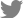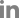|
 |
| Plant Pathol J > Volume 39(3); 2023 > Article |
|
Abstract
Sweet potato symptomless virus 1 (SPSMV-1) is a single-stranded circular DNA virus, belonging to the genus Mastrevirus (family Geminiviridae) that was first identified on sweet potato plants in South Korea in 2012. Although SPSMV-1 does not induce distinct symptoms in sweet potato plants, its co-infection with different sweet potato viruses is highly prevalent, and thus threatens sweet potato production in South Korea. In this study, the complete genome sequence of a Korean isolate of SPSMV-1 was obtained by Sanger sequencing of polymerase chain reaction (PCR) amplicons from sweet potato plants collected in the field (Suwon). An infectious clone of SPSMV-1 (1.1-mer) was constructed, cloned into the plant expression vector pCAMBIA1303, and agro-inoculated into Nicotiana benthamiana using three Agrobacterium tumefaciens strains (GV3101, LBA4404, and EHA105). Although no visual differences were observed between the mock and infected groups, SPSMV-1 accumulation was detected in the roots, stems, and newly produced leaves through PCR. The A. tumefaciens strain LBA4404 was the most effective at transferring the SPSMV-1 genome to N. benthamiana. We confirmed the viral replication in N. benthamiana samples through strand-specific amplification using virion-sense- and complementary-sense-specific primer sets.
Sweet potato (Ipomoea batatas L.) is among the most important food crops worldwide as it contains high levels of vitamins A and C, iron, potassium, fiber, and other nutrients (Kim et al., 2017). Sweet potato production has increased continuously over the last four decades, reaching approximately 105 million metric tons worldwide in 2016 (Food and Agriculture Organization of the United Nations, 2016). However, with the recent spread of several sweet potato viruses, including the sweet potato symptomless virus 1 (SPSMV-1), the productivity of sweet potato has decreased significantly (Loebenstein, 2015). SPSMV-1 was first reported in Peru in 2009 (Cao et al., 2017; Kreuze et al., 2009; Muhire et al., 2013). Since then, the virus has been reported in several countries in the Americas, East Africa, Europe, and Northeast Asia including USA, Uruguay, Brazil, Kenya, Spain, Taiwan, and China (Kwak et al., 2014; Mbanzibwa et al., 2014; Souza et al., 2018). SPSMV-1 was first identified in sweet potatoes in the Korean peninsula in 2012 (Kwak et al., 2014). Since then, SPSMV-1 has been reported to have infected sweet potato plants in many regions of South Korea.
SPSMV-1 is a circular single-stranded DNA (ssDNA) virus belonging to the genus Mastrevirus (family Geminiviridae). The SPSMV-1 genome is 2,599-2,602 nucleotides in size, consisting of five overlapping transcribed open reading frames (ORFs) and two non-transcribed regions called the intergenic regions, and is encapsidated in a twinned icosahedral capsid (Qiao et al., 2020). The large intergenic region (LIR) contains the stem-loop structure, the origin of replication, and transcription start sites, whereas the short intergenic region (SIR) contains the complementary strand replication origin and transcription termination sites (Fondong, 2013; Hayes et al., 1988). LIR contains an abnormal nonanucleotide sequence, TAAGATTCC, which differs from that of most viruses of the family Geminiviridae (Borah et al., 2016; Cao et al., 2017). The virion-sense strand encodes the coat protein (gene V1), which encapsidates the virion-sense ssDNA, and the movement protein (gene V2), which is involved in cell-to-cell movement. The complementary sense encodes three genes: C1, C2, and C3. The replication-associated protein (Rep), expressed in genes C1 and C2 by transcript splicing, initiates rolling-circle replication by introducing a nick into the nonanucleotide sequence in the virion-sense strand. RepA, the protein product of ORF C1, binds to a plant homolog of the retinoblastoma protein to regulate cell cycle progression and support viral DNA replication (Zerbini et al., 2017). ORF C3 is completely embedded within the ORF C1 and appears in all eight other dicot-infecting mastreviruses and in the maize streak virus (MSV) at a similar position (Cao et al., 2017). However, its function remains unclear.
Although SPSMV-1 does not induce major symptoms in sweet potato plants, co-infection with different disease-inducing sweet potato viruses is highly prevalent, and threatens sweet potato production (Souza et al., 2018). In this study, an isolated SPSMV-1 Korean infectious clone was constructed, and agro-inoculation assays with different Agrobacterium tumefaciens strains were performed on the model plant Nicotinana benthamiana to confirm the function of the infectious clone and, later, investigate the interaction between SPSMV-1 and its host plant.
Sweet potato samples were randomly collected from a field in Suwon, Gyeonggi, South Korea during a 2014 survey. Complete sequences of SPSMV-1 isolates from China (KY565235.1, MG603669.1, MG603671.1, MG603668.1, MK802081.1, MG603672.1, and MG603670.1), the USA (KY565232.1), Uruguay (KY565234.1), Brazil (MH375686.1 and MG680260.1), Taiwan (KY565233.1, KY565236.1, and KY565237.1), and Kenya (KY565231.1) were retrieved from NCBI GenBank (https://www.ncbi.nlm.nih.gov/nucleotide). Additionally, the nucleotide sequences of 11 mastreviruses were selected for phylogenetic tree construction to elucidate the genomic characteristics of the Korean SPSMV-1isolate.
Total genomic DNA was isolated from sweet potato tissues using the STE method (Azad and Nematadeh, 2013). Leaf, root, and stem samples were ground using a mortar and pestle in liquid nitrogen. Subsequently, 100 mg of the obtained sample powder was transferred to 1.5 ml microcentrifuge tubes followed by the addition of lysis buffer containing 470 μl STE buffer (0.4 M sucrose, 20 mM Tris-HCl, and 20 mM EDTA), 30 μl of 20% sodium dodecyl sulfate, 200 μl 8 M LiCl, 1 μl of 2-mercaptoethanol, and 100 mg of polyvinylpyrrolidone 40 K. The sample and lysis buffer were vortexed to mix well and incubated at 60°C for 45 min. To separate the DNA from other cellular components, 1 volume (700 μl) of chloroform: isoamyl alcohol (24:1) was added, and the mixture was centrifuged at 13,000 rpm at 4°C for 15 min. The DNA was then precipitated with isopropanol, washed with cool 70% ethanol, air-dried, and dissolved in 50 μl of 1× TE buffer. Viral DNA was amplified via rolling circle amplification (RCA) using a Templiphi Kit (GE Healthcare Life Sciences, Piscataway, NJ, USA) following the manufacturer’s introductions. The RCA products were used as polymerase chain reaction (PCR) templates to obtain full-length sequences of the virus. A 2.6 kb DNA amplicon was ligated into the pGEM-T Easy vector (Promega, Madison, WI, USA) via TA cloning according to the manufacturer’s instructions. A plasmid containing the full-length SPSMV-1 genome was sequenced by Macrogen (Seoul, Korea) and submitted to GenBank.
Multiple alignment of all sequences of the SPSMV-1 isolates and the 11 mastre- viruses (chickpea chlorosis virus [CpCV, GU256530.1]; chickpea chlorotic dwarf virus [CpCDV, AM849097.1]; chickpea redleaf virus [CpRLV, GU256532.1]; eragrostis streak virus [ESV, EU244915.1]; MSV [Y00514.1]; maize striate mosaic virus [MSMV, MF167297.1]; oak dwarf virus [ODV, AM296025.1]; panicum streak virus [PanSV, L39638.1]; sugarcane streak virus [SCSV, M82918.1]; switchgrass mosaic-associated virus [SgMaV, KF806701.1]; tobacco yellow dwarf virus [TYDV, M81103.1]; and wheat dwarf virus [WDV, AJ783960.1]) was conducted using ClustalW tool of MEGA X software (Kumar et al., 2018). A phylogenetic tree was constructed with previously aligned sequences using the neighbor-joining method with a bootstrap value of 1,000. The Rep A, movement protein, coat protein and intergenic regions were compared to identify the relationship of the Korean SPSMV-1 isolate with other mastreviruses using basic local alignment search tool (BLAST; http://blast.ncbi.nlm.nih.gov/Blast.cgi).
An infectious clone of the Korean SPSMV-1 isolate was obtained by constructing a partial tandem repeat of the full-length viral DNA as previously described (Urbino et al., 2008; Vo et al., 2022) (Fig. 1). Specific primer sets were designed with ends containing restriction sites to detect two fragments through PCR amplification (SPSMV-1-IC1-F/SPSMV-1-IC1-R and SPSMV-1-IC2-F/SPSMV-1-IC2-R) (Table 1). Each partial fragment of SPSMV-1 was ligated into the pGEM-T Easy vector (Promega) via TA cloning and sequenced for confirmation. The cloned constructs were digested with the following restriction enzymes: IC1 (XbaI and KpnI) and IC2 (KpnI and HindIII) (Takara, Shiga, Japan). To produce the infectious clone 1.1-mer, two partial fragments were introduced into the pCAMBIA1303 vector (Abcam, Cambridge, UK) and transformed into competent Escherichia coli strain DH5α using the heat shock method. The transformed plasmids were extracted from E. coli using the AccuPrep Nano-Plus Plasmid Mini Extraction Kit (Bioneer, Daejeon, Korea) and cross-checked by digestion with the three restriction enzymes, XbaI, KpnI, and HindIII (The plasmids were then transformed into three different A. tumefaciens strains: GV3101, LBA4404, and EHA105) (Fig. 1). Infectious clones were confirmed by both enzyme digestion and colony PCR using the 2× AccuPower PCR Master Mix (Bioneer) with the primers for IC1 and IC2 SPSMV-1 (Table 1).
N. benthamiana seeds were planted in sterilized soil and cultivated in a growth chamber at the Sungkyunkwan University, Suwon, South Korea. Four-week-old N. benthamiana plants of similar sizes were selected and divided into mock, GV3101, LBA4404, and EHA105. A. tumefaciens strains were cultured in Luria broth media in the presence of the pCAMBIA1303-selective antibiotic, kanamycin (50 mg/l), and other strain-specific selective antibiotics (50 mg/l) (for GV3101: rifampicin and gentamicin; LBA4404: rifampicin; EHA105: rifampicin and streptomycin) at 28°C with agitation for 30 h until the OD value at 600 nm reached 0.8-1.0. The main apical shoot tips were inoculated with 0.5 ml of agrobacterial culture using the pin-pricking method. Plants in the mock group were inoculated with A. tumefaciens containing the empty pCAMBIA1303 plasmid. Photographs were taken for each group four weeks after inoculation, and samples from newly produced leaves, stems, and roots were collected for downstream analysis.
Four weeks after inoculation, samples from young leaves, stems, and roots were collected from the mock- and SPSMV-1-inoculated N. benthamiana plants. Genomic DNA was isolated from the tissues using the STE method described above. To detect the presence of the virus inside the samples, PCR was conducted with the detection primers SPSMV-1-Detection-F/SPSMV-1-Detection-R (Table 1). The mixture was prepared using 10 μl of 1× AccuPower PCR Mastermix (Bioneer), 1 μl of template DNA, 1 μl each of the forward and reverse primers (10 pM), and distilled water to reach a total volume of 20 μl. The conditions for the PCR reaction were as follows: preheating at 94°C (3 min), followed by 35 cycles of 30 s at 94°C, 30 s at 58°C, 1 min at 72°C, and a final extension at 72°C (5 min). The amplified DNA was visualized on a 1% agarose gel by electrophoresis, and sequenced by Macrogen.
Viral accumulation of SPSMV-1 was evaluated in sweet potato samples (young leaves, stems, and roots) and four-week-old-inoculated N. benthamiana. Total DNA was extracted using a DNeasy Plant mini kit (Qiagen, Hilden, Germany) and 1 μg of DNA extracted from leaves was amplified via quantitative real-time PCR (qPCR) using the primer set for the RepA protein (SPSMV-1-F/SPSMV-1-R) (Table 1). The SYBR Green PCR Master mix (Takara) in a final volume of 20 μl was used according to the manufacturer’s protocol. The qPCR experiment was performed in triplicate per sample. The reaction and the detection of the fluorescent signals were performed using a Rotor Gene Q thermocycle (Qiagen). EF1α was used for the internal normalization (Table 1). The data analysis was performed using the 2(−ΔΔCt) method (Livak and Schmittgen, 2001) to determine relative expression levels. An unpaired Student‘s t-test was used to determine significant differences with the data expressed as mean ± standard error, and statistical significance was defined at P < 0.05.
Strand-specific amplification was performed according to the method described by Rodríguez-Negrete et al. (2014) and (Kil et al., 2018) with modification using virion-sense- and complementary-sense-specific primer sets (Table 1). First, extension reactions of single-stranded viral templates with T4 DNA polymerase (Takara) and viral-specific primers (OCS-TAG or OVS-TAG) were performed for strand-specific amplification. The products from this step were purified using the QIAquick PCR Purification Kit (Qiagen). Subsequently, 2 μl of the first-strand reaction product was mixed with 10 μl of 2× AccuPower PCR Master Mix (Bioneer), 1 μl of 10 pM specific primers (TAG, OVS or OCS), and 6 μl of nuclease-free water, following the manufacturer’s protocol. PCR cycling consisted of an initial denaturation step at 95°C for 30 s, 40 cycles of 95°C for 10 s, 60°C for 15 s and 72°C for 20 s in a T100 thermal cycler (Bio-Rad, Hercules, CA, USA).
In 2014, three symptomless sweet potato samples were collected on a farm in Suwon, South Korea, during a survey for sweepovirus detection analysis. Total DNA was extracted and subjected to PCR ampification using SPSMV-1-Detection-F/SPSMV-1-Detection-R (Table 1). An amplicon of the expected 830 nucleotides product was obtained from three of the three sweet potato samples and was sent for sequencing (Fig. 2A). BLAST analysis indicated that the detected viral sequence shared 99.61% similarity with the Chinese SPSMV-1 isolate (KY565235) (Fig. 2B). When PCR was performed directly from the genomic DNA, an amplification product of sufficient concentration could not be obtained, so RCA was performed and then PCR was performed using the RCA product with abutting primers (Table 1) in PCR for full-length amplification of the viral genome. The amplified products were analyzed on a 1% agarose gel, and a target band of 2.6 kb in all three sweet potato samples (Fig. 2C) was cloned into the pGEM-T Easy vector (Promega) and sequenced. BLAST analysis revealed a 99.92% nucleotide identity with the Chinese SPSMV isolate (KY565235). The complete sequence of the Korean SPSMV-1 isolate was deposited in the GenBank (MF148248). qPCR revealed that the relative titer of infected samples was similar (Fig. 2D). Compared to the case where PCR was performed by artificially increasing the amount of virus genome through RCA, the concentration of the amplification product in the previous PCR and the qPCR results showed a similar low level of amplification for each sample (Fig. 2).
The complete sequence of the Korean SPSMV-1 isolate obtained by PCR was 2,599 nucleotides long. The number and arrangement of ORFs and intergenic regions were identical to those of other SPSMV-1 isolates, with five ORFs encoding C1, C2, C3, V1, and V2 (Fig. 3A). The phylogenetic tree shown in Fig. 3B shows the relationship between SPSMV-1 sequences of different origins and mastrevirus sequences. The amino acid sequences of the proteins encoded by the ORFs and the nucleotide sequences of the intergenic sequences of SPSMV-1 were compared with those of other geminiviruses to obtain a better insight into the taxonomic relationship of this virus. The Korean SPSMV-1 isolate was found to be most closely related to the Chinese SPSMV-1isolate. It was confirmed that the ORF sequences for Rep, movement protein, and coat protein showed a certain level of similarity with other dicot-infecting mastreviruses. Interestingly, however, the nucleotide sequences of the LIR and SIR of SPSMV-1 did not show any similarity with any other previously reported sequences of viruses (Table 2). The LIR of all SPSMV-1 isolates was compared at the nucleotide level, and all were found to contain an unusual nonanucleotide sequence TAAGATT↓CC, in contrast to other geminiviruses, which possess the sequence TAATATTAC (Fig. 3C). This means that the IRs of SPSMV-1 have unique characteristics different from those of existing geminiviruses.
Because the choice of A. tumefaciens strain used for the agro-inoculation process can substantially affect the efficiency and expression of viral proteins in plants, we used the three most commonly used A. tumefaciens strains: GV3101, LBA4404, and EHA105. Four weeks after agro-inoculation, N. benthamiana plants showed no visual symptoms in either the mock- or SPSMV-1-inoculated plants. No differences were observed among the three N. benthamiana plants inoculated with the three A. tumefaciens strains (Fig. 4A). Three organs (newly produced leaves, stems, and roots) of the SPSMV-1-inoculated N. benthamiana plants were harvested and analyzed using PCR to investigate their viral replication ability and systemic movement. qPCR was used to evaluate the relative viral titers in N. benthamiana. The results revealed that the virus was present in all three organs 4 weeks after inoculation, and the LBA4404 A. tumefaciens strain showed the highest SPSMV-1 genome transfer ability (Fig. 4B and C). Strand-specific amplification using virion-sense- and complementary-sense specific primer sets was performed to confirm the replication ability of the virus. The assumption that geminiviral DNA replicated and ssDNA was converted into double-stranded DNA (dsDNA) intermediates by a rolling-circle replication mechanism could be confirmed by PCR amplification using OCS and OVS primers and reactions using TAG and OCS or OVS primers (Fig. 5A). Our data showed that dsDNA and two ssDNA molecules (virion and complementary senses) were present in the infected samples, indicating that the viral genomes had replicated in the N. benthamiana plants (Fig. 5B). However, the virus could not be identified through Southern blotting, suggesting that the viral titer was very low (data not shown).
In this study, we identified the whole genome of the Korean SPSMV-1 isolate and constructed an infectious clone, which will contribute to a better understanding of virus-plant interactions and the biological significance of this virus in infected plants. The SPSMV-1 isolate exhibited the typical genome structure (number and location of ORFs) of a mastrevirus. The SPSMV-1 infectious clone was introduced into N. benthamiana plants using Agrobacterium-mediated inoculation, the most commonly used method for plant genetic engineering, owing to its relatively high efficiency. Three widely used A. tumefaciens strains, GV3101, EHA105, and LBA4404, consisting of SPSMV-containing pCAMBIA1303, were evaluated using conventional PCR to determine which A. tumefaciens strain was the best for viral gene transfer to N. benthamiana. Four weeks after inoculation, SPSMV-1 was detected in the young leaves, stems, and roots of N. benthamiana plants. We found that LBA4404 was the most effective strain for SPSMV-1 gene transfer. Therefore, A. tumefaciens strain LBA4404 could be an appropriate strain for efficient viral gene transfer of SPSMV-1 in N. benthamiana plants. Similar result was reported by Bakhsh et al. (2017), who found that LBA4404 was a better choice than EHA105, GV2260, C58C1, GV3101, or AGL1 for the genetic transformation of foreign genes in N. tabacum L. A successful transformation process requires host (plant) genome and A. tumefaciens strain compatibility, which is influenced by several factors, including the type of chromosomal background of A. tumefaciens strains, different opines, mechanism of transfer and integration of T-DNA, and T-DNA copy number containing the gene of interest (Yadav et al., 2014). Therefore, the choice of A. tumefaciens strain for plant transformation can substantially affect the transformation efficiency and expression of foreign proteins (Yadav et al., 2014). However, the detailed mechanism by which LBA4404 favors SPSMV-1 genome transfer to N. benthamiana plants requires further analysis.
The mastrevirus SPSMV-1 has been reported to infect sweet potato plants in several countries worldwide, and a high infection rate has been reported in South Korea (Kwak et al., 2014). Although SPSMV-1 is an asymptomatic mastrevirus and does not appear to play an important pathological role, it may pose a threat due to the occurrence of synergistic interactions among viruses infecting sweet potatoes (Cuellar et al., 2015; Untiveros et al., 2007). In our study, nucleotide sequence analysis revealed that the Korean SPSMV-1 isolate contains an abnormal nonanucleotide sequence and a longer stem-loop region in the LIR, similar to other reported isolates, when compared to other geminiviruses. We hypothesized that this could explain the pathological characteristics of SPSMV-1 (asymptomatic infection and low viral accumulation). Creating mutations in these regions of the genome and constructing their infectious clones are necessary to comprehensively evaluate viral replication and determine whether the plants are asymptomatic owing to the nature of the virus or because a low viral accumulation is insufficient to induce a response in the further studies. This study will help in constructing suitable mutant infectious clones.
Acknowledgments
This work was supported by a grant (project code no. Z-1543086-2017-21-01) from the Animal and Plant Quarantine Agency of South Korea.
Fig. 1
Schematic of the construction of an infectious clone of the Korean sweet potato symptomless virus 1 (SPSMV-1) isolate.

Fig. 2
Detection of sweet potato symptomless virus 1 (SPSMV-1). (A) Detection of SPSMV-1 by polymerase chain reaction (PCR) in leaves (L), stem (S) and roots (R) of sweet potato samples collected from a field in South Korea. Lane +, positive control with plasmid DNA containing partial SPSMV-1 genomic DNA; Lane −, negative control with no DNA template; S1-S3 refer to the different sweet potato samples. (B) Sequencing results showing the detected virus in 3 sweet potato samples with high similarity to the Chinese SPSMV-1 isolate (accession number: KY565235). (C) PCR amplification of the collected sweet potato samples using SPSMV-1 specific primers to obtain complete sequence. Lanes S1-S3 refer to three sweet potato samples. (D) Quantitative real-time PCR analysis indicating SPSMV-1 titer in sweet potato leaves from the fields. Mock plants show virus-free sweet potato samples. The bar graphs indicate the mean±standard deviation (n = 5). The statistical comparison was performed with the unpaired t-test: *P < 0.05.
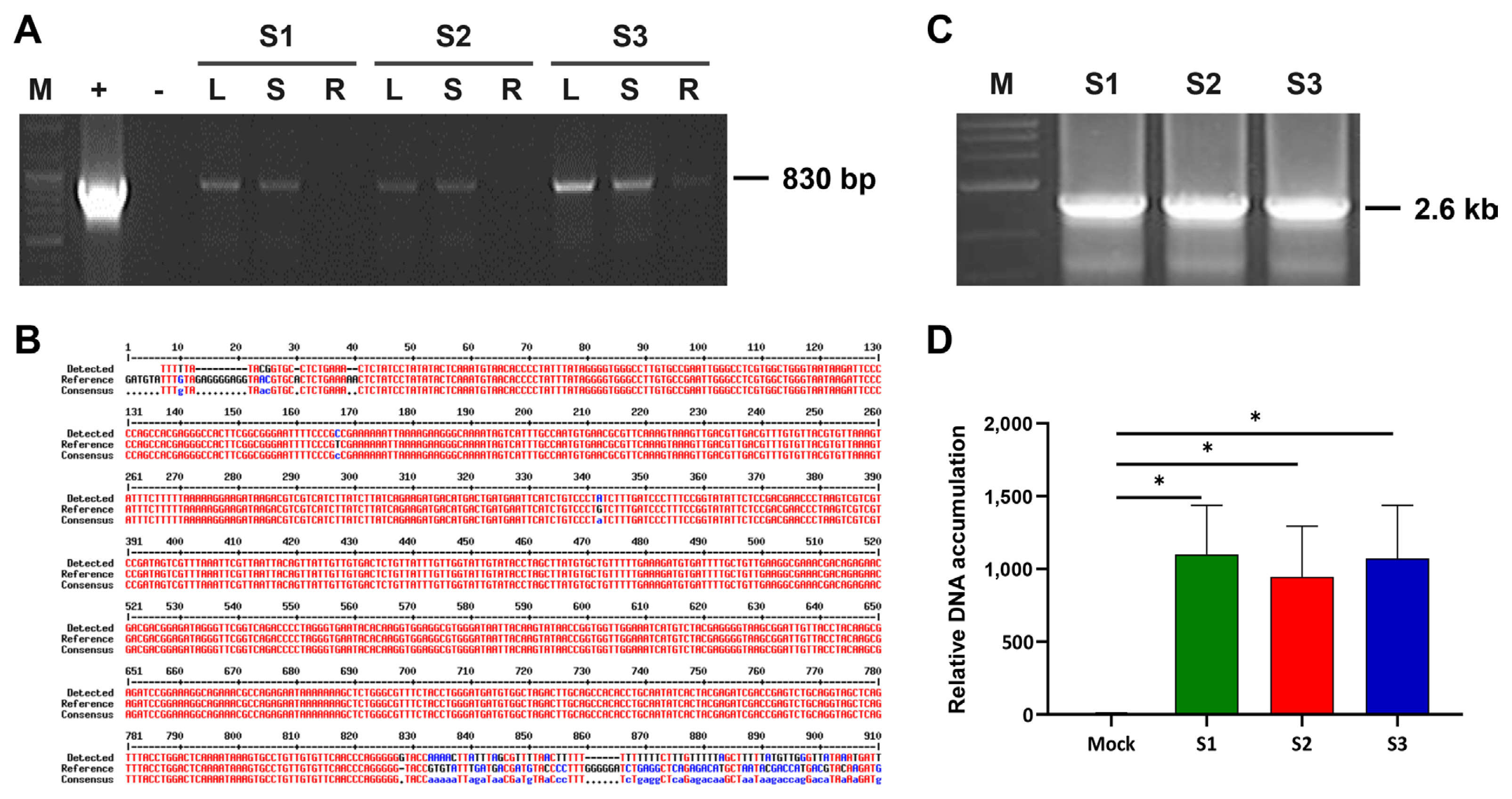
Fig. 3
Sequence analysis of the complete genome of the Korean sweet potato symptomless virus 1 (SPSMV-1) isolate. (A) Schematic diagram of two intergenic regions and five open reading frames. (B) Neighbor-joining phylogenetic tree of the complete genomic sequences of SPSMV-1 and other mastreviruses, constructed using 1,000 bootstrap replicates (MEGA X). (C) The large intergenic region (LIR) with an unusual nonanucleotide sequence (TAAGATT↓CC) in all SPSMV-1 isolates.

Fig. 4
Results of the agro-inoculation test conducted using three Agrobacterium strains vectored with the Korean sweet potato symptomless virus 1 (SPSMV-1) isolate. (A) Appearance of mock Nicotiana benthamiana and infected plants. (B) Detection of SPSMV-1 DNA in N. benthamiana by polymerase chain reaction (+, positive control; −, negative control; number 1-5 indicates infected samples). (C) SPSMV-1 titer in leaf, stem and root of N. benthamiana. The bar graphs indicate the mean±standard deviation (n = 5). The statistical comparison was performed with the unpaired t-test: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, ns: not significant.
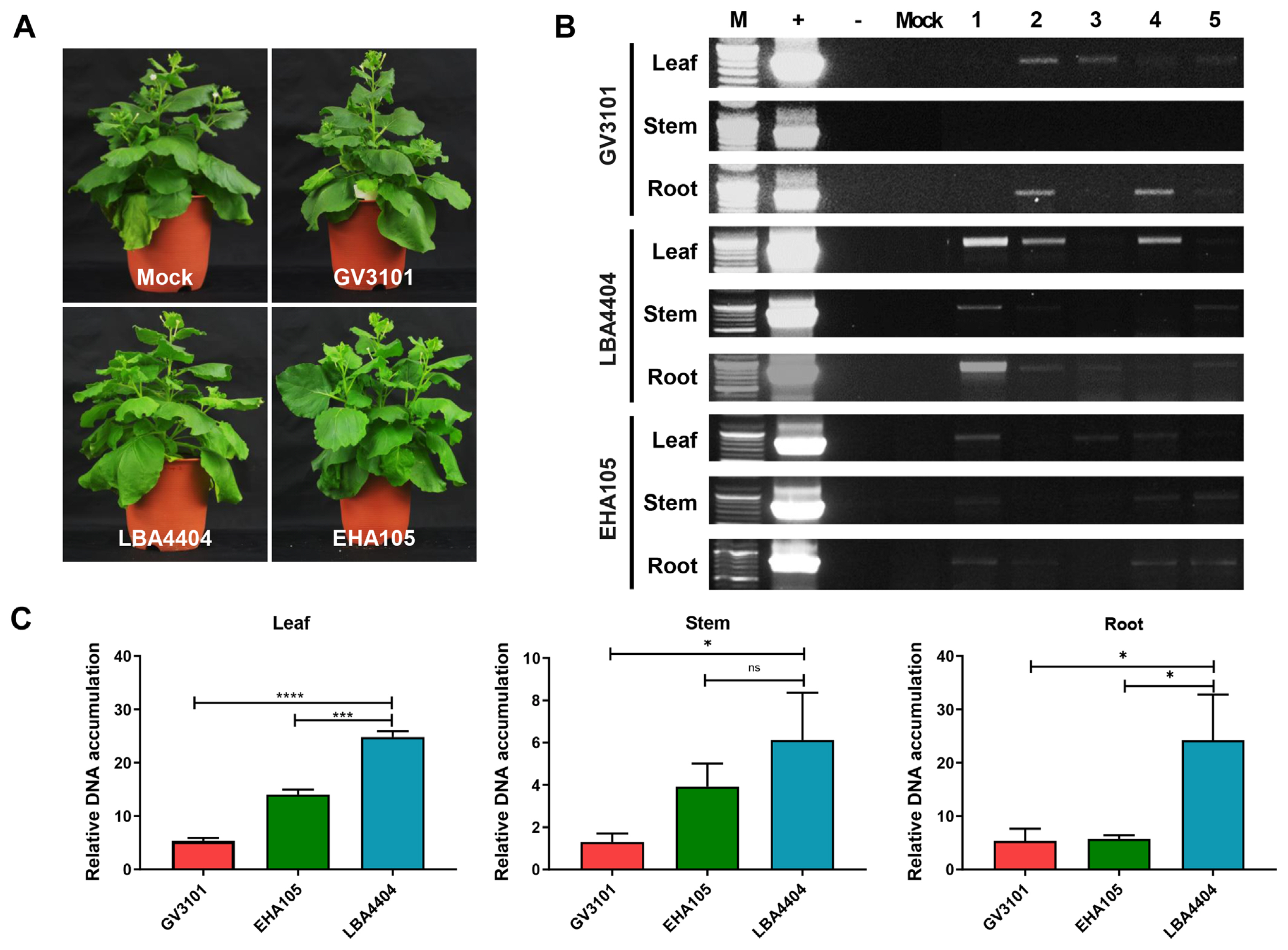
Fig. 5
Strand-specific amplification of sweet potato symptomless virus 1 (SPSMV-1)-infected Nicotiana benthamiana plants. (A) Illustration of strand-specific polymerase chain reaction (PCR). (B) Strand-specific amplification with root samples (1 and 2) from SPSMV-1-inoculated N. benthamiana plants using virion-sense- and complementary-sense-specific primer sets.

Table 1
Primers used in the construction of an infectious clone and the detection of the Korean sweet potato symptomless virus 1 (SPSMV-1) isolate
Table 2
List of mastreviruses sharing open reading frame (ORF) sequence similarities with the Korean sweet potato symptomless virus 1 (SPSMV-1) isolate
| ORF | Virus (similaritya) | Accession no. |
|---|---|---|
| Replication-associated protein (Rep) | Chickpea chlorosis virus (54.04%) | KC172700 |
| Chickpea redleaf virus (52.75%) | NC014739 | |
| Chickpea chlorotic dwarf virus (54.55%) | MN178605 | |
| Chickpea yellow dwarf virus (53.61%) | AIW42780 | |
| Large intergenic region (LIR) | No match with previously reported virus | |
| Movement protein (MP) | Chickpea chlorotic dwarf virus (61.11%) | KY817246 |
| Chickpea redleaf virus (84.38%) | NC014739 | |
| Wheat dwarf virus (45.12%) | KJ473699 | |
| Coat protein (CP) | Chickpea chlorotic dwarf virus (45.31%) | KC172680 |
| Chickpea yellows virus (48.67%) | NC038478 | |
| Chickpea chlorosis virus (51.03%) | JN989424 | |
| Tobacco yellow dwarf virus (49.03%) | JN989446 | |
| Chickpea yellow dwarf virus (50.65%) | NC025475 | |
| Chickpea redleaf virus (46.64%) | MK940528.1 | |
| Short intergenic region (SIR) | No match with previously reported virus |
References
Azad, H. N and Nematadeh, G. H 2013. Introducing a new method of genomic DNA extraction in dicotyledonous plants. Sch. J. Agric. Sci 2:242-248.
Bakhsh, A., Anayol, E. and Ozcan, S. F 2017. Comparison of transformation efficiency of five Agrobacterium tumefaciens strains in Nicotiana tabacum L. Emirates J. Food Agric 26:259-264.

Borah, B. K., Zarreen, F., Baruah, G. and Dasgupta, I. 2016. Insights into the control of geminiviral promoters. Virology 495:101-111.


Cao, M., Lan, P., Li, F., Abad, J., Zhou, C. and Li, R. 2017. Genome characterization of sweet potato symptomless virus 1: a mastrevirus with an unusual nonanucleotide sequence. Arch. Virol 162:2881-2884.



Cuellar, W. J., Galvez, M., Fuentes, S., Tugume, J. and Kreuze, J. 2015. Synergistic interactions of begomoviruses with Sweet potato chlorotic stunt virus (genus Crinivirus) in sweet potato (Ipomoea batatas L.). Mol. Plant Pathol 16:459-471.


Food and Agriculture Organization of the United Nations 2016 FAOSTAT. Statistics URL http://apps.fao.org. 31 December 2022.


Hayes, R. J., Macdonald, H., Coutts, R. H. A and Buck, K. W 1988. Priming of complementary DNA synthesis in vitro by small DNA molecules tightly bound to virion DNA of wheat dwarf virus. J. Gen. Virol 69:1345-1350.

Kil, E.-J., Park, J., Choi, E.-Y., Byun, H.-S., Lee, K.-Y., An, C. G., Lee, J.-H., Lee, G.-S., Choi, H.-S., Kim, C.-S., Kim, J.-K and Lee, S. 2018. Seed transmission of Tomato yellow leaf curl virus in sweet pepper (Capsicum annuum). Eur. J. Plant Pathol 150:759-764.


Kim, J., Yang, J. W., Kwak, H.-R., Kim, M.-K., Seo, J.-K., Chung, M.-N., Lee, H.-U., Lee, K.-B., Nam, S. S., Kim, C.-S., Lee, G.-S., Kim, J.-S., Lee, S. and Choi, H.-S 2017. Virus incidence of sweet potato in Korea from 2011 to 2014. Plant Pathol. J 33:467-477.



Kreuze, J. F., Perez, A., Untiveros, M., Quispe, D., Fuentes, S., Barker, I. and Simon, R. 2009. Complete viral genome sequence and discovery of novel viruses by deep sequencing of small RNAs: a generic method for diagnosis, discovery and sequencing of viruses. Virology 388:1-7.


Kumar, S., Stecher, G., Li, M., Knyaz, C. and Tamura, K. 2018. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol 35:1547-1549.



Kwak, H.-R., Kim, M.-K., Shin, J.-C., Lee, Y.-J., Seo, J.-K., Lee, H.-U., Jung, M.-N., Kim, S.-H and Choi, H.-S 2014. The current incidence of viral disease in Korean sweet potatoes and development of multiplex RT-PCR assays for simultaneous detection of eight sweet potato viruses. Plant Pathol. J 30:416-424.



Livak, K. J and Schmittgen, T. D 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402-408.


Mbanzibwa, D. R., Tugume, A. K., Chiunga, E., Mark, D. and Tairo, F. D 2014. Small RNA deep sequencing-based detection and further evidence of DNA viruses infecting sweetpotato plants in Tanzania. Ann. Appl. Biol 165:329-339.

Muhire, B., Martin, D. P., Brown, J. K., Navas-Castillo, J., Moriones, E., Zerbini, F. M., Rivera-Bustamante, R., Malathi, V. G., Briddon, R. W and Varsani, A. 2013. A genome-wide pairwise-identity-based proposal for the classification of viruses in the genus Mastrevirus (family Geminiviridae). Arch. Virol 158:1411-1424.



Qiao, Q., Zhang, Z., Zhao, X., Wang, Y., Wang, S., Qin, Y., Zhang, D., Tian, Y. and Zhao, F. 2020. Evidence for seed transmission of sweet potato symptomless virus 1 in sweet potato (Ipomoea batatas). J. Plant Pathol 102:299-303.


Rodríguez-Negrete, E. A., Sánchez-Campos, S., Cañizares, M. C., Navas-Castillo, J., Moriones, E., Bejarano, E. R and Grande-Pérez, A. 2014. A sensitive method for the quantification of virion-sense and complementary-sense DNA strands of circular single-stranded DNA viruses. Sci. Rep 4:6438.


Souza, C. A., Rossato, M., Melo, F. L., Boiteux, L. S and Pereira-Carvalho, R. C 2018. First report of sweet potatos symptomless virus 1 infecting Ipomoea batatas in Brazil. Plant Dis 102:2052.
Untiveros, M., Fuentes, S. and Salazar, L. F 2007. Synergistic interaction of Sweet potato chlorotic stunt virus (Crinivirus) with Carla-, Cucumo-, Ipomo-, and Potyviruses infecting sweet potato. Plant Dis 91:669-676.


Urbino, C., Thébaud, G., Granier, M., Blanc, S. and Peterschmitt, M. 2008. A novel cloning strategy for isolating, genotyping and phenotyping genetic variants of geminiviruses. Virol. J 5:135.



Vo, T. T. B., Lal, A., Ho, P. T., Troiano, E., Parrella, G., Kil, E.-J and Lee, S. 2022. Different infectivity of Mediterranean and Southern Asian tomato leaf curl New Delhi virus isolates in cucurbit crops. Plants 11:704.



- TOOLS
-
METRICS

- ORCID iDs
-
Eui-Joon Kil

https://orcid.org/0000-0002-7256-3879 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print