 |
 |
| Plant Pathol J > Volume 32(1); 2016 > Article |
Abstract
Major diseases in grafted cacti have been reported and Fusarium oxysporum, Bipolaris cactivora, Phytophthora spp. and Collectotrichum spp. are known as causal pathogens. These pathogens can lead to plant death after infection. Therefore, some European countries have quarantined imported cacti that are infected with specific fungal pathogens. Consequently, we developed PCR detection methods to identify four quarantined fungal pathogens and reduce export rejection rates of Korean grafted cacti. The pathogen specific primer sets F.oF-F.oR, B.CF-B.CR, P.nF-P.nR, and P.cF-P.CR were tested for F. oxysporum, B. cactivora, P. nicotinae, and P. cactorum, respectively. The F.oF-F.oR primer set was designed from the Fusarium ITS region; the B.CF-B.CR and P.nF-P.nR primers respectively from Bipolaris and Phytophthora ITS1; and the P.cF-P.CR primer set from the Ypt1protein gene region. The quarantine fungal pathogen primer pairs were amplified to the specific number of base pairs in each of the following fungal pathogens: 210-bp (F. oxysporum), 510-bp (B. cactivora), 313-bp (P. nicotinae), and 447-bp (P. cactorum). The detection limit for the mono- and multiplex PCR primer sets was 0.1 ng of template DNA under in vitro conditions. Therefore, each primer set successfully diagnosed contamination of quarantine pathogens in export grafted cacti. Consequently, our methodology is a viable tool to screen contamination of the fungal pathogen in exported grafted cacti.
Most Cactaceae are native to the Americas, including north, central, and south America. Cacti are distributed throughout a broad range of climatic conditions, including xeric deserts of the American southwest to rain forests of South America (Cruz et al., 1997), resulting in a morphologically heterogeneous group that is classified in three subfamilies (Cactoideae, Opuntioideae, and Pereskioideae) composed of approximately 100 genera and over 1,500 species. Recently, cactus farms in Korea, Japan, and China have started grafting cacti. By grafting two different cactus species, including photosynthetic stocks and non-photosynthetic scions are grafted and form decorative colored scions. Currently, grafted-cacti products from Korea exceed 70% of the worldŌĆÖs cacti trading market due to the beauty in cacti appearance and variability in shape and color (Song et al., 2009a, 2009b). The grafted-cacti are cultivated under greenhouse conditions at warm temperatures and high humidity during the entire growing season. Therefore cacti frequently were infected by a variety of fungal diseases (Chang et al., 1998; Choi et al., 2010; Hyun et al., 1998; Kim et al., 2000; Kim et al., 2007). Cactus diseases are a major factor in greenhouse grafted-cacti, particularly stem rot caused by F. oxysporum and B. cactivora (=Helminthosporium cactovorum Petr., (1931) and =Drechslera cactivora) (Petr.) M.B. Ellis, (1971) (Chang et al., 1998; Hyun et al., 1998). Other diseases, anthracnose (Collectotrichum spp.) are also a problem and found worldwide as cactus late blight disease (P. cactorum and P. nicotianae). In 1999 and 2000, a major stem rot disease outbreak was observed in the Suwon regions (National Horticulture Research Institute) and other primary grafted-cacti producing districts, such as Anseong, Eumseong, and Goyang. Stem rot symptoms caused by Alternaria sp., Fusarium sp., and B. cactivora were frequently identified in cacti greenhouses (Kim et al., 2000). Imported counties, including Europe, Israel, and Southern Asia classified the cacti pathogens, that are F. oxysporum, B. cactivora, P. cactorum and P. nicotianae as quarantine pathogens. Therefore, disease or contamination free certification is now required before plants can be imported.
Disease and pathogen diagnosis can be time consuming and sophisticated processes, because most cactus pathogens produce similar disease symptoms. Therefore, there has been an increased demand for rapid diagnosis methods. Polymerase Chain Reaction (PCR)-based detection method has been adopted as the most reliable and rapid technique in the scientific community (Hameed et al., 2014; Kwak et al., 2014). Specific primer sets were developed with fungi unique gene sequences to detect fungal pathogens (Buchman et al., 1990; Kan et al., 1993), Multiplex PCR for pathogen diagnosis, permits simultaneous amplification of several pathogens in a single reaction mixture, and facilitates cost-effective diagnosis (Aguilar et al., 2000; Corias et al., 2003; Fan et al., 1998; Grondahl et al., 1999; Liolios et al., 2001). The objective of this study was to develop a PCR detection method that amplified highly conserved target gene sequences, analyze the detection sensitivity, and the primer specificity to cactus pathogens. Results of this study served to provide a cost effective and rapid method to prove grafted-cacti are disease free for the required certification to export grafted-cacti from Korea.
Four quarantine fungal pathogens were obtained from the Korean Agriculture Culture Collection (KACC), including the standard isolates B. cactivora (#40851), F. oxysporum (#44306), P. cactivorum (#40174), and P. nicotianae (#40403). All isolates used in this study were confirmed by molecular and morphological characteristics (data not shown). All quarantine pathogens cause cactus root or stem rot diseases. Quarantine fungal isolates were cultivated using standard cultivation methods. A pure culture of each fungal isolate was inoculated on Potato Dextrose Agar (PDA: B. cactivora, F. oxysporum) or 10% V8 Juice Agar (VJA: P. cactivorum, P. nicotianae) and subsequently incubated at 27┬░C for 5 days. Following incubation, the spore suspension concentration was adjusted from 1 ├Ś 109 to 1 ├Ś 101 spore/ml using a hemacytometer. A syringe was used to inoculate fungal spore suspension onto the cactus stems in decreasing concentrations. Grafted cactus stems inoculated with fungal spore suspensions were incubated at 25┬░C for 5 days.
For DNA extraction, B. cactivora and F. oxysporum hyphae were grown in PDB at 27┬░C for 5 days at 27┬░C. P. nicotianae and P. cactorum were incubated in 10% V8 juice broth. The hyphae were harvested by centrifugation (5,000 rpm for 5 min). The CTAB method was used to extract genomic DNA from the cultured quarantine fungi (Lee and Taylor, 1990). The extracted DNAs were dissolved in distilled water. Cacti genomic DNA, which was injected with the fungal pathogen, were extracted (100-200 mg frozen weight) using the Qiagen DNeasy Plant Mini Kit. Species-specific primer pairs, including Fo.F-Fo.R (F. oxysporum), BC.F-BC.R (B. cactivora), PN.F-PN.R (P. cactorum), and PC.F-PC.R (P. nicotianae) were designed based on specific sequence data for the internal transcribed spacer (ITS) region and the Ypt1 gene in GenBank. P. cactivora specific primers PN.F and PN.R were previously described (Li et al., 2013). The following reaction mixture was used for PCR with individual primer pairs: l ul (40 ng) of diluted template genomic DNA, 1 ul 10 mM (dNTP), 2 ul of 10├Ś Reaction buffer, 1 ul (10 pmole) primer, and 1 unit of Taq DNA polymerase (Bioneer, Korea) in a total volume of 20 ul. PCR amplification conditions included 5 min of denaturation at 94┬░C; followed by 30 cycles of 94┬░C for 30 s, 55┬░C for 30 s, and 72┬░C for 15s; and a final extension step of 72┬░C for 10 min. Each multiplex contained four primer pairs designed to produce amplicons sufficiently different in size and migration rate to identify the four pathogen species (B. cactivora, F. oxysporum, P. cactivora, and P. nicotianae). Primer information is presented in Table 1. Reaction mixture reagents were the same as described above for single-primer-pair PCR. The PCR tubes were maintained on ice and PCRs were performed in a PTC-100 programmable Thermal Controller (MJ Research, INC. USA). All PCRs were run with the same cycling program used for single-primer-pair PCR. A 4 ul sample of product from each PCR was electrophoresed in a 1.3% agarose gel with 0.3 ug/ml of ethidium bromide for 15 to 20 min. DNA bands were visualized and UV documented with a BioDoc-IT 220 imaging System 3 Door/8.0 LCD With M-20 Transilluminator (Analytik Jena AG, Germany).
The four pairs of species-specific primers were frequently amplified and detected the quarantine pathogens (Table 1). The PCR assay was primarily established as a monospecific assay with individual genomic DNA of the four fungal pathogens. Standard PCR sensitivity was generated with PCR product values obtained and used to optimize PCR efficiency. All tested quarantine pathogens were successfully amplified and visualized by ethidium bromide stained gel analysis (data not shown). More accurately, fungal pathogens (B. cactivora, F. oxysporum, P. cactorum, P. nicotianae) for the species-specific region were also successfully amplified with the defined primer pair (data not present). Sensitivity of the species-specific primers was evaluated with serial diluted DNA concentrations from 100 to 0.01 ng. Under in vitro conditions, the species-specific fungal primers, F.oxF-F.oxR, B.CF-B.CR, and P.NF-P.NR were detectable at 0.01 ng of genomic DNA; and the P.CF-P.CR primer produced an amplicon of the appropriate size in a 1 ng concentration of genomic DNA (Fig. 1). The four different fungal pathogens were detected using 100 ng of DNA by multiplex PCR following ethidium bromide staining. For multiplex PCR, the four primer sets were combined in a single tube to simultaneously identify four fungal pathogens. B. cactivora and F. oxysporum were detected in the agarose gel at a sensitivity level of only 0.01 ng. P. nicotianae sensitivity was 0.1 ng of DNA and 1 ng of DNA for P. cactorum (Fig. 2). The sensitivity of the multiplex PCR was comparable to single-primer-set PCR sensitivity. These results indicated up to three suspected pathogens can be identified in a single PCR.
Cacti were inoculated with spore suspensions (101-9) for detection pathogen from symptomatic cactus. The most of efficiency demonstrated in 107-9 experiments with strains of each of the four fungal pathogens (Fig. 3). Quarantine pathogens inoculated cacti were incubated at room temperature (25┬░C) for 5 days. All pathogens caused typical disease symptoms in cactus species with spore density above 107 cfu/ml. Following sampling from the inoculated site, genomic DNA was extracted to test specificity and sensitivity of the multiplex PCR under in vivo conditions. Multiplex PCRs generated specific amplicons of correct size when templates from corresponding pathogenic fungal species were present. All multiplex PCR conditions were the same as described above. PCR reactions were positive when pathogens caused disease symptoms at 107 cfu/ml, with the exception of F. oxysporum, which was detected at 106 cfu/ml. This sensitivity was achieved only for the quarantine cactus pathogen samples tested using multiplex PCR (data not shown).
For test detection sensitivity of target pathogens with asymptomatic cacti, spore suspension concentration was adjusted from 1 ├Ś 101 to 1 ├Ś 109 spore/ml using a hemacytometer. After dropping fungal spore-stock on cacti stems, inoculated cacti were dried in clean bench for 2 hrs. Inoculated cacti stems were rubbed by a cotton swab. The cotton swab put in 1.5-ml E-tube containing 900 ul distilled water for genomic DNA extraction and multiplex PCR. The multiplex PCR set showed high specificity in four different inoculated spore suspensions on asymptomatic cacti. In this case of P. nicotianae, 313 bp fragment was only generated from 103 to 109 cfu/ml with asymptomatic cacti (Fig. 4A). F. oxysporum and P. cactorum showed 210 bp, 447 bp from 109 to 104 respectively. B. cactivora, where the 510 base-pair appears 105 to 109 in treated cactus.
Our results demonstrated sensitivity similar to that of export grafted cacti cultivation contaminations with a markedly greater accuracy in diagnosis. This method is a valuable addition to the cacti grafting industry, and offers powerful diagnostic tools for the detection and identification of fungal quarantine pathogens.
Acknowledgments
This work was supported by Cooperative Research Program for Agriculture Science & Technology Development (PJ010827) and Plant Quarantine Agency (9513-ARG-0018).
Fig.┬Ā1
Sensitivity testing of different genomic DNA quantities (100 ng, 50 ng, 25 ng, 10 ng, 5 ng, 2.5 ng, 1 ng, 0.1 ng, 0.01 ng/ul). DNA was amplified with specific primers and amplicons detected different DNA quantities. Fungal pathogen sensitivity level is identified in agarose gels. (A) F. oxysporum sensitivity level 0.01 ng. (B) B. cactivora sensitivity level 0.01 ng. (C) P. nicotianae sensitivity level 0.1 ng. (D) P. cactorum sensitivity level 1 ng.
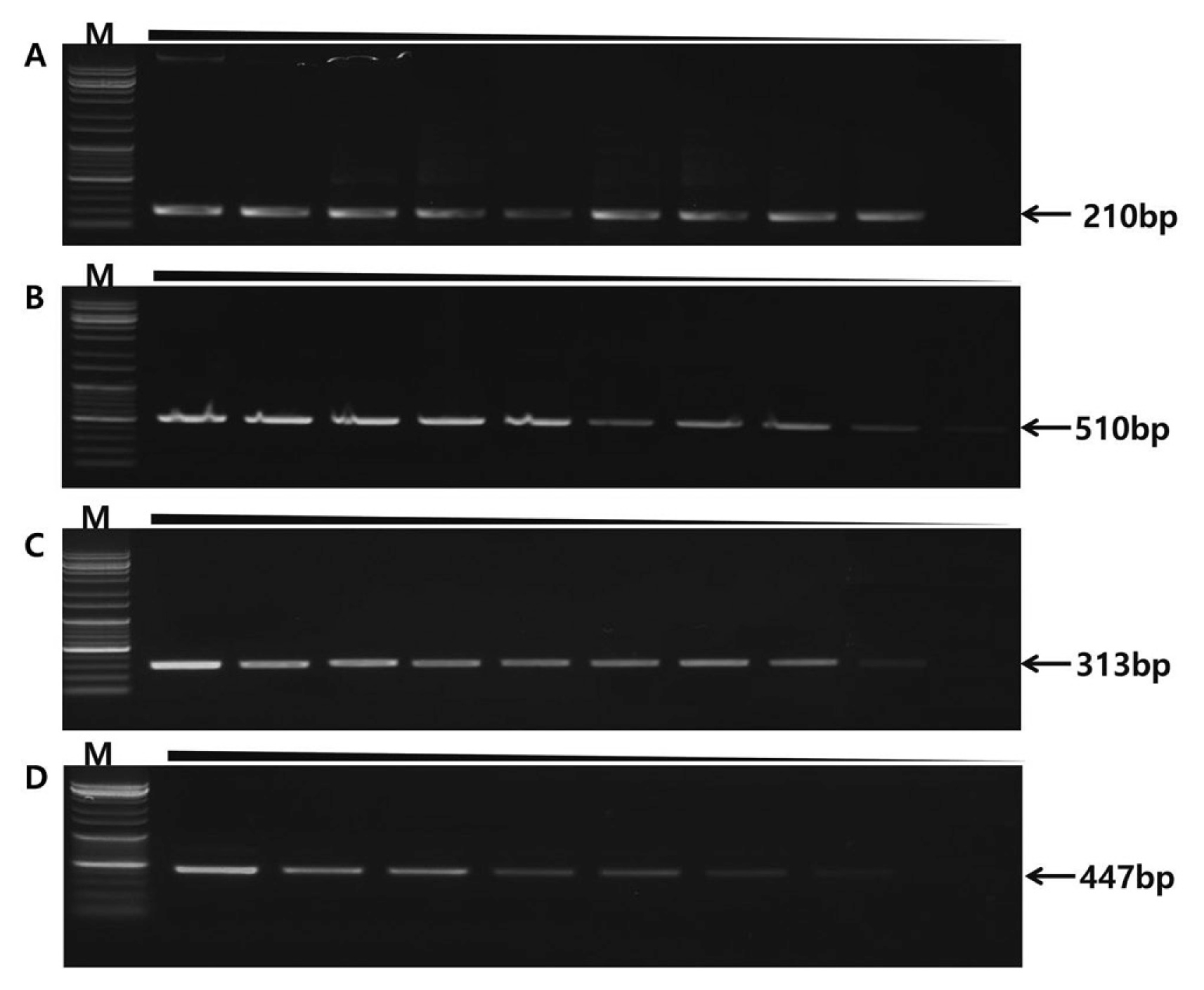
Fig.┬Ā2
Multiplex PCR, four sets of primers were combined in a single tube to identify the four fungal pathogens (100 ng, 50 ng, 25 ng, 10 ng, 5 ng, 2.5 ng, 1 ng, 0.1 ng, 0.01 ng/ul). (A) P. nicotianae sensitivity 0.1 ng DNA. (B) P. cactorum 1 ng DNA. (C) B. cactivora 0.01 ng DNA. (D) F. oxysporum 0.01 ng DNA.
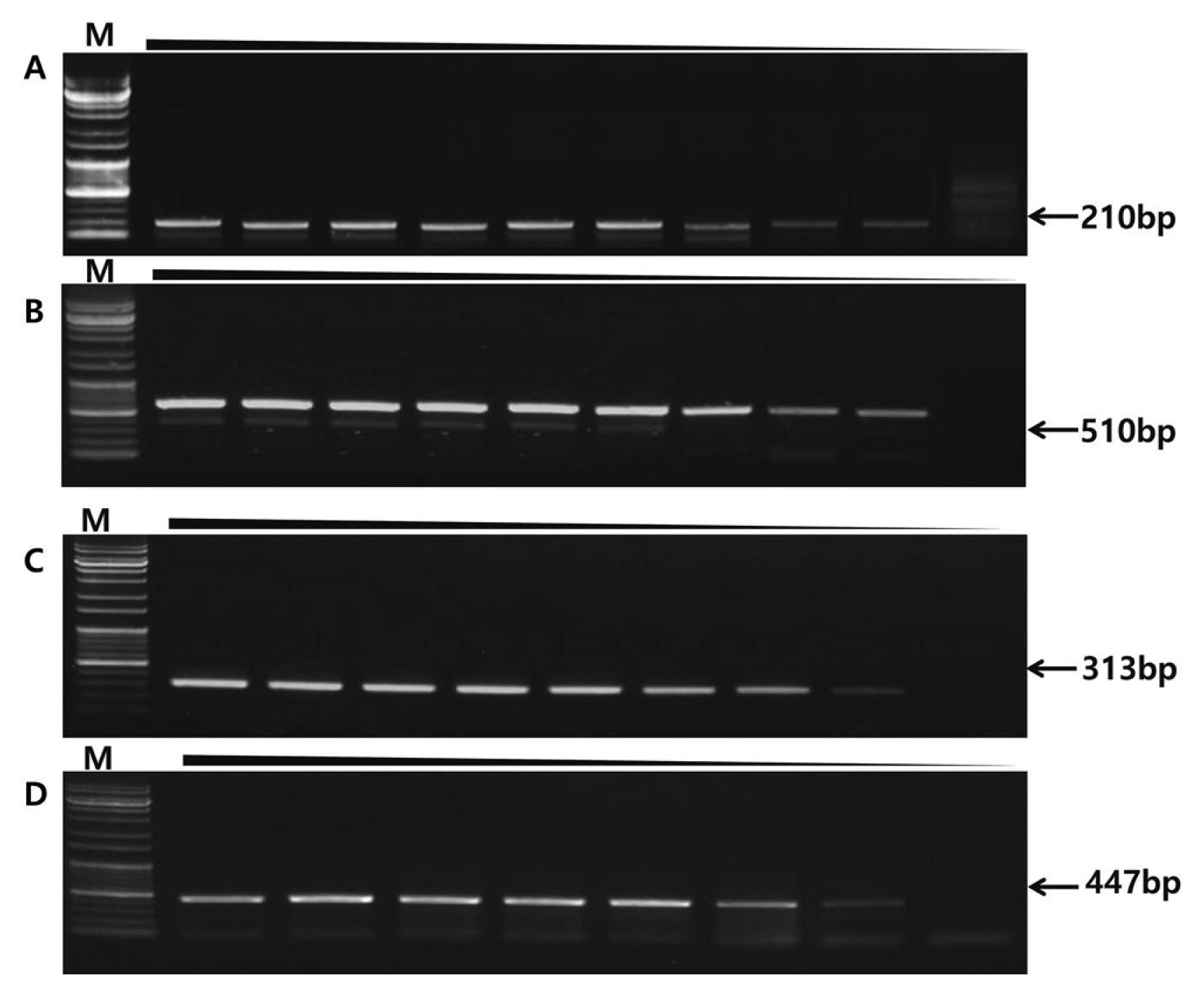
Fig.┬Ā3
Multiplex PCR in planta conditions. After cutting inoculated cactus (109, 108, 107, 106, 105, 104, 103, 102, 101 spore/ml), genomic DNA was extracted for testing multiplex PCR. Nine cacti were inoculated with spore stocks (109, 108, 107, 106, 105, 104, 103, 102, 101 spore/ml) and plant genomic DNA was extracted. (A) F. oxysporum, (B) B. cactivora, (C) P. nicotianae, (D) P. cactorum.
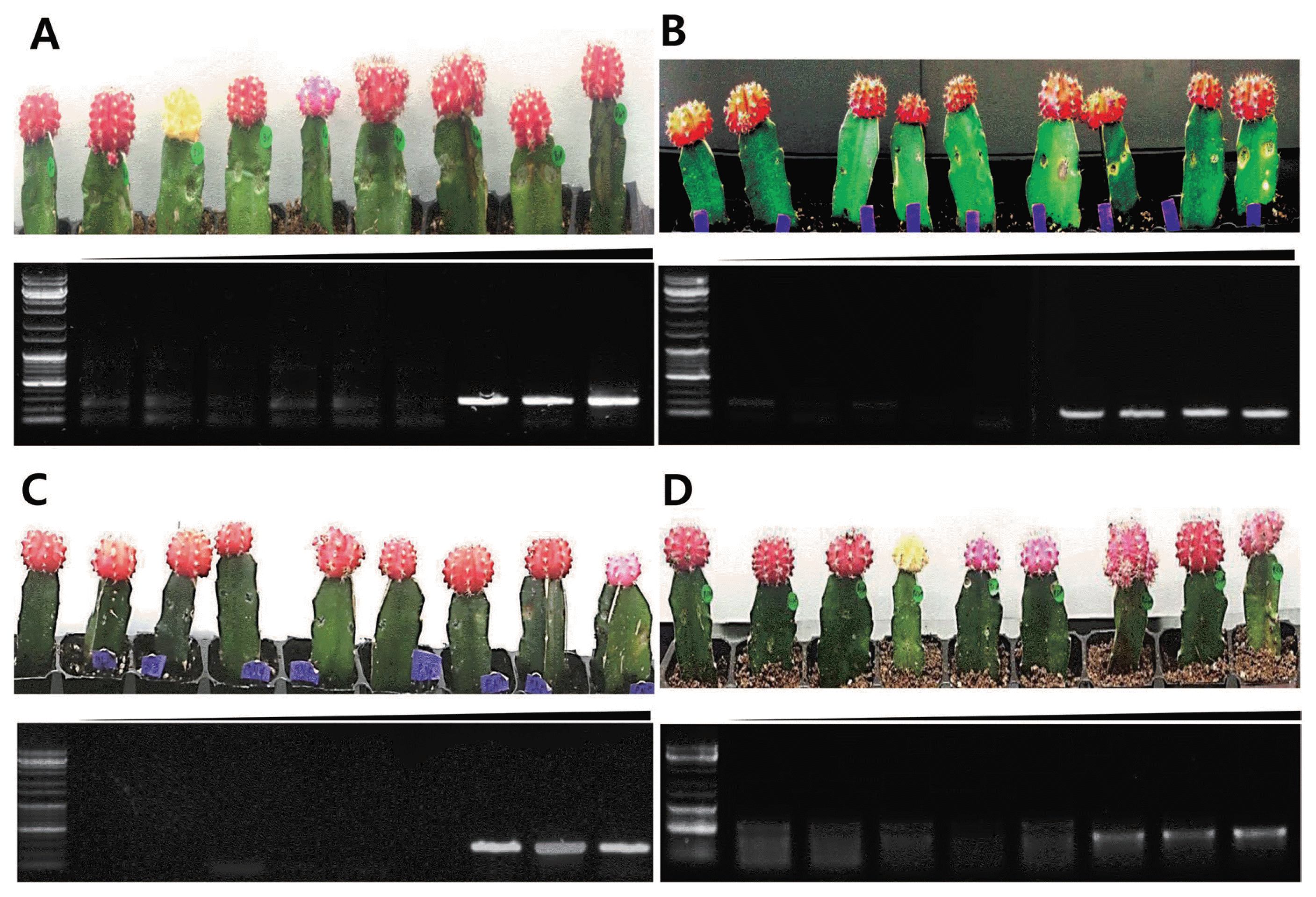
Fig.┬Ā4
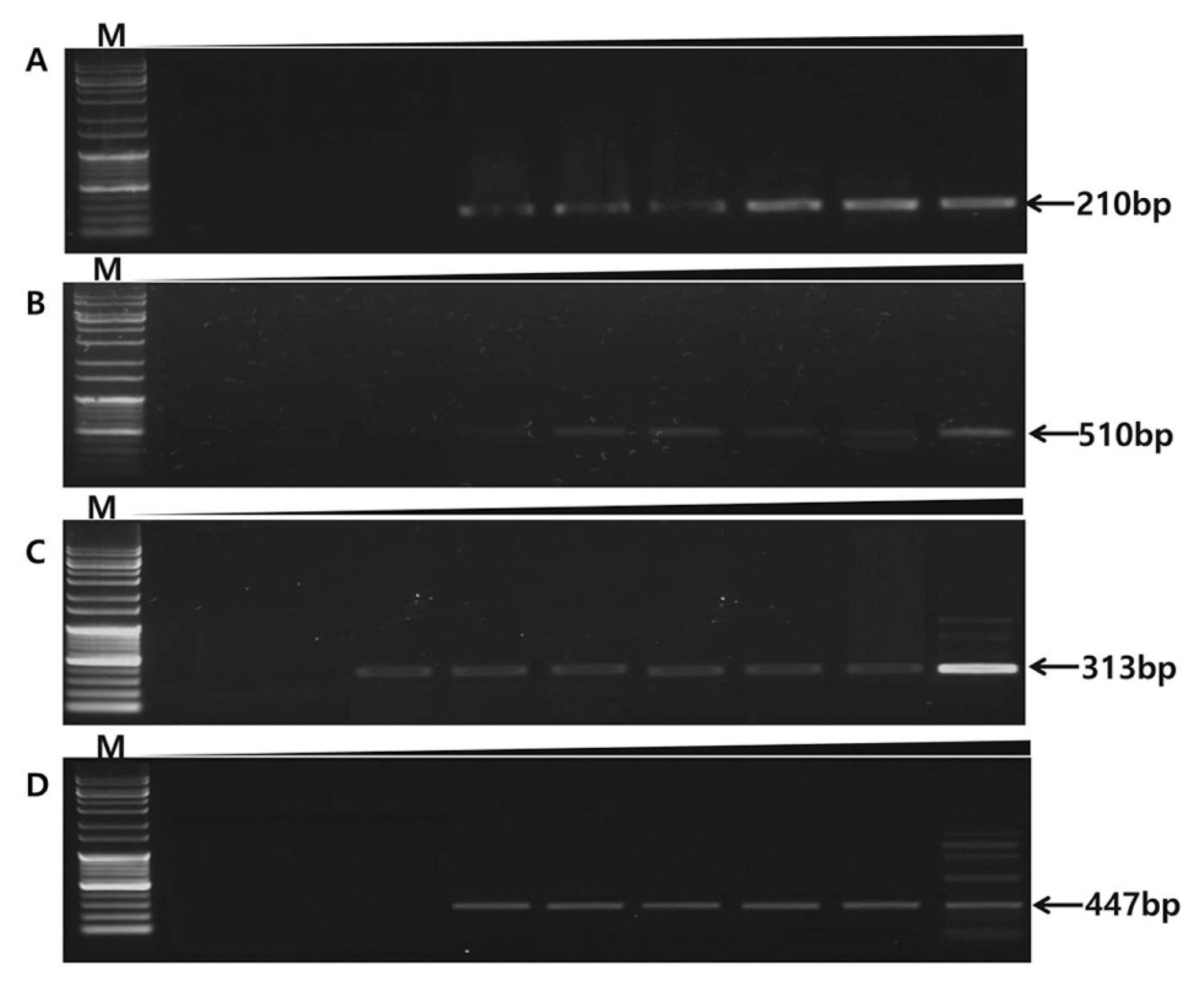
Multiplex PCR in spore-stock conditions. After inoculating spore stocks on cactus (109, 108, 107, 106, 105, 104, 103, 102, 101 spore/ml), total genomic DNA was extracted using cotton swab to test multiplex PCR. Nine cacti were inoculated with spore stocks (109, 108, 107, 106, 105, 104, 103, 102, 101 spore/ml) and plant genomic DNA was extracted. (A) F. oxysporum multiplex PCR showed spore stock ranged from 104 to 109. (B) Cacti inoculated with B. cactivora showed 104 to 109. (C) P. nicotianae multiplex PCR sensitivity is 103 to 109 (D) P. cactorum sensitivity is ranged from 104 to 109 spore stock detected by multiplex PCR in cacti.
Table┬Ā1
Primer sequences and specific amplicon size.
References
Aguilar, JC, Perez-Brena, MP, Garcia, ML, Cruz, N, Erdman, DD and Echevarria, JE 2000. Detection and identification of human arainfluenza viruses 1, 2, 3, and 4 in clinical samples of pediatric patients by multiplex reverse transcription-PCR. J Clin Microbiol. 38:1191-1195.




Buchman, TG, Rossier, M, Merz, WG and Charache, P 1990. Detection of surgical pathogens by in vitro DNA amplification. Part I. Rapid identification of Candida albicans by in vitro amplification of a fungus-specific gene. Surgery. 108:338-347.

Cruz, MDL, Ramirez, F and Hernandez, H 1997. DNA isolation and amplification from cacti. Plant Mol Biol Rep. 15:319-325.
Chang, M, Hyun, IH and Lee, YH 1998. Bipolaris stem rot of cactus caused by Bipolaris cactivora (Petrak) Alcorn. Korean J Plant Pathol. 14:661-663.
Choi, M-O, Kim, SG, Hyun, I-H, Kim, JH, Cho, C-H, Park, MS and Kim, YH 2010. First report of black spot caused by Alternaria alternata on grafted cactus. Plant Pathol J. 26:80-82.

Coiras, MT, Perez-Brena, P, Garcia, ML and Casas, I 2003. Simultaneous detection of influenza A, B, and C viruses, respiratory syncytial virus, and adenoviruses in clinical samples by multiplex reverse transcription nested-PCR assay. J Med Virol. 69:132-144.


Fan, J, Henrickson, KJ and Savatski, LL 1998. Rapid simultaneous diagnosis of infections with respiratory syncytial viruses A and B, influenza viruses A and B, and human parainfluenza virus types 1, 2, and 3 by multiplex quantitative reverse transcription-polymerase chain reaction-enzyme hybridization assay (Hexaplex). Clin Infect Dis. 26:1397-1402.


Grondahl, B, Puppe, W, Hoppe, A, Kuhne, I, Weigl, JA and Schmitt, HJ 1999. Rapid identification of nine microorganisms causing acute respiratory tract infections by single-tube multiplex reverse transcription-PCR: feasibility study. J Clin Microbiol. 37:1-7.




Hameed, A, Iqbal, Z, Asad, S and Mansoor, S 2014. Detection of multiple potato viruses in the field suggests synergistic interations among potato viruses in Pakistan. Plant Pathol J. 30:407-415.


Hyun, IH, Lee, SD, Lee, YH and Heo, NY 1998. Mycological characteristics and pathogenicity of Fusarium oxysporum Schlecht. emend. Snyd. & Hans. causing stem rot of cactus. Korean J Plant Pathol. 14:463-466.
Kim, YH, Jun, OK, Sung, MJ, Shin, JS, Kim, JH and Jeoung, MI 2000. Occurrence of Colletotrichm stem rot caused by Glomerella cingulata on graft-cactus in Korea. Plant Pathol J. 16:242-245.
Kim, JH, Jeon, YH, Kim, SG and Kim, YH 2007. First report on bacterial soft rot of graft-cactus Chamaecereus silvestrii caused by Pectobacterium carotovorum subsp. Carotovorum in Korea. Plant Pathol J. 23:314-317.

Kwak, H-R, Kim, M-K, Shin, J-C, Lee, Y-J, Seo, J-K and et al 2014. The current incidence of viral disease in Korean sweet potatoes and development of multiplex RT-PCR assays for simultaneous detection of eight sweet potato viruses. Plant Pathol J. 30:416-424.



Liolios, L, Jenney, A, Spelman, D, Kotsimbos, T, Catton, M and Wesselingh, S 2001. Comparison of a multiplex reverse transcription-PCR-enzyme hybridization assay with conventional viral culture and immunofluorescence techniques for the detection of seven viral respiratory pathogens. J Clin Microbiol. 39:2779-2783.




Li, Mingzhu, Inada, Minoru, Watanabe, Hideki, Suga, Haruhisa and Kageyama, Koji 2013. Simultaneous detection and quantification of Phytophthora nicotianae and P. cactorum, and distribution analyses in strawberry greenhouses by duplex real-time PCR. Microbes Environ. 28:195-203.


Song, CY, Ahn, DH, Cho, CH, Chung, JW and Nam, SY 2009a. Exporting promotion strategy of grafted cacti. Flower Res J. 17:67-73.
Song, CY, Ahn, DH, Kim, YS, Park, IT and Cho, CH 2009b. Export market trends of grafted cacti. Flower Res J. 17:62-66.



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




