddy soils (Feng et al., 2017). Results by incorporation of rice straws with various proportions of pathogen infested straws indicated that incorporation of diseased straw enhanced pathogen numbers in soil during the decomposition period increased disease severity of sheath blight (Zhu et al., 2014). Our data showed that application of 0.5% S. padanus PMS-702 fermented broth from the culture in the MACC broth in the field soils with R. solani RS1-731 infested rice straws decreased the R. solani RS1-731 population numbers 6 days after treating with S. padanus PMS-702 broth compared to the water and media controls. The data suggested a possible use of S. padanus PMS-702 fermented broth as a field sanitation agent.
Tea seed pomace containing triterpenoid saponins has been shown to change the membrane permeability of R. solani AG4, and reduce the disease incidence of cabbage seedling damping-off caused by R. solani AG4 (Kuo et al., 2010; Yang, 2006). Here, amendment of tea seed pomace with 0.5% S. padanus PMS-702 fermented broth in the MACC broth enhanced the efficacy in inhibition of R. solani RS1-731 survival in the field soil and showed a synergistic effect with S. padanus PMS-702 fermented broth.
Surfactants could be served as pesticide adjuvants, which play an important role in preparing pesticide formulations and give an optimal efficacy of active ingredients (Wang and Liu, 2007). Tea saponins extracted from the tea seed pomace are natural nonionic surfactants which possessed the properties as dispensants, emulsifiers, wetting agents or spreading agents have been reported to have antimicrobial, inseccidal, antihelminthic, and molluscicidal activities (Guo et al., 2018). Our findings suggested that combination use of surfactants including tween 20, EasyClean detergent, tea saponin, and sapindus extract and S. padanus PMS-702 culture broth in SMGC-2 enhanced the inhibition of R. solani RS1-731 mycelial growth compared to the water control. Among the tested surfactants, tea saponins exhibited the best synergistic effect on antifungal activity with S. padanus PMS-702 culture broth. This synergistic effect may be partly attributed to the releasing and dispensing the fungichromin from the S. padanus mycelial pellet by tea saponins (Fan, 2017) or the antifungal efficacy of tea saponins (Kuo et al., 2010; Yang, 2006).
Our results demonstrated that S. padanus PMS-702 produced fungichromin, which was effective in inhibiting mycelial growth of the causal agent of rice sheath blight, R. solani and could also cause leakage of cytoplasm and inhibit the formation of infection structure of R. solani. The concentration of fungichromin reached to 802 mg/l when S. padanus PMS-702 was cultured in MACC broth. Soil amended with 0.5% (v/v) S. padanus PMS-702 broth and 0.5% (w/v) tea seed pomace resulted in the death of R. solani mycelia in the infested rice straws and inhibited the germination of sclerotia. Additionally, S. padanus PMS-702 cultured in soybean meal–glucose (SMGC-2) medium mixing with 2% tea saponin was the most effective in suppressing the pathogen. By foliar application of S. padanus PMS-702 culture broth in SMGC-2 mixing with 2% tea saponin on rice plants significantly reduced rice sheath blight disease severity ranging from 24.04% to 66.67%. Thus, S. padanus PMS-702 is an effective biocontrol agent. The fermentation formula developed for foliar application and soil sanitation in this study could further enhance the biocontrol efficacy of S. padanus PMS-702 for the rice sheath blight disease.
Acknowledgments
This work was supported by the “Innovation and Development Center of Sustainable Agriculture” from the Featured Area Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education in Taiwan, and the Ministry of Science and Technology, Taiwan (MOST 109-2321-B-005-022).
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
