The CsSTE50 Adaptor Protein in Mitogen-Activated Protein Kinase Cascades Is Essential for Pepper Anthracnose Disease of Colletotrichum scovillei
Article information
Abstract
Anthracnose, caused by the ascomycete fungus Colletotrichum scovillei, is a destructive disease in pepper. The fungus germinates and develops an infection structure called an appressorium on the plant surface. Several signaling cascades, including cAMP-mediated signaling and mitogen-activated protein kinase (MAPK) cascades, are involved in fungal development and pathogenicity in plant pathogenic fungi, but this has not been well studied in the fruit-infecting fungus C. scovillei. Ste50 is an adaptor protein interacting with multiple upstream components to activate the MAPK cascades. Here, we characterized the CsSTE50 gene of C. scovillei, a homolog of Magnaporthe oryzae MST50 that functions in MAPK cascades, by gene knockout. The knockout mutant ΔCsste50 had pleiotropic phenotypes in development and pathogenicity. Compared with the wild-type, the mutants grew faster and produced more conidia on regular agar but were more sensitive to osmotic stress. On artificial and plant surfaces, the conidia of the mutant showed significantly reduced germination and failed to form appressoria. The mutant was completely non-pathogenic on pepper fruits with or without wounds, indicating that pre-penetration and invasive growth were both defective in the mutant. Our results show that the adaptor protein CsSTE50 plays a role in vegetative growth, conidiation, germination, appressorium formation, and pathogenicity in C. scovillei.
Colletotrichum scovillei, in the C. acutatum species complex, is a fungal pathogen causing anthracnose disease on pepper fruits (Capsicum annuum L.), leading to significant loss of pepper production (Oo et al., 2017). Combined sequence analysis revealed that C. scovillei is the dominant pepper anthracnose species in many countries, including South Korea (Caires et al., 2014; Diao et al., 2017; Kanto et al., 2014; Khalimi et al., 2019; Noor and Zakaria, 2018; Oo et al., 2017; Zhao et al., 2016). C. scovillei first invades the pepper fruit cuticle layer using the turgor pressure of the appressorium, in which a highly branched structure called the dendroid structure develops and extends toward surrounding cells (Fu et al., 2021; Shin et al., 2021). Subsequently, a thick, invasive hypha emerges from the dendroid structure and penetrates an epidermal cell under the pepper fruit cuticle layer.
Various physical and chemical signals are involved in initiating pathogenic development in plant pathogenic fungi. For example, in the rice blast fungus Magnaporthe oryzae, membrane receptors and sensors recognize hydrophobicity, cyclic adenosine-5′-monophosphate (cAMP), and cutin monomers, inducing appressorium formation (Lee and Dean, 1993; Lee and Lee, 1998; Shin et al., 2019a; Skamnioti and Gurr, 2007). Upon recognizing chemical and physical host signals, intracellular signaling pathways such as the cAMP-dependent protein kinase or mitogen-activated protein kinase (MAPK) cascades are activated leading to appressorium development (Mitchell and Dean, 1995; Xu and Hamer, 1996; Zhao et al., 2007). In C. scovillei, it was shown that hydrophobicity, cAMP, cutin monomers, and CaCl2 are involved in appressorium formation (Fu et al., 2021; Shin et al., 2022). Deletion of CsPMK1 led to defects in appressorium formation and pathogenicity in C. scovillei, indicating that MAPK signaling is important for fungal pathogenicity (Fu et al., 2022). However, the molecular mechanisms of fungal development and pathogenicity in the fruit-infecting fungi C. scovillei are still largely unknown.
MAPKs are serine/threonine protein kinases that mediate signal transduction from a variety of extracellular stimuli to the nucleus. The MAPK cascade involves three protein kinases: MAPK kinase kinase (MAPKKK), MAPK kinase (MAPKK), and MAPK. MAPKKK activates MAPKK, which subsequently activates MAPK. In fungi, MAPK signaling regulates essential developmental processes, stress responses, and pathogenicity (Hagiwara et al., 2009; Mehrabi et al., 2009). In yeast, the adaptor protein Ste50 brings the MAPKKK Ste11 to the plasma membrane, which leads to activation of the high osmolarity glycerol MAPK pathway in response to high osmotic stress (Ramezani-Rad, 2003; Saito and Posas, 2012; Wu et al., 2006). The adaptor protein Ste50 contains sterile-alpha-motif (SAM) and Ras-association (RA) domains. The SAM domain of Ste50 binds to a SAM domain of the MAPKKK Ste11 (Truckses et al., 2006). The RA domain is required for delivering Ste11-Ste50 complexes to the plasma membrane (Truckses et al., 2006). In M. oryzae, Mst50, a homolog of Ste50, functions as an adaptor protein interacting with both MAPKKK Mst11 and MAPKK Mst7 to activate the Pmk1 MAPK pathway, which is required for appressorium formation and plant infection (Park et al., 2006; Xu and Hamer, 1996). These studies indicate that Ste50 interacts with multiple upstream components to activate the MAPK cascades regulating hyperosmotic stress, development, and pathogenicity in fungi.
In this study, we characterized the C. scovillei STE50 gene, a homolog of M. oryzae MST50. The ΔCsste50 mutant exhibited defects in stress tolerance, conidial germination, appressorium formation, and pathogenicity. Interestingly, we found that mycelial growth and conidial production were increased in the ΔCsste50 mutant, unlike ste50 deletion mutants of other fungal pathogens. These results help elucidate the roles of STE50 in fungal development and pathogenicity.
Materials and Methods
Fungal strains and culture conditions
C. scovillei strain KC05 was used as the wild-type strain in this study (Han et al., 2016). The deletion mutant and complementation strain were selected on TB3 agar (200 g of sucrose, 3 g of yeast extract, 3 g of casamino acids, 10 g of glucose, and 8 g of agar per liter) supplemented with 200 μg/ml of hygromycin B (EMD Millipore, Billerica, MA, USA) and 400 μg/ml of G418 geneticin (Gibco, Carlsbad, CA, USA), respectively. CM agar (10 g of sucrose, 6 g of yeast extract, 6 g of casamino acids, and 15 g of agar per liter) supplemented with 0.4 M NaCl or KCl was used to test for osmotic stress tolerance. V8 agar (V8A, 80 ml of V8 juice, 310 μl 10 N NaOH, and 15 g of agar/l) was used to measure conidiation.
Bioinformatics analysis
All DNA and protein sequences were obtained from the Comparative Fungal Genomics Platform (http://cfgp.snu.ac.kr) (Choi et al., 2013; Park et al., 2007), and the BLAST program in the National Center for Biotechnology Information (NCBI, http://www.ncbi.nlm.nih.gov). Protein sequence alignment and phylogenetic analysis were performed using the ClustalW program in MEGA 6.0 (Tamura et al., 2013; Thompson et al., 1994). Domain structure analysis was performed using InterPro Scan v83.0 (http://www.ebi.ac.uk/interpro/) (Mulder et al., 2005). Oligonucleotide primers used in this study were synthesized by Bioneer (Daejeon, Korea).
RNA isolation, reverse transcription polymerase chain reaction, and gene expression analysis
Total RNA was isolated from frozen fungal tissues using the Easy-Spin Total RNA Extraction Kit (Intron Biotechnology, Seoul, Korea) according to the manufacturer’s instructions. First-strand cDNA synthesis was performed from 5 μg total RNA using the oligo(dT) primer with the SuperScript III First-Strand Synthesis System Kit (Invitrogen Life Technologies, CA, USA). Detection of CsSTE50 and β–tubulin (CAP_007327) expression was performed using the primers CsSTE50_RTF/RTR as described by Shin et al. (2021). The primer sets used for reverse transcription polymerase chain reaction (RT-PCR) are listed in Supplementary Table 1. Experiments were conducted in triplicate and repeated three times.
Generation of knockout mutant
Fungal genomic DNA was isolated according to a standard method or the quick method (Chi et al., 2009; Sambrook et al., 1989). Approximately 1.5 kb fragments of upstream and downstream of CsSTE50 were amplified from wild-type KC05 genomic DNA using primers CsSTE50_5F/5R and CsSTE50_3F/3R, respectively. The 1.5 kb hygromycin resistance gene (hyg) cassette was amplified from pBCATPH using primers HPH_F/HPH_R, and fused to the amplified upstream and downstream fragments via the double-joint polymerase chain reaction (PCR) method (Yu et al., 2004). The resulting products were finally amplified using primers CsSTE50_NF/NR and transformed into protoplasts of the wild-type strain by the polyethylene glycol-mediated transformation method (Shin et al., 2019b; Sweigard et al., 1992). Putative knockout mutants were selected by screening PCR using primers CsSTE50_SF/SR and confirmed by southern blot hybridization and RT-PCR (Sambrook et al., 1989). For southern blot hybridization, genomic DNA was digested with MseI restriction enzyme and blot was probed with 0.5 kb downstream cassette. Biotin-High Prime (Roche, Indianapolis, IN, USA) was used to label the probe. ChemiDoc XRS + system with Quantity One software (Bio-Rad Laboratories, Hercules, CA, USA) was used to detect chemiluminescent signal. To complement the mutant, the CsSTE50 gene including 1.7 kb upstream and 500 kb downstream was amplified from wild-type genomic DNA using the primers CsSTE50_CF/CR, and the amplified fragments were co-transformed into protoplasts of the mutant with pII99 that contains a geneticin resistance gene (gen) cassette.
Phenotype analysis
To evaluate vegetative growth, fungal colonies were grown on CM agar and CM agar supplemented with an osmotic stress agent (0.4 M NaCl or KCl) for 5 days at 25°C in the dark. Conidiation was measured by counting the number of conidia harvested with 5 ml of sterile distilled water from 5-day-old V8 agar under continuous light, using a hemocytometer. Conidial morphology was observed under a light microscope, and conidial length was measured using the ZEN imaging software. To measure conidial germination and appressorium formation, conidial drops (5 × 104 conidia/ml) were placed on hydrophobic coverslips and incubated in a moistened box. Exogenous cAMP (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in sterile distilled water to yield a 10 mM solution, and mixed with an equal volume of conidial drops. To induce appressorium formation from hyphal tips, mycelial agar plugs obtained from 5-day-old oatmeal agar were placed on slide glasses, covered with coverslips, and incubated in a moistened box. For conidial penetration and infection assays, conidial drops (5 × 104 conidia/ml or 15 × 104 conidia/ml, respectively) were inoculated onto the surface of pepper fruits and incubated in a moistened box. For mycelial infection assays, mycelial agar plugs were placed onto the surface of pepper fruits and incubated in a moistened box. All experiments were repeated three times with three replicates.
Results
Identification of CsSTE50 in C. scovillei KC05
We identified CsSTE50 (CAP_001511), a homolog of M. oryzae MST50, via BLAST search of the Comparative Fungal Genomics Platform (Choi et al., 2013; Park et al., 2006). The CsSTE50 gene was predicted to encode a 486-amino-acid protein with SAM and RA domains (Fig. 1A and B). Phylogenetic analyses of Ste50 homologs in fungal species revealed that Ste50 homologs are conserved in the subphylum Pezizomycotina, distinct from yeast (Fig. 1A and B). The amino acids of the CsSTE50 and Ste50 homologs share less than 30% amino acid identity in yeast, compared with more than 72% in Pezizomycotina fungi. Ste50 homologs of M. oryzae, Neurospora crassa, Botrytis cinerea, C. gloeosporioides, C. higginsianum, Fusarium oxysporum, F. graminearum, Yarrowia lipolytica, S. cerevisiae, and Candida albicans showed 76.6%, 72.2%, 72.2%, 88.9%, 97.7%, 76.0%, 75.4%, 27.2%, 17.5%, and 20.9% amino acid identity, respectively, with CsSTE50.
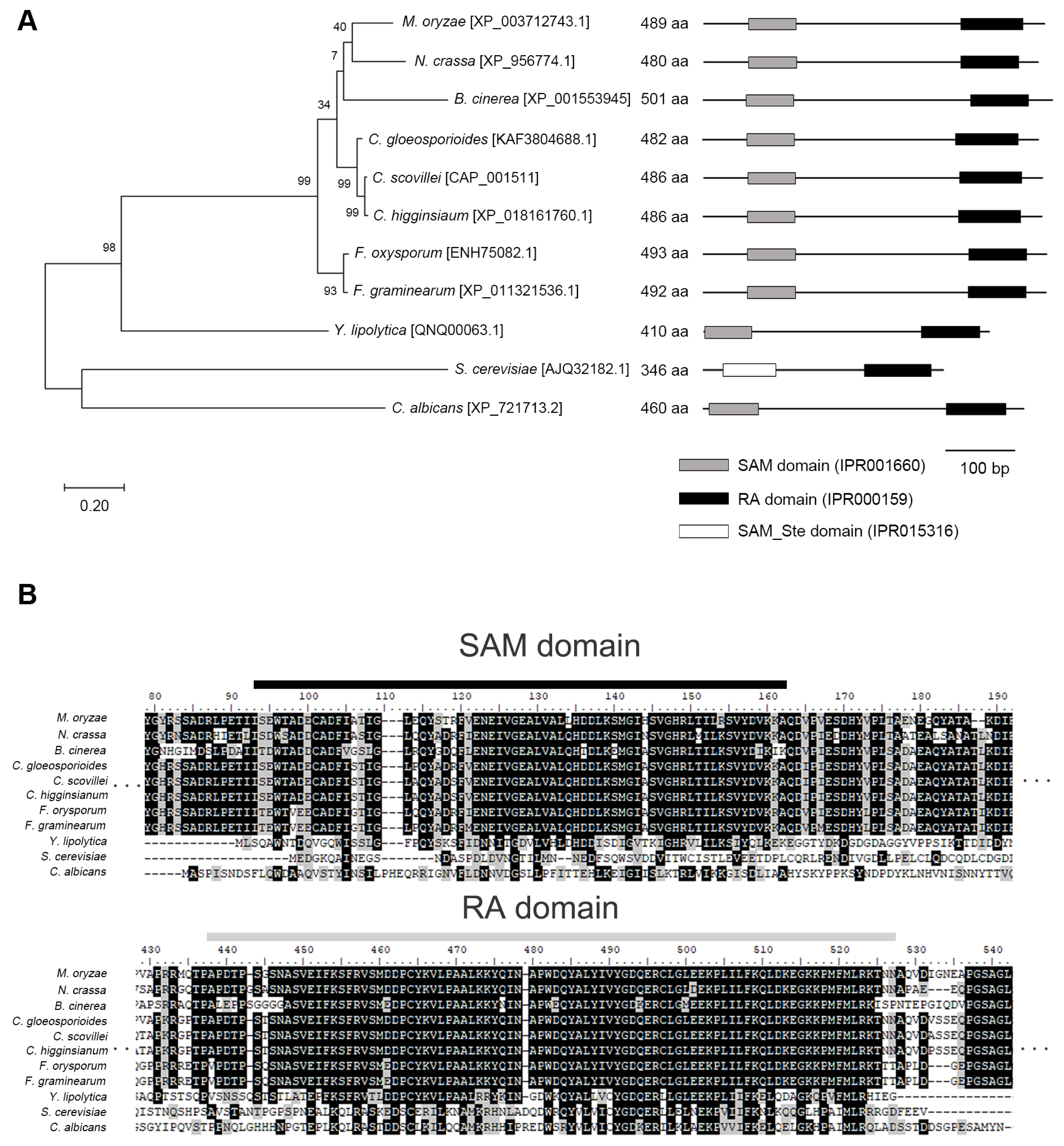
Phylogenetic analysis of Ste50 homologs in fungi. (A) Analysis of phylogenetic relationship. A maximum-likelihood tree (500 bootstrap replicates) was constructed using MEGA 6.0. The scale bar represents the number of amino acid differences per site. (B) Alignment of amino acid sequences. The amino acid sequences of the sterilealpha-motif (SAM) and Ras-association (RA) domains were aligned using ClustalW in MEGA 6.0. Identical amino acids and conserved substitutions are shaded in black and gray, respectively. The black and gray lines are the SAM and RA domains, respectively.
Targeted deletion of CsSTE50
To investigate the functional roles of CsSTE50, a gene replacement construct was generated using double-joint PCR (Fig. 2A). Approximately 1.5 kb upstream and downstream flanking sequences of the CsSTE50 gene were fused to the 1.5 kb hyg cassette via overlapping sequences (Supplementary Table 1, underlined), which generated a 4.5 kb construct. The construct was transformed into C. scovillei KC05 protoplasts. Southern blot hybridization was used to select a ΔCsste50 mutant exhibiting a 1.6-kb band instead of the 2.6-kb band of the wild-type (Fig. 2B), indicating that the CsSTE50 gene was replaced with the hyg cassette without ectopic insertion (Fig. 2B). Finally, RT-PCR confirmed that expression of the CsSTE50 gene was completely abolished in the ΔCsste50 mutant (Fig. 2C). All strains contained a band for the β-tubulin gene, as a positive control (Fig. 2C).

Targeted deletion of CsSTE50 in Colletotrichum scovillei. (A) Deletion strategy of CsSTE50. Double-joint polymerase chain reaction was performed to generate the construct. (B) Confirmation of targeted deletion mutant (ΔCsste50). The restriction enzyme MseI was used to digest genomic DNA, which was hybridized to a probe in Southern blotting. (C) Verification of complemented strain Csste50c. The expression of CsSTE50 was detected in the wild-type and Csste50c but not in the CsSTE50 deletion mutant. The β-tubulin gene was used as a reference.
Role of CsSTE50 in vegetative growth and stress tolerance
To investigate the role of CsSTE50 in the vegetative growth of C. scovillei, mycelial agar plugs of the ΔCsste50 mutant were inoculated on CM agar and incubated for 5 days. The growth of the ΔCsste50 mutant was slightly greater than that of the wild-type (Fig. 3A and B). We also evaluated the osmotic stress tolerance of the ΔCsste50 mutant by assessing the growth of the mutant on CM agar supplemented with an osmotic stress agent. The growth relative to that of the wild-type on CM agar without supplements was assessed. Compared with the wild-type, the ΔCsste50 mutant was highly sensitive to CM agar supplemented with 0.4 M NaCl or 0.4 M KCl (45% or 44% reduction, respectively) (Fig. 3A and B). These results suggest that CsSTE50 is involved in the vegetative growth and stress tolerance of C. scovillei.
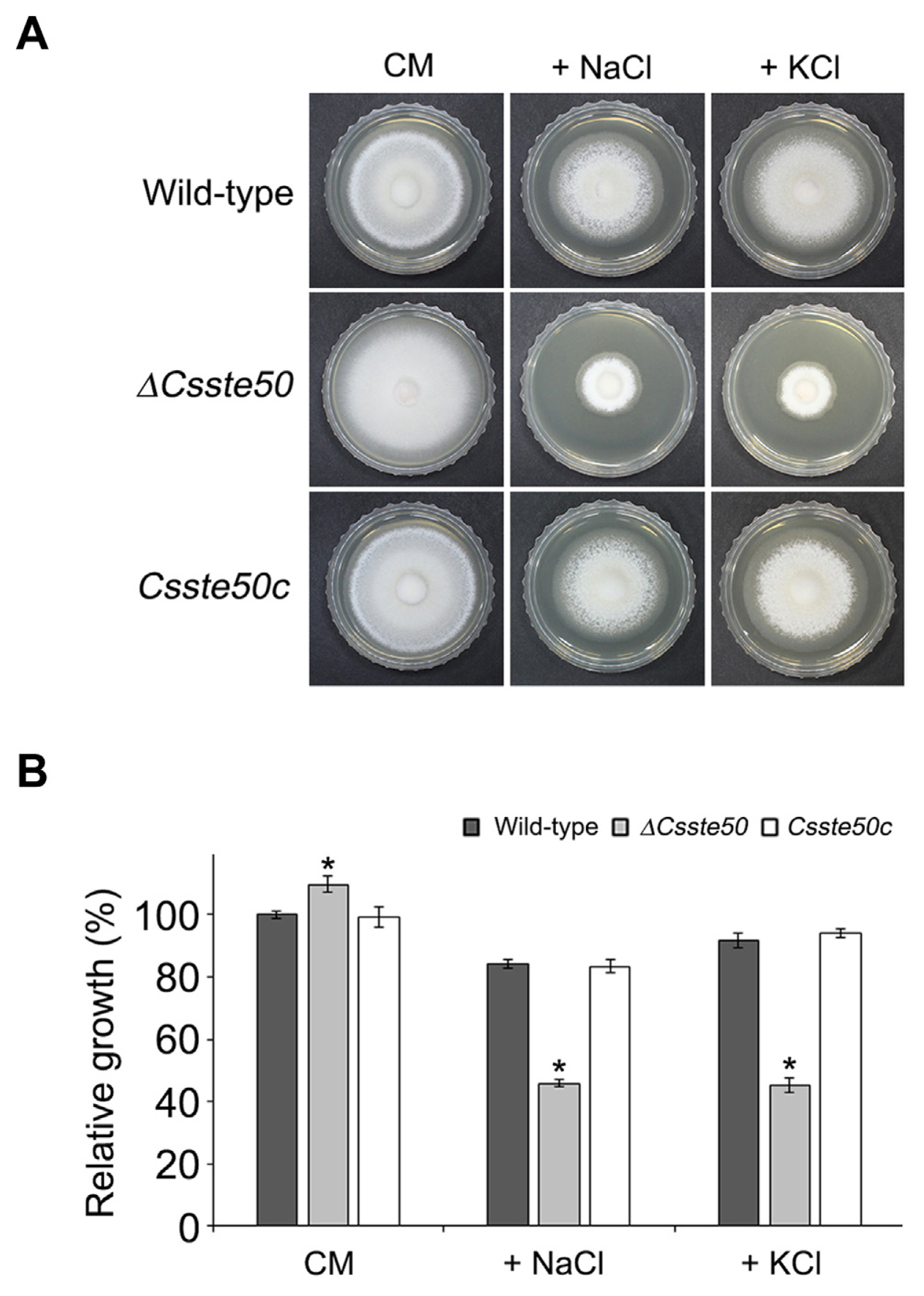
Vegetative growth of ΔCsste50 under chemical stress conditions. (A) Visualization of mycelial growth. Indicated strains were grown on CM agar and CM agar supplemented with osmotic stress agents (0.4 M NaCl or KCl) for 5 days at 25°C in the dark. (B) Quantitative measurements of diameter of colony growth. Growth relative to that of the wild-type on CM agar without an osmotic stress agent (arbitrarily set to 1) was evaluated. The asterisk indicates a significant difference between the wild-type and mutant according to Tukey’s test at P < 0.05.
Role of CsSTE50 in conidiation and conidial morphology
Conidiation is an important developmental process involved in disease dissemination in plant pathogenic fungi (Dean et al., 2012). To assess the role of CsSTE50 in C. scovillei conidiation, the ΔCsste50 mutant was inoculated on V8 agar and incubated for 5 days under continuous light. Remarkably, the ΔCsste50 mutant produced approximately 1.4 times more conidia than did the wild-type (Fig. 4A). The ΔCsste50 mutant also produced much longer conidia (average length 13.5 μm) compared with those of the wild-type (average 10.6 μm) (Fig. 4B). These results indicate that CsSTE50 is involved in conidiation and conidial morphology in C. scovillei.

Conidiation and conidium morphology of ΔCsste50. Conidia were harvested from 5-day-old V8 agar under continuous light. (A) Evaluation of conidiation. Conidiation relative to that of the wild-type (arbitrarily setb to 1) was determined. (B) Visualization of conidium morphology. Photographs were taken and conidium length was measured using a ZEN imaging software. Asterisks indicate significant differences between the wild-type and mutant at P < 0.05 according to Tukey’s test. Scale bars = 10 μm.
Role of CsSTE50 in conidial germination and appressorium formation
We evaluated the conidial germination and appressorium development of the ΔCsste50 mutant on an artificial hydrophobic surface. We placed 20 μl conidial drops (5 × 104 conidia/ml) on coverslips. By 16 h post-inoculation, most of the wild-type conidia (>90%) formed a single germ tube and appressorium (Fig. 5A–C). However, the ΔCsste50 mutant showed significantly delayed germination and failed to form appressoria, indicating that the ΔCsste50 mutant is defective in intracellular signaling or hydrophobic surface recognition for germination and appressorium formation (Fig. 5A–C). We evaluated whether exogenous cAMP, a signaling molecule, could restore appressorium formation in the ΔCsste50 mutant. At 16 h post-inoculation, we found that treatment of conidia with exogenous cAMP did not restore appressorium formation in the mutant, suggesting that CsSTE50 functions in a cAMP-independent manner (Fig. 5D). To observe appressorium formation from hyphal tips, we inoculated mycelial agar plugs of the wild-type and mutant on coverslips. At 72 h post-inoculation, the wild-type formed melanized appressoria on coverslips, but the ΔCsste50 mutant failed to form appressoria (Fig. 5E). Collectively, these results suggest that CsSTE50 is indispensable for appressorium formation in C. scovillei.
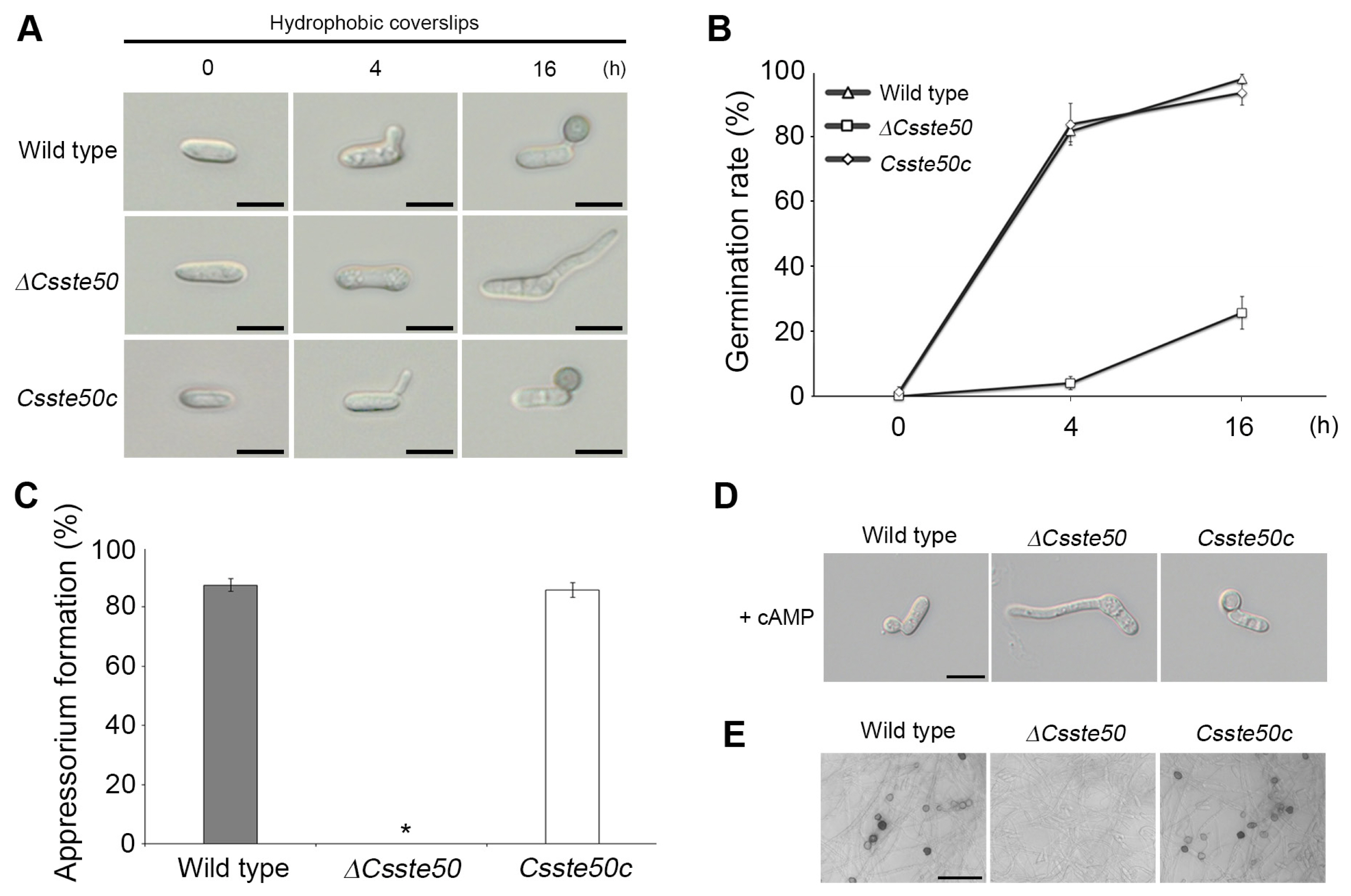
Conidial germination and appressorium formation of the ΔCsste50 on the hydrophobic surface. (A) Visualization of appressorium formation. Conidial drops (5 × 104 conidia/ml) were placed on the hydrophobic surface of coverslips and photographed at 0, 4, and 16 h post-inoculation. Scale bars = 10 μm. (B, C) Quantitative measurements of conidial germination and appressorium formation. A minimum of 100 conidia were examined to assess the conidial germination rate at 0, 4, and 16 h post-inoculation (B) and appressorium formation rate at 16 h post-inoculation (C). The asterisk (*) indicates a complete defect in appressorium formation of ΔCsste50. (D) Recovery of appressorium formation with exogenous treatment of cAMP. The cAMP (5 mM) was added to the conidial drops at 2 h post-inoculation. Photographs were taken at 16 h post-inoculation. Scale bar = 10 μm. (E) Visualization of appressorium like structure (ALS) formation. Mycelial agar plugs grown on 5-day-old oatmeal agar were placed on glass slides and covered with coverslips. Photographs were taken at 72 h post-inoculation. All experiments were conducted in triplicate and repeated three times. Scale bar = 30 μm.
Role of CsSTE50 in pathogenicity
To investigate whether the ΔCsste50 mutant can form appressoria on pepper fruit, we inoculated the fruit with conidial drops (5 × 104 conidia/ml). At 3 days post-inoculation, the wild-type conidia formed appressoria and penetrated the plant cuticle (Fig. 6A and B), whereas the ΔCsste50 mutant failed to form appressoria and formed long germ tubes on the peppers (Fig. 6A and B). This indicates that CsSTE50 is important for appressorium formation on pepper fruits. To determine fungal pathogenicity, the surfaces of pepper fruit were inoculated with conidial drops (15 × 104 conidia/ml) or mycelial agar plugs. At 8 days post-inoculation, the wild-type developed anthracnose lesions on both intact (left panel) and wounded (right panel) fruits (Fig. 6C). However, the ΔCsste50 mutant failed to develop disease lesions on intact peppers, and only small spots were observed on wounded fruit (Fig. 6C). Collectively, these results indicate that CsSTE50 is important for appressorium formation and invasive growth in C. scovillei.
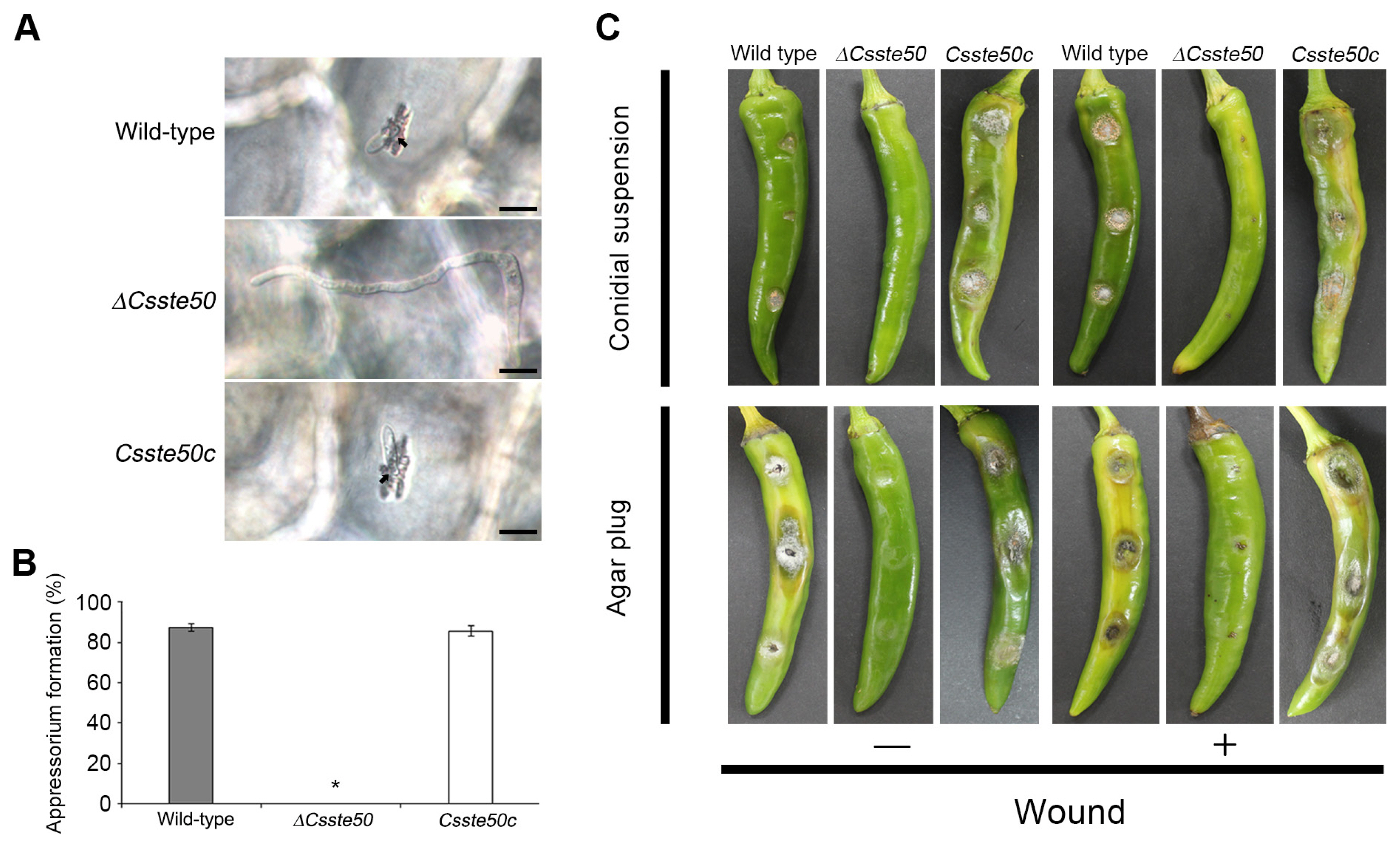
Pathogenicity assays of the ΔCsste50. (A) Appressorium-mediated penetration. Conidial drops (5 × 104 conidia/ml) were inoculated onto the surface of pepper fruits, incubated in a moistened box, and photographed at 72 h post-inoculation. Scale bars = 10 μm. (B) Appressorium formation on the surface of pepper fruits. At least 100 conidia were counted to assess the appressorium formation rate. The asterisk (*) indicates a complete defect in appressorium formation of ΔCsste50. (C) Plant infection assay of the ΔCsste50. Conidial drops (15 × 104 conidia/ml) (upper panels) and mycelial agar plugs (lower panels) were inoculated onto the surface of pepper fruits with or without wound. Photographs were taken at 8 days post-inoculation. All experiments were conducted in triplicate and repeated three times.
Discussion
The MAPK pathways play important roles in controlling cellular functions in fungi (Hagiwara et al., 2009; Mehrabi et al., 2009). Thus, studies of the roles of proteins involved in MAPK pathways will help elucidate the molecular mechanisms underlying the cellular processes of phytopathogenic fungi, including growth, development, and pathogenicity. This study analyzed the functional roles of a gene encoding the adaptor protein CsSTE50 in the anthracnose fungus C. scovillei. To analyze its functional roles in fungal development and pathogenicity, we deleted the gene via homology-dependent gene replacement and observed the resultant phenotypes.
STE50 homologs in many fungi are involved in osmoregulation, regulating the activation of high osmolarity glycerol response signaling (Chen et al., 2020; Saito and Posas, 2012). For example, deletion of the STE50 gene in C. fructicola and M. oryzae results in reduced vegetative growth under osmotic stress (Chen et al., 2020; Park et al., 2006). Consistently, the ΔCsste50 mutant was hypersensitive to osmotic stress (NaCl or KCl) during vegetative growth, indicating that CsSTE50 is involved in C. scovillei osmoregulation (Fig. 3).
Reduced conidiation is frequently observed in ste50 deletion mutants of fungal pathogens, including C. fructicola, F. graminearum, and M. oryzae (Chen et al., 2020; Gu et al., 2015; Park et al., 2006). However, the ΔCsste50 mutant showed increased conidiation on regular agar medium (Fig. 4A), unlike the reduced conidiation observed in the ste50 mutants of C. fructicola, F. graminearum, and M. oryzae (Chen et al., 2020; Gu et al., 2015; Park et al., 2006). Notably, deletion of the STE50 gene in the grey mold fungus B. cinerea results in excessive microconidia production (Schamber et al., 2010). Thus, we postulate that there are species-specific differences in the role of STE50 in the conidiation of fungal pathogens. Similarly, the role of STE50 in conidial germination differs depending on the fungal species. In our study, the ΔCsste50 mutant had a significantly delayed and reduced germination rate (Fig. 5A and B). In the southern corn leaf blight fungus Bipolaris maydis, STE50 deletion reduced the germination rate compared with the wild-type (Sumita et al., 2020). However, in M. oryzae, the mst50 (STE50 homolog) mutant had a similar germination rate to that of the wild-type strain (Li et al., 2017).
Ste50 homologs are important in intracellular signaling pathways for appressorium formation in many fungi, including C. fructicola, F. graminearum, and M. oryzae (Chen et al., 2020; Gu et al., 2015; Park et al., 2006). For example, in M. oryzae, the Ste50 homolog Mst50 regulates Pmk1 MAPK pathway activation, and the mst50 deletion mutant failed to form appressoria on both coverslips and plant surfaces (Park et al., 2006). The addition of exogenous cAMP, a signaling molecule, also failed to induce appressorium formation by the mst50 deletion mutant. Consistently, the ΔCsste50 mutant failed to form appressoria on coverslips or plant surfaces, and exogenous cAMP did not induce appressorium formation. Therefore, CsSTE50 may play a crucial role in intracellular signaling pathways for appressorium formation in C. scovillei, resulting in loss of pathogenicity on pepper fruits. Previously, we showed that the CsPMK1 gene in C. scovillei is important for stress tolerance, conidial germination, appressorium formation, and pathogenicity (Fu et al., 2021). The ΔCspmk1 and ΔCsste50 mutants had similar development and pathogenicity phenotypes. Although protein interaction or phosphorylation experiments are required for verification, we speculate that the CsSTE50 gene is involved in the activation of CsPMK1.
Acknowledgments
This study was supported by Basic Science Research Program through the National Research Foundation of Korea grant (NRF-2020R1A2C100550700) funded by the Ministry of Education, Science and Technology.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Electronic Supplementary Material
Supplementary materials are available at The Plant Pathology Journal website (http://www.ppjonline.org/).
