 |
 |
| Plant Pathol J > Volume 39(1); 2023 > Article |
|
Abstract
Fire blight, caused by Erwinia amylovora, is one of the major bacterial disease of apple and pear, causing enormous economic losses worldwide. Several control measures against E. amylovora have been reported till date, however, none of them have proved to be effective significantly against the pathogen. In this study, mechanisms of the copper-based control agents (CBCAs): copper oxychloride (COCHL), copper oxide (COX), copper hydroxide (CHY), copper sulfate basic (CSB), and tribasic copper sulfate (TCS) and their disease severity reduction efficacy against E. amylovora were analyzed. Bis-1,3-dibutylbarbituric acid trimethine oxonol, carboxyl fluorescein diacetate succinimidyl ester, and 5-cyano-2,3-ditolyl tetrazolium chloride staining were used to check the damage of membrane potential, cytoplasmic pHin, and respiration of CBCAs-treated E. amylovora, respectively. High disturbance in the membrane potential of E. amylovora was found under COX and COCHL treatments. Similarly, higher significant changes in the inner cytoplasmic pHin were observed under COX, COCHL, and TCS treatment. CHY and COCHL-treated E. amylovora showed a significant reduction in respiration. In vitro bioassay results revealed that CHY, CSB, and TCS at 2,000 ppm reduced the severity of fire blight both in pre- and post-treatment of CBCAs in immature apple fruits and seedlings. Overall, the most effective CBCAs against E. amylovora could be CHY at 2,000 ppm as its showed inhibition mechanisms and disease severity reduction.
Erwinia amylovora (Winslow et al., 1920) is a plant pathogenic bacterium that causes fire blight of apple and pear trees, which in turn causes enormous economic losses to fruit industries worldwide. The disease is indigenous to North America and has disseminated to over 50 countries since its discovery in the 1870s (Zhao et al., 2019). In Korea, fire blight was first reported in apple and pear orchards in the cities of Anseong, Cheonan, and Jecheon in 2015 (Myung et al., 2016; Park et al., 2016). Recent reports have indicated that fire blight is currently prevalent in Kyrgyzstan and Kazakhstan, posing a huge threat to the worldŌĆÖs leading producers of apples and pears (A─ćimovi─ć et al., 2015).
Control of E. amylovora is difficult due to the lack of specific synthetic compounds that directly affect the pathogen (Myung et al., 2016). Also, E. amylovora spreads rapidly and develops resistance to antibiotics (McGhee and Sundin, 2011). The use of antibiotics in the field for plant protection is under scrutiny since spray-drifted antibiotics affect the environment and soil; in addition, commensal microorganisms act as reservoirs of antibiotic resistance genes, which poses challenges to clinical medicine (Lamichhane et al., 2018; McManus, 2014). Preventive spraying of copper-based control agents (CBCAs) is one of the cultural practices for the management of fire blight; copper compounds are used as a bactericides in low concentrations for the control of shoot blight during summer (Acimovic and Meredith, 2017; McManus, 2014). Copper compounds help in disease management as they reduce inoculum buildup on susceptible leaf tissues, thereby preventing infection (Lamichhane et al., 2018).
The wide availability of CBCAs has facilitated the management of foliar diseases (Lamichhane et al., 2018). Foliar diseases that are difficult to manage without the use of fungicides are controlled using copper-based compounds; this is evident in the case of apple scab (Hindorf et al., 2015). Copper-based compounds play pivotal roles even in conventional agriculture because of their low costs and they offer a safe alternative to fungicides some of which are banned due to their detrimental effects on human health and biodiversity (Lamichhane et al., 2018). Also, unlike a wide range of fungicides available for crop management, very few bactericides are available to protect crops. Therefore, copper compounds are often used to manage diseases caused by plant pathogenic bacteria including fire blight of pome fruits (Elkins et al., 2015).
Copper compounds act as broad-spectrum biocides due to their interactions with nucleic acids, interference with the energy transport system, disruption of enzyme activities, and disruption of the integrity of cell membranes (Fleming and Trevors, 1989). In addition, bactericidal activity of copper compounds is mainly attributed to the release of ions; this further disrupts the bacterial membrane or the wall, resulting in intracellular oxidative stress and genotoxic, eventually leading to the death of bacterial cells (Vincent et al., 2018).
Copper compounds differ widely in terms of the availability of free copper ions released on moist plant surfaces (Zitter, 2013). CBCAs inhibit bacteria through different mechanisms: they cause inhibition of DNA synthesis, changes in membrane potential, cell morphology, intracellular pH, and swarming motility. DNA synthesis inhibition is an effective antibacterial mechanism of action (Dastidar et al., 2000; Feng et al., 2000); DNA synthesis inhibition coupled with an apoptosis-like response in the cells create a dual bactericidal effect (Lee et al., 2014). CBCAs cause a change in the bacterial membrane potential, which induces an early response to injury in bacteria; the membrane integrity is assessed using fluorescent probes, which diffuse through disrupted membranes but fail to enter intact membranes (S├Īnchez et al., 2010). Another factor is membrane integrity for a change in cytoplasmic pH in bacteria, which is crucial for many physiological activities (Olsen, 1993). The capacity of bacterial cells to maintain a pH gradient may also provide information regarding cellular activity (Chitarra et al., 2000). The above observations indicate that bacterial vital responses (intracellular pH, ATP production, membrane potential) can be used to validate antimicrobial agents (Olsen, 1993). However, in the case of fire blight, no studies have been conducted till date to determine the effects of CBCAs on E. amylovora response mechanisms. Therefore, the main purpose of this study was to identify the most effective copper compound among the CBCAs (copper oxychloride [COCHL], copper oxide [COX], copper hydroxide [CHY], copper sulfate basic [CSB], and tribasic copper sulfate [TCS]), registered under the authority of the Rural Development Administration of Korea (RDA), against fire blight. The objective of our study are (1) to examine the response mechanisms (changes in membrane potential, cell morphology, and intracellular pH and respiration inhibition) of E. amylovora to CBCAs and (2) to investigate the in vitro inhibition efficacy of CBCAs against the bacteria on immature apple fruits and seedlings.
Strain E. amylovora TS3128 isolated from Anseong was used in this study. The strain was freshly sub-cultured on a daily basis in mannitol glutamate yeast extract (MGY) media (mannitol 10 g, glutamic acid 2.0 g, KH2PO4 0.5 g, MgSO4 0.2 g, NaCl 0.2 g, yeast extract 1.0 g/l, pH 7.0). The culture was grown overnight in a rotary shaker at 220 rpm at 28┬░C. The CBCAs such as COCHL, COX, CHY, CSB, and TCS were used in this study. Different concentrations of each of the CBCAs at 10, 20, 25, 50, 100, 250, 500, and 2,000 ppm were used to determine the growth conditions of E. amylovora TS3128.
Fresh overnight culture of E. amylovora TS3128 was diluted serially to obtain an absorbance (OD600nm) range of 0.5-0.7. The cells were then harvested using centrifugation at 4,000 rpm for 10 min at 4┬░C. The bacterial pellet was then re-suspended in freshly prepared MGY media to obtain an absorbance of 1.0 at OD600nm. E. amylovora TS3128 was exposed to different concentrations of all the five CBCAs (COCHL, CHY, COX, CSB, and TCS). minimum inhibitory concentrations (MICs) were determined using 0.1 bacterial suspensions at OD600 nm using 10, 25, 50, 100, 250, 500, and 2,000 ppm of each CBCA that suspended in distilled water.
Disturbance of membrane potential of E. amylovora TS3128 was carried out using the method described by S├Īnchez et al. (2010) with minor modifications. E. amylovora TS3128 was grown overnight in MGY media and diluted culture was grown for 24 h. A suspension of 24 h old bacterial culture was diluted to 108-109 using 0.01 M of phosphate-buffered saline (PBS; pH 7.4). To 1 ml of bacterial suspension, 1 ╬╝M bis-1,3-dibutylbarbituric acid trimethine oxonol (DiBAC4(3) (Molecular Probes, Eugene, OR, USA) was added, and the mixture was incubated for 5 min at 28┬░C. This was followed by the addition of each of the CBCAs (COCHL and COX were used at a final concentration of 250 ppm, and CHY, CSB, and TCS were used at a final concentration of 25 ppm). As COCHL and COX showed similar MIC in 25 and 250 ppm, thus, 250 ppm was used for COCHL and COX. DiBAC4(3) stained bacteria were observed by using fluorescent-activated cell sorter (FACS; FACS-Calibur, BD Biosciences, San Jose, CA, USA). Fluorescence intensity of the stained bacteria was measured using 10,000 cells per every count using excitation wavelength of 490 nm and emission wavelength of 520 nm.
E. amylovora TS1328 was grown overnight and further inoculated into 5 ml each of fresh MGY broth with 250 ppm COCHL and COX, and 25 ppm CHY, CSB, and TCS and grown at 28┬░C in a rotary shaker until O.D600 nm of 0.6 was reached. The cells were then harvested using centrifugation at 4,000 rpm, at 4┬░C. The cells were washed twice in PBS buffer with 5 ╬╝M EDTA, pH 7.4, and re-suspended in 5 ml PBS buffer. A total of 1 ml of E. amylovora TS1328 from each CBCAs treatment was aliquoted into 1.5 ml microcentrifuge tubes. Carboxyl fluorescein diacetate succinimidyl ester (cFSE; Invitrogen, Carlsbad, CA, USA) was then added to the bacterial suspensions at a final concentration of 1 ╬╝M and the mixture was incubated for 10 min at 28┬░C. The cells were then washed with PBS buffer to eliminate non-conjugated cFSE. Then the cells were incubated with 10 ╬╝M glucose for 30 min at 28┬░C and washed twice in PBS buffer and placed on ice until further analysis. cFSE-stained bacteria were observed by using FACS (FACS-Calibur, BD). Fluorescence intensity of stained bacteria was measured using 10,000 cells per count using excitation wavelength of 490 nm and emission wavelength of 520 nm.
An overnight culture of E. amylovora TS3128 was used for the respiratory inhibition test. The bacterial culture was diluted to 108-109 with PBS buffer and treated with CBCAs: 250 ppm COCHL and COX, and 25 ppm CHY, CSB, and TCS. The cultures were incubated at 28┬░C in a rotary shaker until O.D600 nm of 0.6 was reached. Twenty ╬╝l of 5-cyano-2,3-ditolyl tetrazolium chloride (CTC; Bacstain-CTC staining kit, Dojindo, Kumamoto, Japan) and 5 ╬╝l of an enhancing reagent B were added per milliliter of the bacterial suspension and incubated for 30 min at 28┬░C. After CTC staining, the samples were diluted once and 10 ╬╝l was placed on a hemocytometer (depth, 0.10 mm and dimensions, 0.0025 mm2) and observed under a fluorescence microscope (Olympus BX53F2, Tokyo, Japan) at 480 nm.
Inocula of E. amylovora TS3128 were prepared at OD600nm of 0.5 in 10 ╬╝M MgCl2 with Silwet (final concentration of 0.02%). A 1 ml syringe needle was used to make wounds over the round surface of a half-sectioned immature apple fruit (Fuji) and upper three leaves of an apple seedling (M26). The bacterial suspension was then applied over immature apple fruits and upper leaves. The experiment was carried out in three replications. The experiment was conducted with a pre-treatment and post-treatment. Pre-treatment involved spraying of CBCAs 3 h before inoculation; post-treatment involved spraying of CBCAs 3 h after inoculation.
The concentration of CBCAs used was as follow: 2,000 ppm of CSB, TCS, and CHY; 25 ppm of CHY, TCS, and CSB; and 250 ppm of COX and COCHL. After treatment with CBCAs and inoculation, the immature apple fruits and seedlings were immediately transferred to a plant growth chamber maintained at 26-28┬░C and 60-80% humidity. Disease severity was ranked by scoring the lesion size on immature apple fruits and disease index after the 6 days of inoculation on apple seedlings, respectively. Population test was carried out immediately after ranking the disease. Disease severity rank on apple seedlings after pre- and post-treatment of different CBCAs inoculated with E. amylovora TS3128 was carried out in the order from 0 to 5. Disease severity ranking 0 to 5 in indicated as: 0, no symptoms; 1, only oozing out of lesion; 2, browning of upper leaves and oozing; 3, start of necrosis on upper leaves; 4, necrosis and browning on lower leaves; and 5, necrosis in half of the seedlings. To estimate the bacterial population, the whole plant was put in an extraction bag with 2 ml of MgCl2 and smashed with a hammer. After smashing, the extract was serially diluted till 10ŌłÆ6 and 100 ╬╝l each of the 10ŌłÆ6 dilution were plated on MGY media. Colony forming unit (cfu/ml) of bacteria was checked after 24 h of incubation at 28┬░C.
Growth of E. amylovora TS3128 in different concentrations of CBCAs showed a gradual reduction in a dosage-dependent manner in the case of four tested CBCAs (CSB, CHY, TCS, and COCHL) except COX. Thus, 25 ppm, which showed a constant OD600 nm of 0.7 that substrate each growth level of four CBCAs by control (E.a) was decided as MIC except for COX (Fig. 1). In the case of COX, concentrations of 10 and 50 ppm showed greater inhibitory effect than concentrations of 250 and 500 ppm; 25 ppm showed similar inhibition level to that of 250 and 500 ppm and 250 ppm was used as the MIC for COX (Fig. 1). Streptomycin (100 ╬╝g/ml) used as positive control showed the highest growth inhibition of the tested pathogenic bacterial strain when compared to all of the CBCAs.
For determination of disturbance in membrane potential of E. amylovora TS3128 after treatment with CBCAs, a dye DiBAC4(3), was firstly used to monitor the changes in membrane depolarization of the pathogen after treatment with CBCAs. Results indicated a significantly high disturbance in the membrane potential of E. amylovora TS3128 in treatments with COX and COCHL compared to other CBCAs (Fig. 2A). The increased in fluorescence intensity led to a corresponding increase in membrane potential disturbance in E. amylovora TS3128 treatment of COX and COCHL (Fig. 2A). Flow cytometry analysis of DiBAC4(3) staining before and after treatment of E. amylovora TS3128 with COCHL showed a shift in auto-fluorescence, which indicated membrane disturbance due to high auto-fluorescence (Fig. 2B and C). In particular, fluorescence light intensity of 200 arbitrary unit (au) was shown to be effective for potential membrane disturbance (Fig. 2A). COCHL-treated E. amylovora TS3128 exhibited higher auto-fluorescence than COCHL-untreated E. amylovora TS3128 (Fig. 2D and E). Moreover, CSB, CHY, and TCS showed reduced membrane potential damage of the isolate in comparison with COX and COCHL (Fig. 2A). Live and dead cells of E. amylovora TS3128 that were used as controls showed no damage in membrane potential in the live cells, while a highly significant membrane damage was observed in dead cells (Fig. 2A).
Similarly, changes in cytoplasmic pHin of pathogen were also screened in CBCAs-treated E. amylovora TS3128 by staining with cFSE. A highly significant change in cytoplasmic pHin was observed in COCHL, COX, and TCS compared to CSB and TCS (Fig. 3A). However, CSB and TCS treatment showed significant changes in cytoplasmic pHin of the study isolate when compared with live cells of the isolate (Fig. 3A). In particular, fluorescence light intensity of >1,200 arbitrary unit (au) was shown to be effective for potential membrane disturbance (Fig. 3A). The results clearly demonstrated that cytoplasmic pHin of E. amylovora TS3128 increased with an increase in fluorescence intensity.
Furthermore, CBCAs-treated E. amylovora TS3128 was stained with CTC to screen the respiration effect on E. amylovora TS3128. The results were expressed as percentage of fluorescent cells out of the total cells, which indicated that there was no significant difference (P Ōēż 0.05) among control (E. amylovora TS3128 grown in an untreated MGY media) and treatment with COX, CSB, and TCS (Fig. 3B). However, significant difference (P Ōēż 0.05) was found in the case of COCHL and CHY treatment when compared with the control (Fig. 3B).
Cultivars of apple Fuji and M26 were used for the bioassay on immature fruits and seedlings, respectively, to screen the pre- and post-treatment efficacy of different CBCAs (COCHL, COX, CHY, CSB, and TCS) on E. amylovora TS3128 inoculations before and after CBCAs application. The results obtained from pre-treatment of CBCAs on immature apple fruits suggested that 2,000 ppm of CSB and CHY showed significant lower necrosis symptoms in comparison with other treatments (Fig. 4). Negative control (treated with E. amylovora TS3128 only) reflected significantly higher necrosis symptoms compared to all the CBCA treatments. These results clearly illustrated that in comparison with negative control, (treated with E. amylovora TS3128 only) all the CBCAs significantly reduced the necrosis on immature apple fruits (Fig. 4).
Results from post-treatments of CBCAs against E. amylovora TS3128 showed that compared with other CBCAs, 2,000 ppm CHY significantly suppressed the necrotic lesions (Fig. 4). However, 25 ppm CSB and 25 and 2,000 ppm TCS also significantly reduced the necrotic lesions in comparison with negative control (Fig. 4).
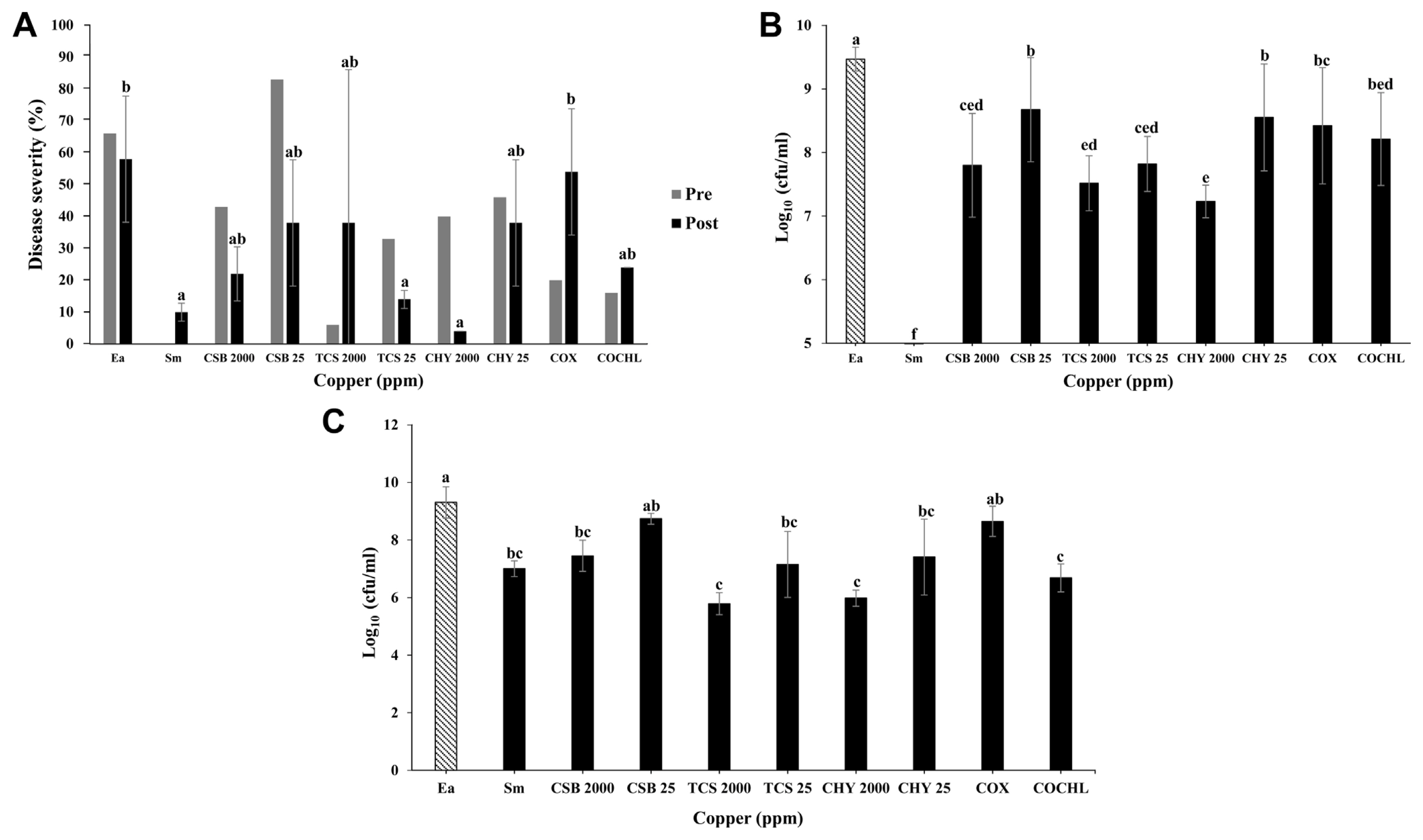
Pre-treatment of CBCAs in apple seedlings before E. amylovora TS3128 inoculation showed that disease severity was significantly reduced with the treatment of 2,000 ppm TCS when compared with that of other CBCAs (Fig. 5A). Similarly, streptomycin used as positive control also showed significant low disease severity in comparison with CBCAs (2,000 ppm CHY, 25 ppm CHY, 25 ppm TCS, and 25 ppm CSB) (Fig. 5A).
Apple seedlings treated with CBCAs after E. amylovora TS3128 inoculation (post-treatment) also showed varying results. Those with 2,000 ppm CHY treatment significantly suppressed the disease symptoms and reduced the disease severity (Fig. 5A), followed by 25 ppm TCS. Seedlings treated with streptomycin showed no severity (Fig. 5A). Negative control treated with E. amylovora TS3128 only) expressed significant high disease severity among all other treatments (Fig. 5A). The bacterial population test showed a significantly low E. amylovora TS3128 population in seedlings treated with 2,000 pm CHY when compared to other treatments (Fig. 5B). Streptomycin-treated seedlings used as positive control showed no bacterial populations (Fig. 5B). Followed by 2,000 ppm CHY, 2,000 ppm TCS-treated seedlings exhibited low E. amylovora TS3128 populations in comparison with negative control (seedlings treated with E. amylovora TS3128 only) (Fig. 5B). However, results indicated that compared to the negative control, 2,000 ppm CSB, 250 ppm COCHL and COX, 25 ppm TCS and CHY also showed significantly low E. amylovora TS3128 population (Fig. 5B). Furthermore, E. amylovora TS3128 population was also screened after post-treatment of CBCAs in apple seedlings. The results showed that 2,000 ppm CHY- and TCS-treated seedlings exhibited significantly low population followed by 250 ppm COCHL (Fig. 5C). Compared to the 2,000 ppm TCS- and CHY- and 250 ppm COCHL-treated seedlings, streptomycin-treated seedlings used as positive control showed higher bacterial population (Fig. 5C).
The emergence and transmission of bacterial resistance and the deterioration and residual effects of registered antibiotics in the environment pose challenges to crop protection (Buttimer et al., 2017; OŌĆÖNeil et al., 2003). Therefore, it is imperative to develop effective plant protection strategies. Since the first discovery of antibacterial effects of copper, many changes in structure of antibacterial copper compounds have been made. Copper compounds have proven to be ideal candidates capable of controlling formidable pathogens such as E. amylovora. However, CBCAs have been evaluated according to their efficacies assessed in small scale experiments under laboratory and field conditions. Therefore, only a limited information is available on the mechanism of action of CBCAs with respect to target organisms such as E. amylovora. In this study, we screened the potential antibacterial activity of different copper compounds officially registered under RDA against fire blight. The first step was to determine the MIC of copper compounds using co-culture in broth media; MIC of five CBCAs on E. amylovora TS3128 showed results that were similar to those of a previous report (Lee et al., 2018).
Several mechanisms of copper compounds effect changes at the physiological and cellular level in bacteria. Flow cytometry was used to analyze the physiological state of individual cells, which has been successfully applied in recent years in studies involving microorganisms (Kennedy and Wilkinson, 2017). Multipara metric measurements allow different physiological stages to be detected at the cellular and population level: metabolically active cells (living cells), compromised membranes (injured cells), and damaged membranes (dead cells) (D├Łaz et al., 2010). The analysis of physiological damage on a pathogen by way of treatment methods such as copper, involves the detection of potential membrane damage using fluorescent dyes and flow cytometry. In this study, we used the oxonol derivative DIBAC4(3) dye, which is an anionic dye that enters depolarized cells and binds to intracellular proteins, thus presenting an enhanced green fluorescence and a red spectral shift. This red spectral shift is used to observe membrane damage of E. amylovora TS3128 treated with CBCAs using flow cytometry. This enables the differentiation of metabolically active cells (with a polarized membrane) and dead cells (with a depolarized membrane) (D├Łaz et al., 2010; Str├żuber and M├╝ller, 2010). In this study, cell membrane of E. amylovora TS3128 treated with COX and COCHL was found damaged, indicating the change in membrane polarization of E. amylovora TS3128 (Fig. 2A). We found that E. amylovora TS3128 when treated with different CBCAs (COCHL) exhibited increased auto-fluorescence when analyzed using flow cytometry (Fig. 2B), which explains the altered cell morphology of E. amylovora TS3128. Flow cytometry can be used to detect changes in the morphology of bacterial cells (Wickens et al., 2000). These alterations are estimated by forward and side scatter (FSC-H and SSC-H). The magnitude of FSC-H is proposed to be roughly proportional to the size of the cell, and SSC-H was assumed to be related to the internal granularity of the cell (Tracy et al., 2010; Walberg et al., 1996). In this study, changes in cell morphology of E. amylovora TS3128 indicated auto-fluorescence resulting from the changes in FSC-H and SSC-H of COCHL treatments (Fig. 2B and C). COCHL-treated E. amylovora TS3128 showed increased auto-fluorescence, which altered the cell morphology and damaged the membrane potential of E. amylovora TS3128 (Fig. 2D and E). Similar results were also reported by Renggli et al. (2013) where the cell morphology of Escherichia coli was altered after treatment with bactericidal antibiotics; the cells were visualized using flow cytometric analysis. The fluorescence emitted by the dye is enhanced when the dye crosses the cell membrane because of membrane depolarization (less negative charge inside the cell) (Whiteaker et al., 2001); this is one of the reasons for membrane damage of E. amylovora TS3128 treated with CBCAs (COCHL, COX, and TCS) (Fig. 2A).
The cFSE staining technique was used to measure the cytoplasmic pHin based on intracellular conjugation of the succinimidyl group of cFSE. The dye cFSE is a fluorescent cell staining dye, which is permeable; it covalently couples through its succinimidyl group with intracellular molecules (Parish, 1999) and was developed to determine the intracellular pH of bacteria (Breeuwer et al., 1996). pH is pivotal for the control of many cellular activities, such as DNA transcription, enzyme activities, and protein synthesis (Breeuwer et al., 1996). In this study, the fluorescent probe cFSE was evaluated for determination of the pHin of different copper compounds treated E. amylovora TS3128. cFSE has been previously used to determine cell division in bacteria (Ueckert et al., 1995); however, it can be potentially used as a pH probe because its fluorescence is probe dependent (Breeuwer et al., 1996). In this study, COCHL, COX, and TCS showed high cytoplasmic pHin indicating membrane damage of E. amylovora TS3128 (Fig. 3A). The physiological responses to pHin variations bacteria are due to stress conditions such as exposure to detergents and high temperatures (Parish, 1999). In this study, membrane damage of E. amylovora TS3128 with high cytoplasmic pHin is attributed to the severe stress caused by copper compounds (COCHL, COX, and TCS).
Furthermore, CTC is another fluorescent probe used in flow cytometry to assess the physiological state to measure respiration of bacterial cells (Rezaeinejad and Ivanov, 2011). CTC was used to screen the respiration inhibition of E. amylovora TS3128 by CBCAs. In a normal respiratory system, CTC turns into CTC-formazan, accumulates in bacterial cells and is detected at 630 nm. However, bacteria with physiological malfunctions do not accumulate CTC-formazan (Kobayashi et al., 2012). In the present study, E. amylovora TS3128 treated with COCHL and CHY expressed significantly low CTC levels, indicating low respiration (Fig. 3B). Thus, the reduction of CTC in a cell is considered as an indicator of bacterial cell respiration (Belkova et al., 2007; Creach et al., 2003).
Copper-containing compounds are inorganic antibacterial materials, which have been shown to inhibit the growth of microorganisms and exert antimicrobial properties (Ghidan and Al Antary, 2019). In this study, bioassay experiments with immature apple fruits and seedlings showed E. amylovora TS3128 inhibition efficacy of CBCAs. Results of pre- and post-treatment of immature apple fruits and seedlings using CBCAs against E. amylovora TS3128 indicated varying disease inhibition activities (Figs. 4 and 5). However, 25 ppm TCS and 2,000 ppm CHY exhibited higher disease suppression efficacy among other CBCAs under post-treatment of immature apple fruit and seedlings. Although, other CBCAs (COCHL, CSB, and TCS) showed low disease inhibition than 25 ppm TCS and 2,000 ppm CHY, their disease inhibition efficacy was significantly higher compared to that of the control. In conclusion, COCHL showed better inhibition mechanisms while staining with different dyes, and 2,000 ppm CHY showed higher disease reduction. The reason behind the variations in mechanisms and bioassays might be due to the direct contact killing activity of copper compounds on immature apple fruits and seedlings. To the best of our knowledge this is the first report in Korea that present the mechanisms of disease inhibition by CBCAs. However, further investigations are required to understand the detailed molecular and physiological interactions of CBCAs with E. amylovora.
Acknowledgments
This work was carried out with the support of Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry (IPET) through Agri-Bio industry Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (No. 320041-05-3-SB010) and was also supported by a grant from Farmhannong.
Fig.┬Ā1
Bacterial growth inhibition assay of CBCAs on MGY broth medium. Growth test of CBCA-treated Erwinia amylovora TS3128 in MGY broth medium with different concentrations of CBCAs. Note: Positive control (streptomycin, Sm) and negative control (E. amylovora, Ea). CSB, copper sulfate basic; CHY, copper hydroxide; CBCA, copper-based control agent; TCS, tribasic copper sulfate; COX, copper oxide; COCHL, copper oxychloride.

Fig.┬Ā2
Effects of CBCAs on the membrane potentials of Erwinia amylovora TS3128. (A) Membrane potential damage of E. amylovora TS3128 treated with CBCAs. (B, D) Dot plots of forward and side scatter of E. amylovora TS3128 and light intensity before COCHL treatment. (C, E) Dot plots of forward and side scatter of E. amylovora TS3128 and light intensity after COCHL treatment. Ea, non-treated E. amylovora; Ea Dead, 70% EtOH treated E. amylovora; Ea stain, DiBAC4(3) stained with non-treated E. amylovora; CSB, CHY, TCS, COX, and COCHL, E. amylovora treated with each CBCAs and stained with DiBAC4(3). CSB, copper sulfate basic; CHY, copper hydroxide; CBCA, copper-based control agent; TCS, tribasic copper sulfate; COX, copper oxide; COCHL, copper oxychloride. Data shown are from three replications. Letters with data indicate that the means are significantly different if given the different letter (P = 0.05). Red box indicates the means of auto fluorescence light intensity.
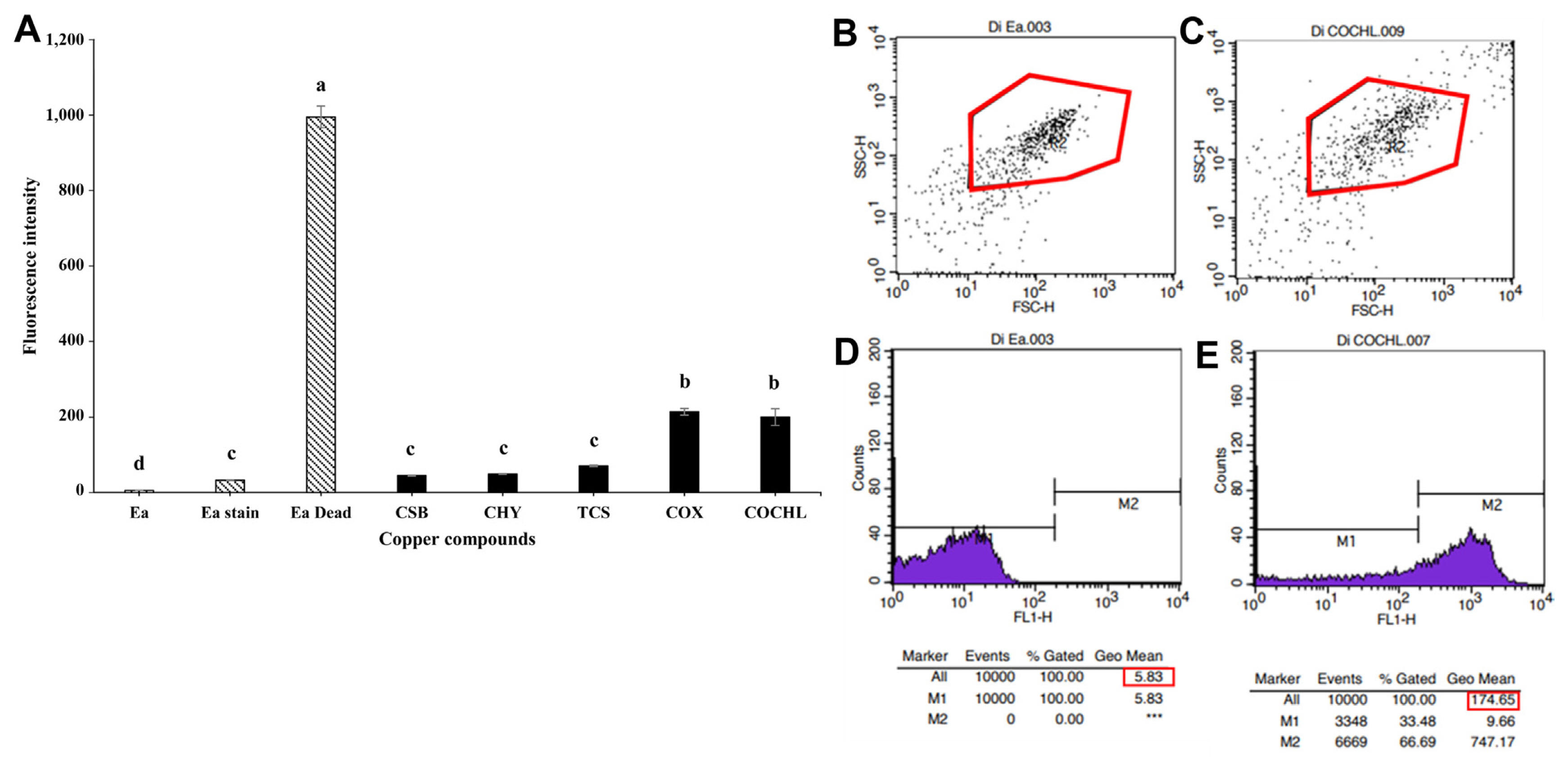
Fig.┬Ā3
cFSE and CTC staining to compare the inhibition of Erwinia amylovora TS3128 by CBCAs. (A) Effects of CBCA-treated E. amylovora TS3128 on the intracellular pHin using cFSE staining. (B) Respiration effect on E. amylovora TS3128 treated with CBCAs using CTC staining. Ea, non-treated E. amylovora; Ea Dead, 70% EtOH treated E. amylovora; Ea stain, cFSE stained with non-treated E. amylovora; CSB, CHY, TCS, COX, and COCHL, E. amylovora cells were treated with each of the CBCAs and stained with cFSE. CSB, copper sulfate basic; CHY, copper hydroxide; CBCA, copper-based control agent; TCS, tribasic copper sulfate; COX, copper oxide; COCHL, copper oxychloride; cFSE, carboxyl fluorescein diacetate succinimidyl ester; CTC, 5-cyano-2,3-ditolyl tetrazolium chloride. Data shown are from three replications. Letters with data indicate that the means are significantly different if given the different letter (P = 0.05).

Fig.┬Ā4
Bioassay screening of pre-treatment and post-treatment efficacy of different CBCAs (COCHL, COX, CHY, CSB, and TCS) on immature apple fruits inoculated with Erwinia amylovora TS3128. Ea, non-treated E. amylovora; Sm, streptomycin; CSB, copper sulfate basic; TCS, tribasic copper sulfate; CHY, copper hydroxide; COX, copper oxide; COCHL, copper oxychloride. Data shown are from three replications. Letters with data indicate that the means are significantly different if given the different letter (P = 0.05).
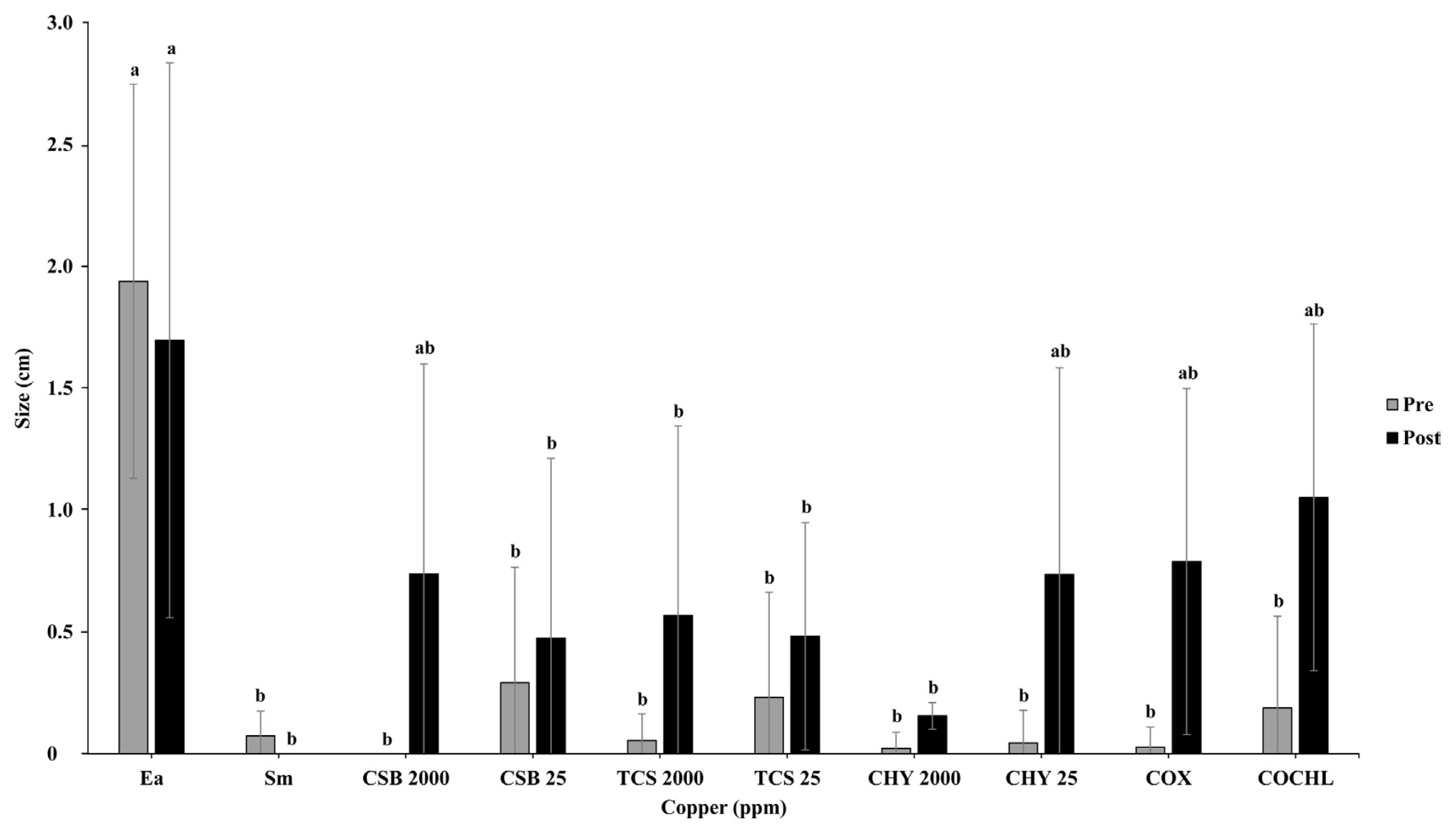
Fig.┬Ā5
Disease severity screening and colony forming unit (cfu/ml) of Erwinia amylovora TS3128 in apple seedlings showing pre- and post-treatment efficacy of different CBCAs. (A) Disease severity screening of apple seedlings on pre- and post-treatment efficacy of different CBCAs (CSB, TCS, CHY, COX, and COCHL) in apple seedlings inoculated with E. amylovora TS3128. (B) Cfu of E. amylovora TS3128 after pre-treatment of apple seedlings with different CBCAs. (C) Cfu of E. amylovora TS3128 after post-treatment of apple seedlings with different CBCAs. Ea, non-treated E. amylovora; Sm, streptomycin; CSB, copper sulfate basic; TCS, tribasic copper sulfate; CHY, copper hydroxide; COX, copper oxide; COCHL, copper oxychloride; CBCA, copper-based control agent. Data shown are from three replications. Letters with data indicate that the means are significantly different if given the different letter (P = 0.05).
References
A─ćimovi─ć, S. G. and Meredith, C. L. 2017. Evaluation of dormant copper sprays with bark penetrating surfactants in reduction of Erwinia amylovora in cankers and of low-rate copper sprays in blossom blight control. Fruit Q 25:15-20.
A─ćimovi─ć, S. G., Zeng, Q., McGhee, G. C., Sundin, G. W. and Wise, J. C. 2015. Control of fire blight (Erwinia amylovora) on apple trees with trunk-injected plant resistance inducers and antibiotics and assessment of induction of pathogenesis-related protein genes. Front. Plant Sci 6:16.



Belkova, N. L., Tazaki, K., Zakharova, J. R. and Parfenova, V. V. 2007. Activity of bacteria in water of hot springs from Southern and Central Kamchatskaya geothermal provinces, Kamchatka Peninsula, Russia. Microbiol. Res 162:99-107.


Breeuwer, P., Drocourt, J., Rombouts, F. M. and Abee, T. 1996. A novel method for continuous determination of the intracellular pH in bacteria with the internally conjugated fluorescent probe 5 (and 6-)-carboxyfluorescein succinimidyl ester. Appl. Environ. Microbiol 62:178-183.




Buttimer, C., McAuliffe, O., Ross, R. P., Hill, C., OŌĆÖMahony, J. and Coffey, A. 2017. Bacteriophages and bacterial plant diseases. Front. Microbiol 8:34.



Chitarra, L. G., Breeuwer, P., Van Den Bulk, R. W. and Abee, T. 2000. Rapid fluorescence assessment of intracellular pH as a viability indicator of Clavibacter michiganensis subsp. michiganensis. J. Appl. Microbiol 88:809-816.


Cr├®ach, V., Baudoux, A. C., Bertru, G. and Rouzic, B. L. 2003. Direct estimate of active bacteria: CTC use and limitations. J. Microbiol. Methods 52:19-28.


Dastidar, S. G., Ganguly, K., Chaudhuri, K. and Chakrabarty, A. N. 2000. The anti-bacterial action of diclofenac shown by inhibition of DNA synthesis. Int. J. Antimicrob. Agents 3:249-251.

D├Łaz, M., Herrero, M., Garc├Ła, L. A. and Quir├│s, C. 2010. Application of flow cytometry to industrial microbial bioprocesses. Biochem. Eng. J 48:385-407.

Elkins, R. B., Temple, T. N., Shaffer, C. A., Ingels, C. A., Lindow, S. B., Zoller, B. G. and Johnson, K. B. 2015. Evaluation of dormant-stage inoculum sanitation as a component of a fire blight management program for fresh-market bartlett pear. Plant Dis 99:1147-1152.


Feng, Q. L., Wu, J., Chen, G. Q., Cui, F. Z., Kim, T. N. and Kim, J. O. 2000. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus
. J. Biomed. Mater. Res 52:662-668.


Flemming, C. A. and Trevors, J. T. 1989. Copper toxicity and chemistry in the environment: a review. Water Air Soil Poll 44:143-158.


Ghidan, A. Y. and Al Antary, T. M. A. 2019. Applications of nanotechnology in agriculture. In: Applications of nano biotechnology, eds. by M. Stoytcheva and R. Zlatev, IntechOpen, London, UK.

Hindorf, H., Tadesse, M., Pohlan, J., Weeden, O. and Finckh, M. R. 2015. Organic coffee disease management. In: Plant disease and their management in organic agriculture, eds. by M. R. Finckh, A. H. C. van Bruggen and L. Tamm, pp. 367-382. APS Press, St. Paul, MN, USA.
Kennedy, D. and Wilkinson, M. G. 2017. Application of flow cytometry to the detection of pathogenic bacteria. Curr. Issues Mol. Biol 23:21-38.


Kobayashi, T., Mito, T., Watanabe, N., Suzuki, T., Shiraishi, A. and Ohashi, Y. 2012. Use of 5-cyano-2,3-ditolyl-tetrazolium chloride staining as an indicator of biocidal activity in a rapid assay for anti-Acanthamoeba agents. J. Clin. Microbiol 5:1606-1612.


Lamichhane, J. R., Osdaghi, E., Behlau, F., K├Čhl, J., Jones, J. B. and Aubertot, J.-N. 2018. Thirteen decades of antimicrobial copper compounds applied in agriculture. A review. Agron. Sustain. Dev 38:28.


Lee, M. S., Lee, I., Kim, S. K., Oh, C.-S. and Park, D. H. 2018.
In vitro screening of antibacterial agents for suppression of fire blight disease in Korea. Res. Plant Dis 23:41-51 (in Korean).


Lee, W., Kim, K. J. and Lee, D. G. 2014. A novel mechanism for the antibacterial effect of silver nanoparticles on Escherichia coli
. Biometals 27:1191-1201.



McGhee, G. C. and Sundin, G. W. 2011. Evaluation of kasugamycin for fire blight management, effect on nontarget bacteria, and assessment of kasugamycin resistance potential in Erwinia amylovora
. Phytopathology 101:192-204.


McManus, P. D. 2014. Does a drop in the bucket make a splash? Assessing the impact of antibiotic use on plants. Curr. Opin. Microbiol 19:76-82.


Myung, I.-S., Lee, J.-Y., Yun, M.-J., Lee, Y.-H., Park, D.-H. and Oh, C.-S. 2016. Fire blight of apple caused by Erwinia amylovora, a new disease in Korea. Plant Dis 100:1774.

OŌĆÖNeil, R. J., Yaninek, J. S., Landis, D. A. and Orr, D. B. 2003. Biological control and integrated pest management. In: Integrated pest management in the global arena, eds. by K. M. Maredia, D. Dakouo and D. Mota-Sanchez, pp. 19-30. CABI Publishing, Wallingford, UK.

Parish, C. R. 1999. Fluorescent dyes for lymphocyte migration and proliferation studies. Immunol. Cell Biol 77:499-508.



Park, D. H., Yu, J.-G., Oh, E.-J., Han, K.-S., Yea, M. C., Lee, S. J., Myung, I.-S., Shim, H. S. and OH, C.-S. 2016. First report of fire blight disease on Asian pear caused by Erwinia amylovora in Korea. Plant Dis 100:1946.

Renggli, S., Keck, W., Jenal, U. and Ritz, D. 2013. Role of autofluorescence in flow cytometric analysis of Escherichia coli treated with bactericidal antibiotics. J. Biotechnol 195:4067-4073.

Rezaeinejad, S. and Ivanov, V. 2011. Heterogeneity of Escherichia coli population by respiratory activity and membrane potential of cells during growth and long-term starvation. Microbiol. Res 166:129-135.


S├Īnchez, E., Garc├Ła, S. and Heredia, N. 2010. Extracts of edible and medicinal plants damage membranes of Vibrio cholerae
. Appl. Environ. Microbiol 76:6888-6894.




Str├żuber, H. and M├╝ller, S. 2010. Viability states of bacteria: specific mechanisms of selected probes. Cytometry A 77:623-634.

Tracy, B. P., Gaida, S. M. and Papoutsakis, E. T. 2010. Flow cytometry for bacteria: enabling metabolic engineering, synthetic biology and the elucidation of complex phenotypes. Curr. Opin. Biotechnol 21:85-99.


Ueckert, J., Breeuwer, P., Abee, T., Stephens, P., von Caron, G. N. and ter Steeg, P. F. 1995. Flow cytometry applications in physiological study and detection of foodborne microorganisms. Int. J. Food Microbiol 28:317-326.


Vincent, M., Duval, R. E., Hartemann, P. and Engels-Deutsch, M. 2018. Contact killing and antimicrobial properties of copper. J. Appl. Microbiol 124:1032-1046.



Walberg, M., Gaustad, P. and Steen, H. B. 1996. Rapid flow cytometric assessment of mecillinam and ampicillin bacterial susceptibility. J. Antimicrob. Chemother 37:1063-1075.


Whiteaker, K. L., Gopalakrishnan, S. M., Groebe, D., Shieh, CC., Warrior, U., Burns, D. J., Coghlan, M. J., Scott, V. E. and Gopalakrishnan, M. 2001. Validation of FLIPR membrane potential dye for high throughput screening of potassium channel modulators. J. Biomol. Screen 6:305-312.



Wickens, H. J., Pinney, R. J., Mason, D. J. and Gant, V. A. 2000. Flow cytometric investigation of filamentation, membrane patency, and membrane potential in Escherichia coli following ciprofloxacin exposure. Antimicrob Agents Chemother 44:686-687.


Winslow, C. E. A., Broadhurst, J., Buchanan, R. E., Krumwiede, C., Rogers, L. A. and Smith, G. H. 1920. The families and genera of the bacteria: final report of the committee of the Society of American Bacteriologists on characterization and classification of bacterial types. J. Bacteriol 5:191-229.




Zhao, Y.-Q., Tian, Y.-L., Wang, L.-M., Geng, G.-M., Zhao, W.-J., Hu, B.-S. and Zhao, Y.-F. 2019. Fire blight disease, a fast-approaching threat to apple and pear production in China. J. Integr. Agric 18:815-820.

Zitter, T. A. 2013 How copper sprays work and avoiding phytotoxicity URL https://enych.cce.cornell.edu/submission.php?id=140&crumb=organic%7Corganic
. 14 December 2022.
- TOOLS
-
METRICS

- ORCID iDs
-
Mahesh Adhikari

https://orcid.org/0000-0001-9646-8633Duck Hwan Park

https://orcid.org/0000-0001-8486-9544 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



