Evaluation of Antimicrobial Properties of Lichen Substances against Plant Pathogens
Article information
Abstract
Plant pathogens pose major threats on agriculture and horticulture, causing significant economic loss worldwide. Due to the continuous and excessive use of synthetic pesticides, emergence of pesticide resistant pathogens has become more frequent. Thus, there is a growing needs for environmentally-friendly and selective antimicrobial agents with a novel mode of action, which may be used in combination with conventional pesticides to delay development of pesticide resistance. In this study, we evaluated the potentials of lichen substances as novel biopesticides against eight bacterial and twelve fungal plant pathogens that have historically caused significant phytopathological problems in South Korea. Eight lichen substances of diverse chemical origins were extracted from axenic culture or dried specimen, and further purified for comparative analysis of their antimicrobial properties. Usnic acid and vulpinic acid exhibited strong antibacterial activities against Clavibacter michiganensis subsp. michiganensis. In addition, usnic acid and vulpinic acid were highly effective in the growth inhibition of fungal pathogens, such as Diaporthe eres, D. actinidiae, and Sclerotinia sclerotiorum. Intriguingly, the growth of Rhizoctonia solani was specifically inhibited by lecanoric acid, indicating that lichen substances exhibit some degrees of selectivity to plant pathogens. These results suggested that lichen substance can be used as a selective biopesticide for controlling plant disease of agricultural and horticultural significance, minimizing possible emergence of pesticide resistant pathogens in fields.
Microbial pathogens cause phythopathological problems in agricultural production worldwide, resulting in an annual estimated loss of 10–15% of the world’s major crops with economic losses of up to hundreds of billions of dollars (Rizzo et al., 2021; Savary et al., 2019). Synthetic chemical pesticides have been used to prevent and minimize the occurrence of plant diseases caused by bacteria, fungi and insects. However, extensive use of synthetic chemical pesticides poses environmental and ecological threats, including increased pathogen populations resistant to pesticides, soil compaction, and environmental pollution (Peng et al., 2021). Therefore, there have been extensive efforts to search for environmentally-friendly, effective, and novel antimicrobial compounds derived from nature (Atanasov et al., 2021). Microbial organisms are rich sources of natural products with antimicrobial activities, inhibiting the growth and metabolic activities of competing microbes (Ghorbanpour et al., 2018; Ongena and Jacques, 2008; Raaijmakers and Mazzola, 2012; Thomashow et al., 1997).
Lichens are complex organisms that form symbiotic associations between a fungus (so-called lichen-forming fungi) and a photosynthetic partner which can be a green algae or a cyanobacteria (Honegger, 1991). Lichens are known to produce more than a thousand of structurally diverse compounds, many of which are exclusively found in lichens (Calcott et al., 2018; Goga et al., 2018; Stocker-Wörgötter, 2008). These lichen-specific substances have received considerable attention for their unique chemical structures and diverse biological activities (Boustie and Grube, 2005; Calcott et al., 2018; Dayan and Romagni, 2001; Lawrey, 1986; Molnár and Farkas, 2010; Stocker-Wörgötter, 2008). Specifically, baeomycesic acid is a potent dose-dependent inhibitor of 5-lipoxygenase (Ingólfsdóttir et al., 1997); lecanoric acid is used as an antioxidant (Luo et al., 2009); lobaric acid exhibits various biological activities, including antitumor, anti-proliferation, anti-inflammation, and antioxidant activities (Hong et al., 2018); salazinic acid is known to be antimicrobial (Candan et al., 2007); structurally-related squamatic acid and thamnolic acid also exhibit antimicrobial activity (Cankiliç et al., 2017); usnic acid is antimicrobial and anticancer (Lee et al., 2020; Yang et al., 2016); vulpinic acid exerts antifungal, anticancer, and antioxidative effects (Yi et al., 2019).
Although crude lichen extracts have been used for comparative study on antimicrobial activities (Halama and Van Haluwin, 2004; Huneck, 1999; Molnár and Farkas, 2010; Oh et al., 2006; Ranković and Mišić, 2008), there were only a few comparative studies of biological activities of purified lichen substances against a set of organisms of interest (König and Wright, 1999). In addition, little is known about the antagonistic effect of lichen substances against bacterial and fungal pathogens that have caused phythopathological problems in South Korea (Oh et al., 2006). Therefore, in this study, we conducted comparative analysis of the above mentioned eight lichen-specific substances to evaluate their antimicrobial activities against bacterial and fungal plant pathogens notorious for their diseasecausing abilities to crops and orchard trees of agricultural and horticultural importance in South Korea.
Materials and Methods
Preparation of purified lichen substances
Baeomycesic acid, thamnolic acid, and squamatic acid were extracted, and purified from dried specimens of Thamnolia vermicularis (aka. snow tea in Tibet). In brief, lichen thalli were soaked in acetone, and then subject to sonication for 30 min. The acetone extracts were evaporated to dryness on a Rotavapor and reconstituted with a reduced volume of acetone. The concentrated extract was subjected to preparative thin-layer chromatography (TLC) with toluene-dioxaneacetic acid (18:4.5:0.5, v/v) as eluent. Four fractions (T1–T4) containing UV-active compounds were collected separately by scraping silica on preparative TLC plates. T2 and T4 fraction was further separated by preparative TLC to purify squamatic acid (6 mg) and baeomycesis acid (11 mg), respectively. To purify thamnolic acid, T1 and T3 fractions were mixed, evaporated to dryness, reconstituted with methanol, and subjected to preparative high-pressure liquid chromatography (HPLC). Separation was achieved on a HPLC instrument at a flow rate of 2.2 ml/min, using the Kromasil C18-column (250 × 10 mm, 5 μm, 10 nm; at 40°C), with UV monitoring at 254 nm. Thamnolic acid (t R = 32.0 min; 1.2 mg) was purified using a gradient program: 0–30 min, 20–100%; 30–40 min, 100%; 40–52 min, 20% of pump B. The mobile phase was composed of distilled water/trifluoroacetic acid (99.9:0.1, v/v) for pump A, and methanol/trifluoroacetic acid (99.9:0.1, v/v) for pump B.
Lobaric acid was extracted and purified from axenic culture of a lichen-forming fungus isolated from thalli of Stereocaulon alpinum (Institutional accession number: KoLRI022277) (Kim et al., 2021) The lichen-forming fungus was cultured in 200 ml of potato dextrose broth (PDB; Difco, Detroit, MI, USA) at 20°C with 12-h light and dark intervals for 1 month. The PDB culture was homogenized and mixed with an equal volume of ethyl acetate. The solvent layer was separated and evaporated to dryness on a Rotavapor. The crude extract was reconstituted with methanol and subjected to preparative HPLC. Separation was achieved on a preparative HPLC as described above except for the followings: gradient elution of 60–70% A (v/v) over 52 min; a flow rate of 2.5 ml/min; the column temperature at 25°C.
Salazinic acid with purity above 95% was obtained from Dr. Chang, Dong-jo (College of Pharmacy, Sunchon National University, Suncheon, South Korea). Lecanoric acid (Gaia Chemical Corp., New Milford, CT, USA), usnic acid (Sigma-Aldrich, St. Louis, MO, USA), and vulpinic acid (Toronto Research Chemicals Inc., North York, ON, Canada) were purchased from the commercial vendors. Chemical identities of all the purified and purchased lichen substances were confirmed by comparing their relative retention time and the UV spectra to those in our in-house lichen substance database (Yoshimura et al., 1994). Thirty microliters of lichen substances (1 mg/ml) were injected for HPLC analysis.
Bacterial and fungal plant pathogens
Bacterial and fungal strains were obtained from the Korean Agricultural Culture Collection (KACC) (http://genebank.rda.go.kr/microbeMain.do), unless otherwise mentioned. Bacterial strains used in this study were as follows: Clavibacter michiganensis subsp. michiganensis (KACC 16995), Dickeya chrysanthemi (KACC 10165), Erwinia pyrifoliae (KACC 13946), Pseudomonas syringae pv. actinidiae (KACC 10593), Ralstonia solanacearum (KACC 10672), Xanthomonas arboricola pv. pruni (KACC 18156), X. euvesicatoria (KACC 18723), and X. citri (KACC 10444). The bacterial strains were maintained on tryptic soy broth (TSB; Becton, Dickinson and Company, Sparks, MD, USA).
Fungal strains used in this study were as follows: Alternaria alternata (KACC 40019), A. mali (KACC 44418), Colletotrichum gloeosporioides (KACC 40896), Diaporthe actinidiae (KACC 48275), Fusarium oxysporum f. sp. lycopersici (KACC 40038), Pythium ultimum (KACC 40705), Rhizoctonia cerealis (KACC 40154), R. solani AG-2-2 (KACC 40151), and Sclerotinia sclerotiorum (KACC 40457). Also, fungal strains of Botrytis cinerea, Botryosphaeria dothidea, and D. eres were obtained from Dr. Yang, Kwang-Yeol (Chonnam National University, Gwangju, South Korea). The fungal strains were maintained on potato dextrose agar (PDA; Difco).
Paper disk diffusion assay
Bacterial strains were grown in TSB overnight, and the suspensions were standardized to a final concentration of 1 × 105 colony-forming units (cfu)/ ml. One milliliter of the bacterial suspension was added to 100 ml of tryptic soy agar (TSA; Becton, Dickinson and Co.), and mixed well before distribution to a petri dish (90-mm in diameter). For paper disk assay, 40 μl of purified lichen substances dissolved in methanol (1 mg/ml) were applied to an 8 mm paper disc. Disks containing the same volume of methanol and 200 μg/ml of streptomycin sulfate (Sigma-Aldrich) were prepared as a negative control and a positive control, respectively. Disks were dried in fume hood and placed onto TSA. The plates were incubated for 1–3 days at 30°C until bacterial growth was visible. Fungal strains were grown on quarter-strength PDA (QPDA) in a 90-mm petri dish for 1–3 days until they cover about half of the plate. Disks containing each lichen substance were placed onto the margins of the plate. The plates were incubated for additional 1–3 days at 23°C until fungi completely cover the plates. Methanol and 200 μg/ml of chlorothalonil (Sigma-Aldrich) were used as a negative control and a positive control, respectively. The experiments were conducted twice independently, each with three replicate plates.
Determination of minimal inhibitory concentration
Minimal inhibitory concentration (MIC) values of lichen substances that inhibited the growth of C. michiganensis subsp. michiganensis in paper disk assay were determined by a broth microdilution method in 96-well microtiter plates (Wiegand et al., 2008). Serial dilution of four lichen substances (lecanoric acid, lobaric acid, usnic acid, and vulpinic acid) was made to obtain final concentrations ranging from 1 to 500 μg/ml. These dilution series were added to bacterial suspensions adjusted to 5 × 105 cfu/ml in TSB. Oxolinic acid and oxytetracycline hydrochloride (Sigma-Aldrich) were used as positive controls. The microtiter plates were incubated at 30°C for 24 h, and the optical density (620 nm) was measured using a microplate reader (BioTek Instruments, Winooski, VT, USA). The MIC value was defined as the lowest concentration of compounds that completely inhibited bacterial growth after 24-h incubation. MIC values were determined by three replicates.
Determination of median effective concentration (EC50)
Fungal strains whose growth was inhibited by lichen substances were grown on QPDA containing two-fold dilution of lichen substances, ranging from 50 to 2.5 μg/ml. Colony diameter of fungal strains grown on QPDA containing lichen substance was measured when fungal strains completely covered QPDA containing 5% of methanol. The inhibition percentages were calculated using the formula: I (%) = ([(C – d) – (T – d)])/ ((C – d)) × 100, where d is the diameter of the initial agar plug containing mycelia, I is the inhibition (%), and C and T are the average colony diameters of the negative control and treatment, respectively. The average inhibition percentage of the triplicates was used to calculate the EC50 of the lichen substance.
Results
Purification of lichen substances
To test antimicrobial properties of lichen substances, we prepared eight well-known lichen substances with diverse chemical structures from different biological sources (see Materials and Methods). Included were four depside-class compounds (baeomycesic acid, lecanoric acid, squamatic acid, and thamnolic acid), two depsidone-class compounds (lobaric acid and salazinic acid), a dibenzofuran (usnic acid), and vulpinic acid (Fig. 1). Lecanoric acid and lobaric acid are compounds of the orcinol series, while baeomycesic acid, salazinic acid, squamatic acid, and thamnolic acid are compounds of the β-orcinol series. Usnic acid is a product of dimerization of two methylphloroacetophenone molecules, and vulpinic acid is a product of dimerization of two aromatic amino acids. The authenticity and purity of the eight lichen substances compounds were confirmed by HPLC analyses in which the relative retention time and UV spectra of lichen substances were compared to those in our in-house lichen substance database (Yoshimura et al., 1994) (Fig. 1).
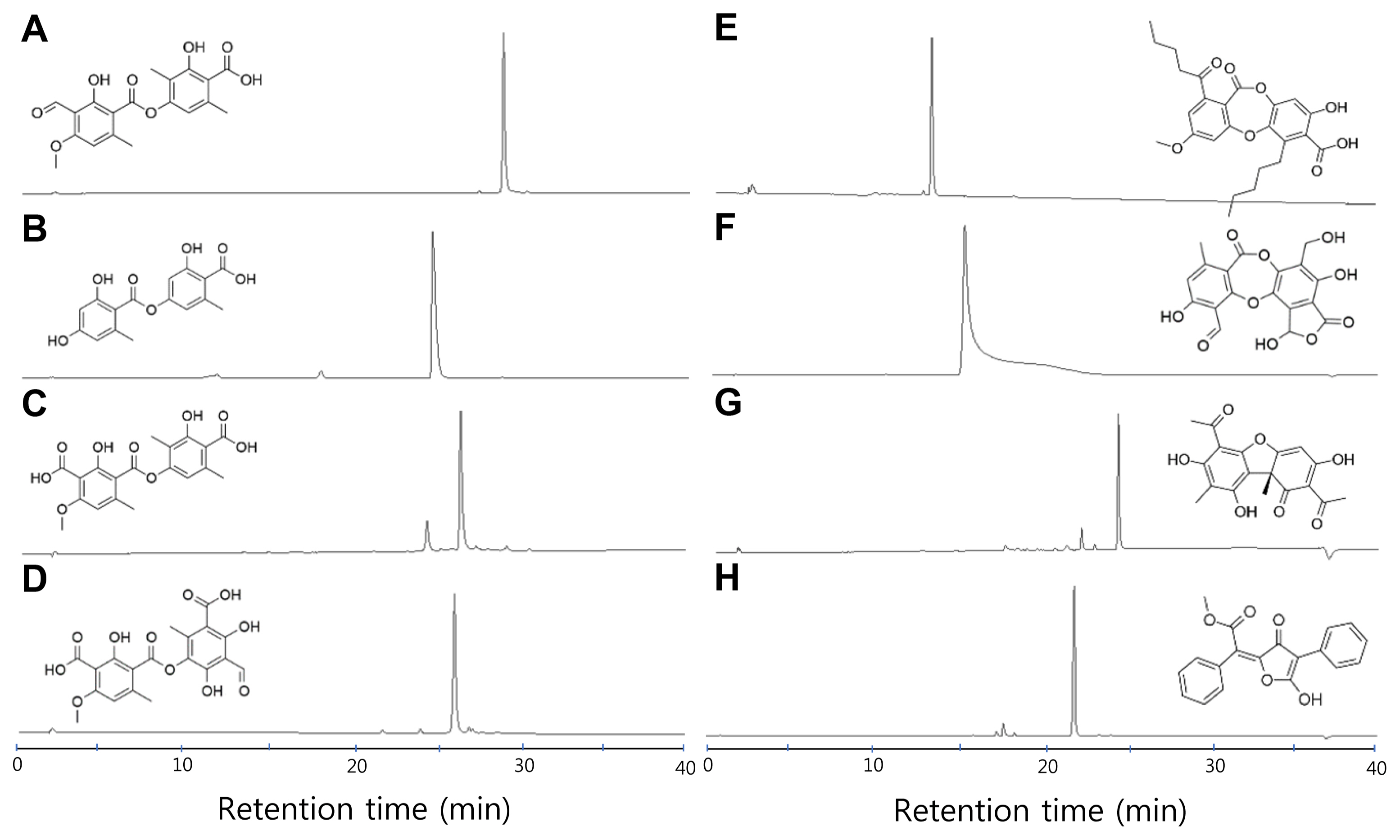
High-pressure liquid chromatography and chemical structures of purified lichen substances. The major peaks in each chromatogram represent the indicated lichen substances: baeomycesic acid (A), lecanoric acid (B), squamatic acid (C), thamnolic acid (D), lobaric acid (E), salazinic acid (F), usnic acid (G), or vulpinic acid (H).
Antibacterial activities of lichen substances
We tested the antibacterial activities of the eight purified lichen substances, using a paper disk diffusion method. As a result, four lichen substances, lecanoric acid, lobaric acid, usnic acid, and vulpinic acid, were antibacterial to the Gram-positive C. michiganensis subsp. michiganensis (Fig. 2A). However, the eight lichen substances did not exhibit any significant activities against the other Gram-negative bacteria. Notably, usnic acid and vulpinic acid exhibited strong antibacterial activities against C. michiganensis subsp. michiganensis (Fig. 2B). The antibacterial activity of lobaric acid was comparable to that of streptomycin that was used as a positive control in this analysis.
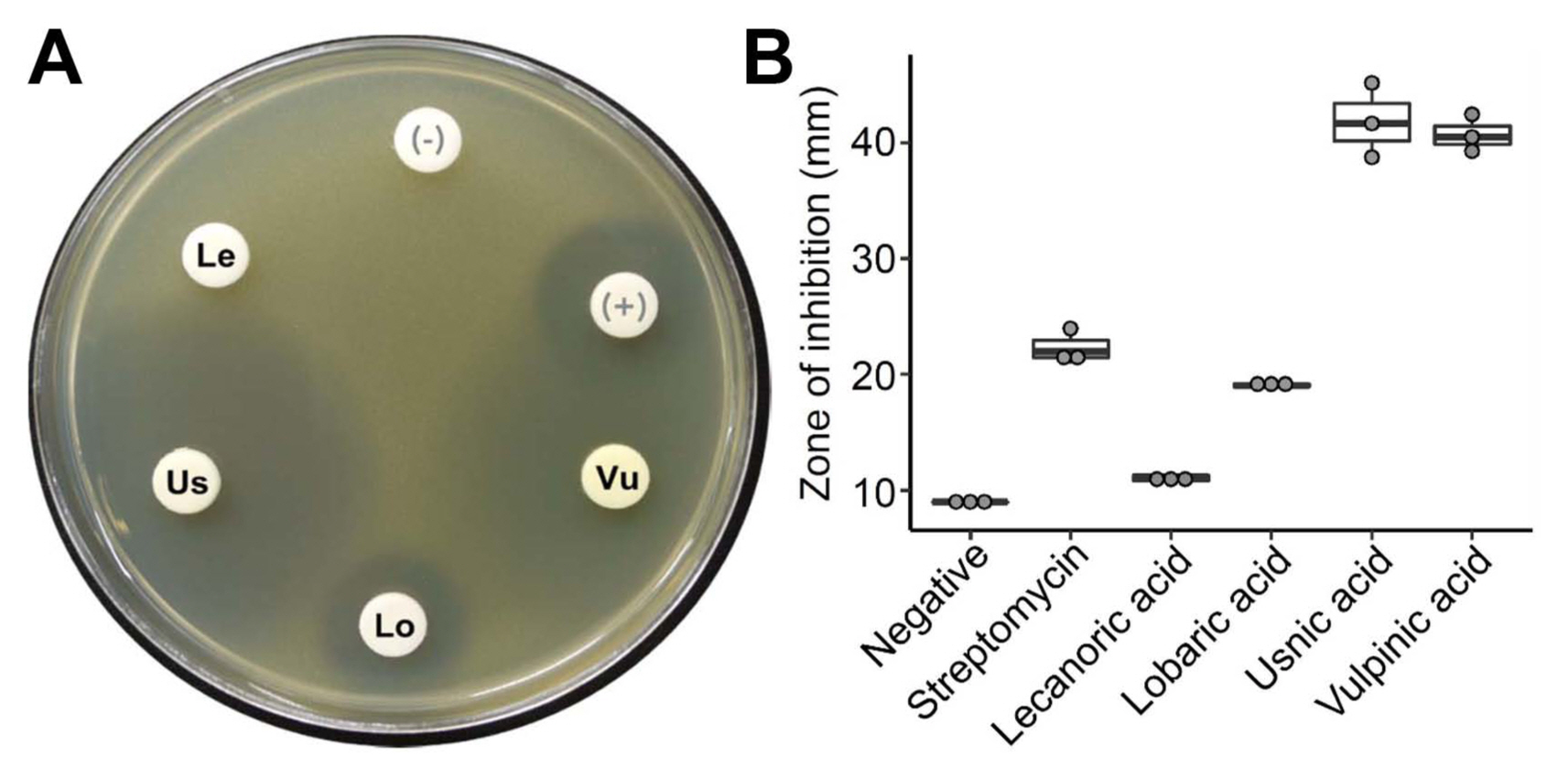
Antibacterial activity of lichen substances. (A) An example of the culture plate of Clavibacter michiganensis subsp. michiganensis showing zone of inhibition by lichen substances: lecanoric acid (Le), lobaric acid (Lo), usnic acid (Us), vulpinic acid (Vu), methanol as a negative control (−), streptomycin as a positive control (+). (B) Relative growth inhibition of Clavibacter michiganensis subsp. michiganensis by lichen substances. Zone of inhibition was measured as diameter of a clear zone of bacterial growth inhibition including a paper disk. Circles indicate data points from three replicate experiments.
Antifungal activities of lichen substances
Antifungal activities of purified lichen substances were tested against a panel of plant pathogenic fungi representing diverse fungal taxa (Fig. 3). As a result, the tested lichen substances, except for salazinic acid, exhibited strong or weak antifungal activities. Lichen substances, such as usnic acid, vulpinic acid, and lecanoric acid, displayed a broad spectrum of fungal growth inhibition, while lichen depsides of the β-orcinol series (baeomycesic acid, squamatic acid, and thamnolic acid) have a narrow range of antifungal activities, marginally inhibiting the growth of only fast-growing fungi, such as B. cinerea and S. sclerotiorum that belong to the fungal class Leotiomycetes. Lobaric acid, a depsidone of the orcinol series, was only active against S. sclerotiorum. It is notable that usnic acid and vulpinic acid strongly inhibit the growth of plant pathogenic fungi, D. actinidae, D. eres, and S. sclerotiorum. In addition, the growth of B. dothidea and R. solani were inhibited by vulpinic acid and lecanoric acid, respectively. None of the tested lichen substance was active against A. alternata and P. ultimum.
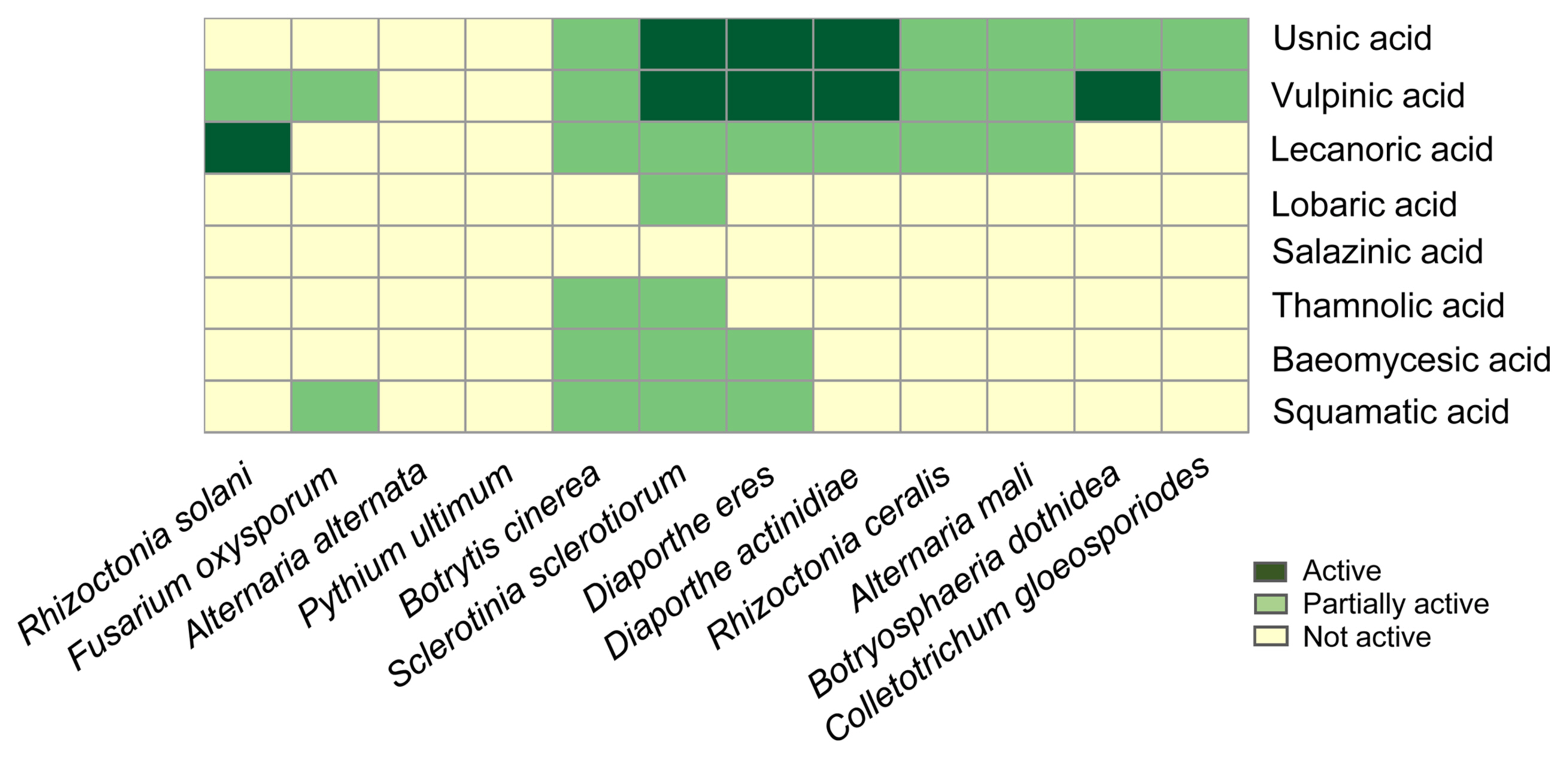
Antifungal activity of lichen substances. Relative antifungal activities of lichen substances against 12 plant pathogenic fungi are shown as heatmap. The antifungal activities were considered ‘Active’ when fungal mycelia did not touch paper discs containing lichen substances, or considered ‘Partially active’ when fungal mycelia did touch, but cannot cover paper discs containing lichen substances.
Potentials of lichen substances as novel biopesticides
To further investigate antimicrobial properties of lichen substances, we determined MIC of lichen substances that showed significant antibacterial activities against C. michiganensis subsp. michiganensis. Vulpinic acid (MIC 3.9 μg/ml) and usnic acid (MIC 7.812 μg/ml) exhibited lower MIC values than commercially available antibiotics, such as oxytetracycline and oxolinic acid (Table 1). Although lobaric acid was active against C. michiganensis subsp. michiganensis in our paper disk diffusion assay (Fig. 2), its MIC value was 250 μg/ml. This difference may be attributable to relatively low solubility of the compounds in the growth media where the bacteria were cultured. Also, we determined EC50 values for antifungal lichen substances. Fungal strains that were sensitive to lichen substances were grown in media containing varying concentrations of lichen substances. Usnic acid strongly inhibited the growth of two related fungal pathogens, D. actinidae and D. eres, and vulpinic acid was highly effective in growth inhibition of S. sclerotiorum (Fig. 4). It is noteworthy that R. solani (the sole basidiomycetous fungus among the tested fungal pathogens) was sensitive only to lecanoric acid (Fig. 4).
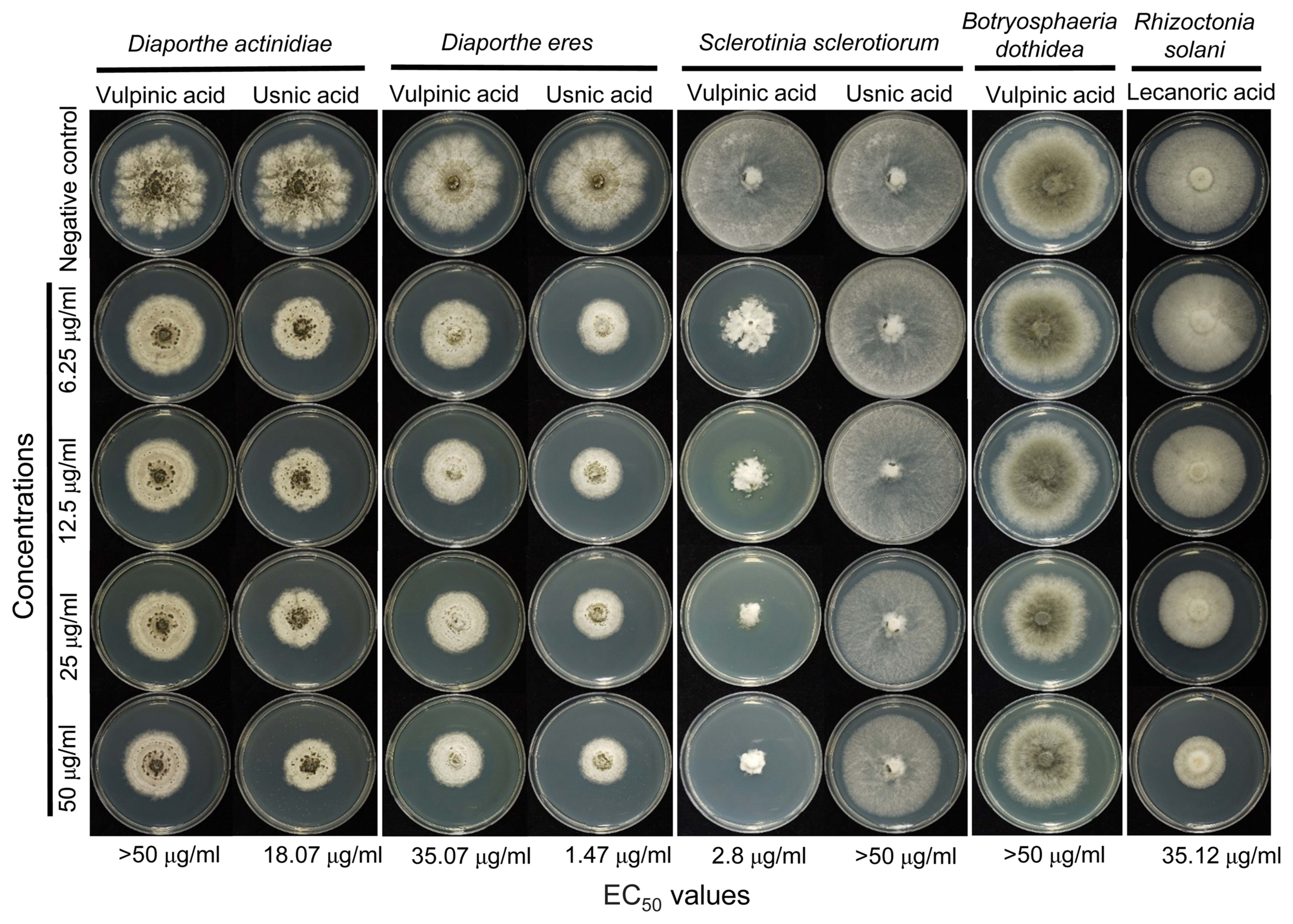
The effective concentration (EC50) of lichen substances against plant pathogenic fungi. Antifungal activities were measured by growth rate on quarter-strength potato dextrose agar containing indicated concentrations of lichen substances. The EC50 for each combination of fungi and lichen substances were estimated and indicated at the bottom. For negative controls, media were supplemented with methanol as a solvent control (5%).
Discussion
Newly emerging plant pathogens and frequent occurrence of pesticide-resistant strains adversely affect agricultural production worldwide. To cope with these global issues, there is a growing needs for discovering antimicrobial agents with a novel mode of action that selectively inhibits specific plant pathogens. Ever since the antimicrobial properties of extracts of lichen thalli were reported (Burkholder et al., 1944), numerous studies have confirmed a variety of biological activities of purified lichen substances as well as lichen extracts (Boustie and Grube, 2005; Calcott et al., 2018; Dayan and Romagni, 2001; Halama and Haluwin, 2004; Huneck, 1999; Kokubun et al., 2007; Lawrey, 1986; Manojlovic et al., 2012; Molnár and Farkas, 2010; Oh et al., 2006; Ranković and Mišić, 2008; Stocker-Wörgötter, 2008). However, there have been limited studies on antimicrobial activities of lichen substances against bacterial and fungal pathogens that have caused significant phytopathological problems in South Korea. In this study, we compared antimicrobial properties of eight purified lichen substances against a panel of bacterial and fungal pathogens, and found that lichen substances exhibit strong activities and high degrees of selective toxicity to specific plant pathogens, suggesting use of lichen substances as novel biopesticides.
Among the eight bacterial pathogens, lichen substances tested in this study showed antibacterial activities only to a Gram-positive bacterium, C. michiganensis subsp. michiganensis. In particular, usnic acid and vulpinic acid were potent antibacterial agents for controlling bacterial canker of tomato caused by the bacterial pathogen, having estimated MIC values below 10 μg/ml. The purified lichen substances did not show any activities against seven Gram-negative bacterial pathogens tested in this study. Previous studies also have reported that lichen substances tend to be inactive to Gram-negative bacteria (Francolini et al., 2004; Ingólfsdóttir, 2002; Lauterwein et al., 1995; Melgarejo et al., 2008; Paudel et al., 2012; Yilmaz et al., 2005).
Usnic acid showed strong antifungal activities against D. actinidiae and D. eres that cause postharvest kiwifruit rots in South Korea (Lee et al., 2001; K.-Y. Yang, personal communication, Aug 5, 2021). Although moderate antifungal activities of usnic acid to different fungal species have been reported (Schmeda-Hirschmann et al., 2008), our finding suggested that usnic acid is a selective antifungal agent against these fruit rot pathogens. Although the mode of action for the antifungal activity of usnic acid is currently unknown, inhibition of the biosynthesis of nucleic acids in Gram-positive bacteria, such as Bacillus subtilis and Staphylococcus aureus, was thought to be the primary cause of antibacterial activity of usnic acid (Maciąg-Dorszyńska et al., 2014). Vulpinic acid exhibited a broad range of antifungal activities against plant pathogenic fungi, as previously reported (Kowalski et al., 2011). The antifungal activity was highest against S. sclerotiorum with an estimated EC50 value of 2.8 μg/ml, which indicates that vulpinic acid is potent and may be used for controlling the Sclerotinia rot diseases on different vegetables and fruits. Although there has been no study on the mode of action at a molecular level, vulpinic acid is known to be involved in disruption of membrane integrity and cell division (Kwon et al., 2016; Shrestha et al., 2016). Lecanoric acid selectively inhibit the growth of R. solani with an estimated EC50 value of 35.12 μg/ml. Use of this lichen substance in golf courses may be an environmentally-friendly option for managing brown patches caused by R. solani on creeping bentgrass (Agrostis palustris Huds.) and perennial ryegrasses grown in fairway. Our comparative analysis of antimicrobial properties of lichen substances revealed a high degree of selective toxicity, some of which exhibited strong activities against Gram-positive C. michiganensis subsp. michiganensis and specific fungal pathogens. These lichen substances need to be tested in greenhouse and field conditions to prove their effectiveness in controlling the diseases caused by the plant pathogens. Given the observed selectivity, lichen substances likely have potential for use as biopesticides with novel mode of actions. Nevertheless, precise mechanisms of antimicrobial activities of lichen substances need to be determined in order for this chemicals to be safely used as pesticides. This study reinvestigated potentials of lichen substances as promising candidates for environmental-friendly pesticides that can be incorporated into integrated pest management programs.
Acknowledgments
This work was supported by the Korea Research Fellowship Program funded by the Ministry of Science, ICT, and Future Planning (2018H1D3A1A01074888) and by The Korean National Research Resource Center Program (2017M3A9B8069471), through the National Research Foundation of Korea (NRF).
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.

