 |
 |
| Plant Pathol J > Volume 39(5); 2023 > Article |
|
Abstract
Seed-borne diseases reduce not only the seed germination and seedling growth but also seed quality, resulting in the significant yield loss in crop production. Plant seed harbors diverse microbes termed endophytes other than pathogens inside it. However, their roles and application to agricultures were rarely understood and explored to date. Recently, we had isolated from soybean seeds culturable endophytes exhibiting in-vitro antagonistic activities against common bacterial and fungal seed-borne pathogens. In this study, we evaluated effects of seed treatment with endophytes on plant growth and protection against the common seed-borne pathogens: four fungal pathogens (Cercospora sojina, C. kikuchii, Septoria glycines, Diaporthe eres) and two bacterial pathogens (Xanthomonas axonopodis pv. glycines, Pseudomonas syringae pv. tabaci). Our experiments showed that treatment of soybean seeds with seed endophytes clearly offer protection against seed-borne pathogens. We also found that some of the endophytes promote plant growth in addition to the disease suppression. Taken together, our results demonstrate agricultural potential of seed endophytes in crop protection.
Soybean (Glycine max) is an economically important leguminous crop that is grown in all over the world (Karges et al., 2022). However, expansion of cultivation area has been concomitant of various plant diseases, hindering the spread of soybean cultivation in paddies (Kang et al., 2015). Although it is rare that commonly occurring soybean diseases destroy the entire fields, they can cause dramatic decrease in overall yield. Prevention of soybean diseases are important, as they decrease the quantity and quality of overall crops grown for consumption by humans and animals, as well as for industrial products such as biofuel production (Allen et al., 2017). Prevention of seed-borne diseases are, however, particularly challenging, because (1) many seed-borne pathogens reside within the seeds without obvious visible symptoms, and (2) it is therefore difficult to diagnose without relying on detecting molecular markers.
Seeds harbor diverse endophytic microbial community, and their interactions that take place in seed have a profound influence on host plant ecosystem, condition, and productivity (Nelson, 2018). Seed endophytes often have ability to improve seed germination and plant growth, and thus are agriculturally important resources that can have important agricultural applications (Li et al., 2019a). For example, they enhance the plant growth by producing hormones that control nutrient uptake and suppress plant diseases by producing antibiotics (Harman et al., 2021). There is increasing demand for the development of the biotechnological applications of seed endophytes to phytoremediation and the sustainable production of crops (Xavier et al., 2021).
In our previous study, we had isolated culturable endophytes from soybean seeds and tested their in-vitro antagonistic activities against the selected set of seed-borne pathogens of soybean plants (Kim et al., 2022). One promising way for these endophytes to be utilized would be to inoculate soybean seeds with endophytes, anticipating that the endophytes might provide protection against the pathogens during seed germination and seedling growth. Such application of endophytes would open up the possibility to better exploit the potential of seed endophytes for sustainable agriculture.
Seed-borne pathogens that are used in our study include four fungal pathognes (Cercospora sojina, C. kikuchii, Septoria glycines, and Diaporthe eres) and two bacterial pathogens (Xanthomonas axonopodis pv. glycines [Xag] and Pseudomonas syringae pv. tabaci [Pst]). Currently, there are two fungal species of Cercospora that infect soybeans: C. sojina and C. kikuchii causing frogeye leaf spot and purple seed stain, respectively (Soares et al., 2015). Symptoms of frogeye leaf spot appear as light brown circular spots surrounded by a deep brown to red circle, while in case of purple seed stain, the diseased seeds show a pink to purple stain, ranging from a small spot to covering the entire seed coat (Li et al., 2019b). Septoria brown spot, caused by the fungus Septoria glycines, is also the highly common foliar disease of soybean. Small and brown lesions infected by S. glycines progress on leaves in the lower canopy and continue up the plant as the season progresses to the upper canopy (Cruz et al., 2010). There is also a report that soybean seed decay can be caused by the fungus Diaporthe eres (Petrović et al., 2015). The result in the Petrović’s work shows that isolates of D. eres caused 72% seed decay in their experiment, while isolates of D. conorum and Phomopsis occulta did not cause seed decay. Bacterial pathogens are as serious a problem in soybean plants as fungal pathogens. Two prevalent bacterial diseases are bacterial leaf pustule and wildfire caused by Xag and Pst, respectively. Bacterial leaf pustule is characterized by distinctive symptoms on soybean leaves, exposing yellow to brown lesions with a raised pustule in the center, whereas wildfire is recognized by brown leaf spots surrounded by yellow haloes with distinctive margins (Hong et al., 2012).
Here, we tested whether those isolated seed endophytes showing antagonistic activities can provide soybean plants with protection during seed germination and seedling growth against the pathogens via seed treatment. In addition, we also monitored whether the seed treatment with endophytes can promote plant growth.
In order to assess plant protection of endophytes against seed-borne pathogens, soybean seeds (cultivar Daewon) were treated with microbial suspension from individual endophytic isolates, followed by treatment with seed-borne pathogen suspension. Then, vegetative growth and resistance to pathogenicity were assessed through pot experiment (Fig. 1). Soybean seeds were washed with 70% ethanol and dipped into 1% sodium hypochlorite solution for 5 min to disinfect the surface and rinsed with sterilized water before microbial treatment. Effectiveness of surface sterilization was confirmed by no colony formation on the agar media plated with distilled water used to rinse the surface-sterilized seeds.
The seed-borne pathogens C. sojina (KACC No. 49847), S. glycines (KACC no. 43091), X. axonopodis pv. glycines (KACC no. 10491) and P. syringae pv. tabaci (KACC no. 17820) were acquired from the Korean Agriculture Culture Collection (KACC). All the pathogens obtained from the KACC were described in the website as being isolated from the seeds. Diaporthe sp. and Cercospora sp. were isolated from diseased soybean seeds and identified by sequencing of PCR product using the primers EF1-728F (5′-CATCGAGAAGTTCGAGAAGG-3′) and EF1-986R (5′-TACTTGAAGGAACCCTTACC-3′) (Gomes et al., 2013) and the primers cfp_F1 (5′-GCCGATCGATTGCAGGAGTTGGC-3′) and cfp_R1 (5′-TTGCTGATCCAAGTAGTCGGACG-3′) (Soares et al., 2015) for identification of Diaporthe sp. and Cercospora sp., respectively. Then they were identified as D. eres and C. kikuchii corresponding to accession no. KJ490498 and no. KP752257 each with 100% similiarity. Seed endophytes were selected from our previous study, based on their in-vitro antagonistic activities against the seed-borne pathogens (Kim et al., 2022). We tested three species of bacteria (Bacillus sp. DWB21155, Pseudomonas koreensis DWB21122, and Stenotrophomonas maltophilia DWB21134) and three species of fungi (Alternaria hungarica DWF21130, Cladosporium pseudocladosporioidies DWF21127, and Penicillium steckii DWF21107).
Three soybean seeds were planted in one experimental pot with six replications per one treatment (pot size: 11.3 cm upper diameter, 6.5 cm bottom diameter, 9.7 cm high). The pot trials were conducted in a phyto-chamber at 25°C and 70% relative humidity with a 12-h light and dark alternation from a fluorescent lamp. Before planting, seeds were treated with endophyte isolates for 2 h by immersing them in bacterial suspension or spore suspension, followed by another immersion in bacterial suspension or spore suspension of seed-borne pathogens for 2 h. Then, the treated seeds were moved on the to the petri dishes with wet paper disc on the bottom and incubated for 3 days to allow seed germination. The endophytic treatments consisted of the bacterial isolates DWB21155, DWB21235, and DWB21122 and the fungal isolates DWF21130, DWF21232 and DWF21107 obtained from the previous study (Kim et al., 2022). The soil used to grow the plants consisted of 70% cocopeat, 8.5% peatmoss, 9.5% pearlite, 6% vermiculite, 3.748% zeolite, 0.01% humic acid, 0.01% wooden charcoal and 0.18% fertilizer. Bacterial suspensions for seed treatment were prepared with tryptic soy broth (Difco, Detroit, MI, USA) incubated for approximately 24 h at 30°C, 120 rpm from cultures grown for 24 h in trypsin soy agar at 30°C, until the suspension concentration of each of the bacteria reached an OD600 = 0.5-0.6 in spectrophotometer. Then, the cultures were centrifuged at 1,500 ×g for 5 min at 4°C, the bacterial pellet was resuspended in distilled water. To obtain fungal suspension, each of the fungal isolates (5 mm in diameter of a cylindrical agar plug) was inoculated on the center of potato dextrose agar medium and incubated at 25°C until mycelia reached the edge of the plate (9 cm in diameter). Next, the fungi were scraped with sterile water from the medium before being transferred to a sterile falcon tube, and filtered through a Miracloth layer (Millipore, Billerica, MA, USA). Then, the number of spores in the spore suspension was counted using a hemocytometer. Concentration of spore suspensions were adjusted to a final concentration of 4-5 × 104 spores/ml before seed treatment. The soybean seeds were immersed in the suspension for 2 h under 50 rpm at 30°C and 25°C for bacterial and fungal treatment, respectively. The control group with no inoculation was treated with distilled water instead of bacterial or fungal suspensions.
We measured shoot length (cm), root length (cm), number of leaves, fresh leave weight (g), fresh stem weight (g), fresh root weight (g), dry leave weight (g), dry stem weight (g), and dry root weight (g), as the indicator of plant development 14 days after planting the soybean seeds. To compare each measured value, we used the following formula; (Vc − Vexp)/Vc, where Vc represents each measured value of control group and Vexp represents each measured value of experimental group treated with microbes. Then, the averages of resulting values were taken and shown in heatmaps generated using the R program (version 4.2.1). The control was referred to healthy control (HC), and the control with pathogen inoculation was referred to infected control (IC).
The control seeds treated with distilled water swelled up a few hours after the treatment without any color change, while seeds treated with some pathogens showed morphological changes after the treatment in addition to discoloration. In the D. eres-treated seeds, for example, seeds looked quite different from the controls at six hours after treatment. D. eres-treated seeds were brown in whole or in part on the surface of the seed, compared to control (‘De-trt’ in Fig. 2A). The width and length of the seeds was comparable to the control, but D. eres-treated seeds exhibited difference in thickness (Fig. 2B, Supplementary Table 1). We also often observed brown spots or cracks in the hilum up of the D. eres-treated seeds.
When comparing C. kikuchii-treated seeds with control seeds, there was little difference in length, width, and thickness. However, the difference in color was clearly visible at six hours after treatment. We found pink to purple stain in every seed coat treated with C. kikuchii. Some of C. kikuchii-treated seeds displayed relatively small range of stain, while other seeds showed stain over the entire area of seed coat (‘Ck-trt’ in Fig. 2A). The seeds treated with Pst did not differ significantly from the control seeds, except that the seed surface tended to become slightly darker (‘Pst-trt’ in Fig. 2A). The seeds treated with C. sojina, S. glycines, and Xag showed no clear difference from the control at the seed stage.
To gauge the effects of seed treatment with endophytes, we first observed the effect of seed inoculation with pathogens on seed germination and seedling growth. After C. kikuchii inoculation, only 3 out of 6 soybean seeds succeeded in germination, and 2 of those did not reach the leaf development stage (Fig. 3 Trt (P) row and Supplementary Table 2). We also observed dark discoloration in the leaves of C. kikuchii-treated group (‘Ck-trt’ in Supplementary Fig. 1B).
When seeds were inoculated with C. sojina, five out of six seeds germinated, which was the highest germination rate among pathogen-only treated groups. Three out of six seeds (50%) were able to germinate after inoculation with S. glycines. Of the six D. eres-treated seeds, three showed severe decay symptoms in consistent with the observed abnormalities in D. eres-treated seeds, and three others also did not develop normally (Supplementary Fig. 1A). Only 1 out of 6 seeds inoculated with D. eres succeeded in germination, and even 14 days after planting, the germinated seed did not succeed in leaf release.
Only half of the Xag-treated seeds germinated, and the growth of soybean plant from the seed was very poor. Notably, the leaves of Xag-treated group exhibited yellow to brown lesions with pustules (‘Xag-trt’ in Supplementary Fig. 1B). Similarly, three out of six Pst-treated seeds germinated and showed relatively poor growth. In the leaves of plants, yellow halos were also observed at the edges (‘Pst-trt’ in Supplementary Fig. 1B).
Given the responses of plants to seed-borne pathogens, we set out to test whether the seed endophytes, which we had isolated in the previous work (Kim et al., 2022), would improve or remedy such responses of plants to the pathogen treatment. To do the test, seeds were treated first with the endophytes before being challenged with the pathogens and planted in the pot. The endophytes we tested in this study include three bacteria (P. koreensis DWB21122, Bacillus sp. DWB21155, and S. maltophilia DWB21134) and three fungi (A. hungarica DWF21130, C. pseudocladosporioidies DWF21127, and P. steckii DWF21107), which will be referred to as their isolation number (for example, DWB21122 for P. koreensis) (Fig. 3, Supplementary Table 2).
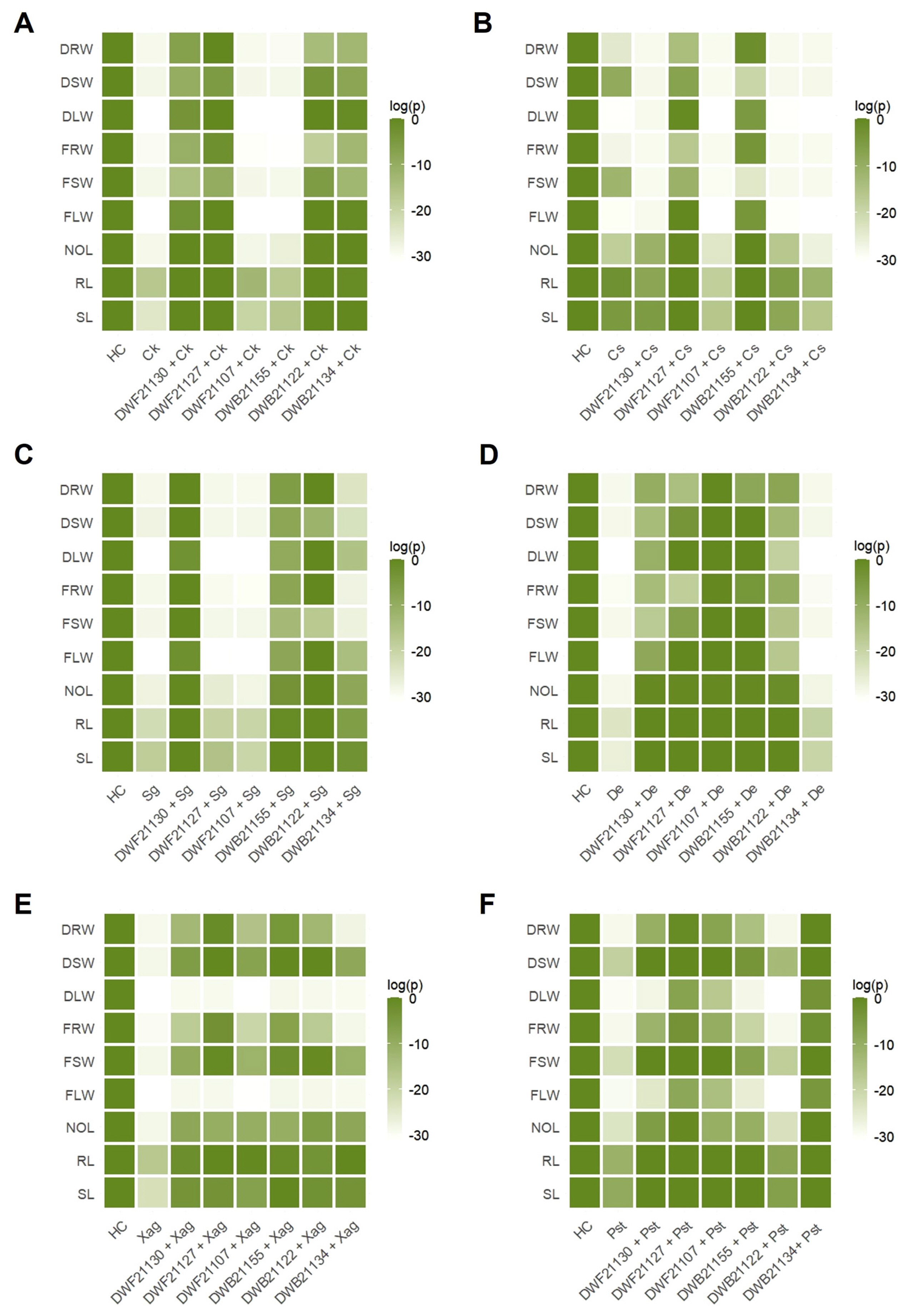
For C. kikuchii, treatment with DWF21107 and DWB21155 did not show noticeable difference, compared to the C. kikuchii-treated plant (Trt(P)). DWB21155 treatment even reduced the stem weight lightly more (0.275 g and 0.113 g of fresh stem weight and 0.175 g and 0.738 g of dry stem weight at Trt(P) and Bacillus sp. DWB21155, respectively). In contrast, the treatment with DWF21130, DWF21127, DWB21122 and DWB21134 improved plant growth, especially in shoot length, number of leaves, leaves weight, and root weight (Figs. 3 and 4A, Supplementary Table 2).
For C. sojina, DWF21127 and DWB21155 had a positive effect on seed germination and plant growth. Especially, DWF21127 increased shoot length, fresh leaves weight, and dry leaves weight (52%, 80%, and 81%, respectively). In contrast, DWF21107 appeared to have a negative effect, resulting in a decrease of fresh and dry stem weight (86% in both) (Figs. 3 and 4B, Supplementary Table 2).
For S. glycine, the germination of soybean seeds was improved by treatment with DWF21130, DWB21155, DWB21122 and DWB21134 (100%, 83%, 100%, and 67%), but not in seeds treated with DWF21127 and DWF21107. The most significant effect was observed in plants grown out of seeds inoculated with DWF21130 (77%, 83%, 76%, 94%, 73%, 83%, 94%, 77%, and 85% increase in shoot length, root length, number of leaves, fresh leave weight, fresh stem weight, fresh root weight, dry leave weight, dry stem weight, and dry root weight, respectively). DWF21127 and DWF21107 had little effect against S. glycines in the pot experiments (Figs. 3 and 4C, Supplementary Table 2).
Among all the pathogens we tested in our work, D. eres had the most devastating effects on soybean plants, causing seed decay and suppressing the plant growth and development (Fig. 3). Seed treatment with endophytes except DWB21134 dramatically recovered the germination rate and plant growth (Figs. 3 and 4D, Supplementary Table 2). Leaf weight in both of fresh and dry condition was stimulated positively by five strains. In particular, DWB21155 displayed plant growth promoting effect even in comparison with the non-treatment control.
For Xag, treatment with endophytes was able to improve shoot length, root length, number of leaves, fresh and dry stem weight, and fresh and dry root weight (Figs. 3 and 4E, Supplementary Table 2). In addition, the germination rate was 100% in plant groups inoculated with endophytes, while it was 50% in infected control. However, none of the endophytes could recover the stem length and leaf weight (both fresh and dry).
For Pst, the seed treatment with endophytes increased seed germination rate in all cases (Figs. 3 and 4F, Supplementary Table 2). DWF21130, DWF21134 and DWF21127 was most effective in promoting plant growth, compared to the Trt(P). Especially, when seeds were treated with DWB21134, all measurements showed increase in value with fresh root weight even higher than non-treatment control (31%).
Crop seeds contain a multitude of microorganisms that are called endophytes (Card et al., 2015; Lugtenberg et al., 2016; Verma, 2019). There is a large volume of documentations on seed endophytes, although how they get into the seed, what they do inside it and whether they are stable inhabitants or just passers-by are still open questions. Despite such scarcity of our knowledge, seed endophytes opened up new possibilities for application of microbes to crop protection (Chaudhary et al., 2022; Watts et al., 2023). Seed endophytes, many of which show antagonism to plant pathogens, reside in seed, and get first access to the developing plants upon seed germination. Such precedence may have long-term effects on assembly and structure of endosphere as well as rhizosphere microbiomes, which in turn affect the overall fitness and productivity of plants. This is known as ‘priority effect’ in microbial ecology (Debray et al., 2022).
In our previous study, we had isolated and identified 30 species of bacteria and 15 species of fungi from soybean seeds (Kim et al., 2022). Evaluation of those endophytes for their antagonistic activities against a set of common seed-borne pathogens using plate assays had revealed candidate seed endophytes, of which we can take advantage for seed treatment. In order to examine whether such plate assay results can be translated into actual plant protection, in this study, we tested effects of seed treatment with endophytes on seed germination and subsequent plant growth. We first showed that the common seed-borne pathogens could cause disease symptoms typical of pathogens by dipping the soybean seeds in solutions containing either bacteria or fungal spores.
Our treatment of seeds with endophytes clearly demonstrated that seed endophytes we used in this study can protect the soybean plants from seed-borne pathogens. In particular, regardless of pathogen species, endophyte treatment tended to significantly improve seed germination rate. However, protection provided by endophytes after the seed germination appear to be varying across different pathogen species. For example, DWB21155 seems to be highly effective against D. eres, but it was not against C. kikuchii. Therefore, none of the endophytes showed broad-spectrum of plant protection activity. Interestingly, some endophytes showed plant-growth promoting effects. For example, DWB21134 increased fresh root weight of the plant.
For many crops, seed treatment with systemic fungicide before sowing is a common and widespread practice (Ayesha et al., 2021). However, there has been growing concerns about inadvertent effect of such chemicals on non-target organisms in seeds and soil (Vasanthakumari et al., 2019). In light of this, use of endophytes for crop protection and improvement of seedling growth is a good alternative to the chemicals. As there is no single endophyte providing protection against all the pathogens in this work, it would be worth trying combination of endophytes in seed treatment for more broad-spectrum protection in future study. Together with combining endophytes during seed treatment, changes in microbiome structures should be monitored in order to see if the seed treatment with endophyte(s) has long-lasting ‘priority effect’ in endosphere and rhizosphere during plant growth and development.
In summary, information gleaned from our study offers clear strategies with which the seed endophytes can be deployed through seed treatment. Recently, seed coating is gaining attention as a delivery system for plant-beneficial microbes (Javed et al., 2022). Our endophytes would serve as a stepping stone for development of seed coating technology beyond seed treatment in the future.
Acknowledgments
This study was supported by a grant of Rural Development Administration (PJ01576801-2021).
Electronic Supplementary Material
Supplementary materials are available at The Plant Pathology Journal website (http://www.ppjonline.org/).
Fig. 1
Experimental design for seed treatment. Surface-sterilized soybean seeds were treated with microbial suspension from endophytic isolates (treatment 1), followed by treatment with seed-borne pathogen suspension (treatment 2). The treated seeds were sown in the pot to compare vegetative growth and resistance to pathogenicity.
Fig. 2
Effects of seed inoculation with pathogens on soybean seeds. (A) Seed morphology after treatment. Control, seeds without microbial treatment; De-trt, seeds treated with Diaporthe eres; Ck-trt, seeds treated with Cercospora kikuchii; Pst-trt, seeds treated with Pseudomonas syringae pv. tabaci. (B) Difference in seed thickness after treatment. Control and D. eres-treated seeds are shown in left and right panel, respectively.
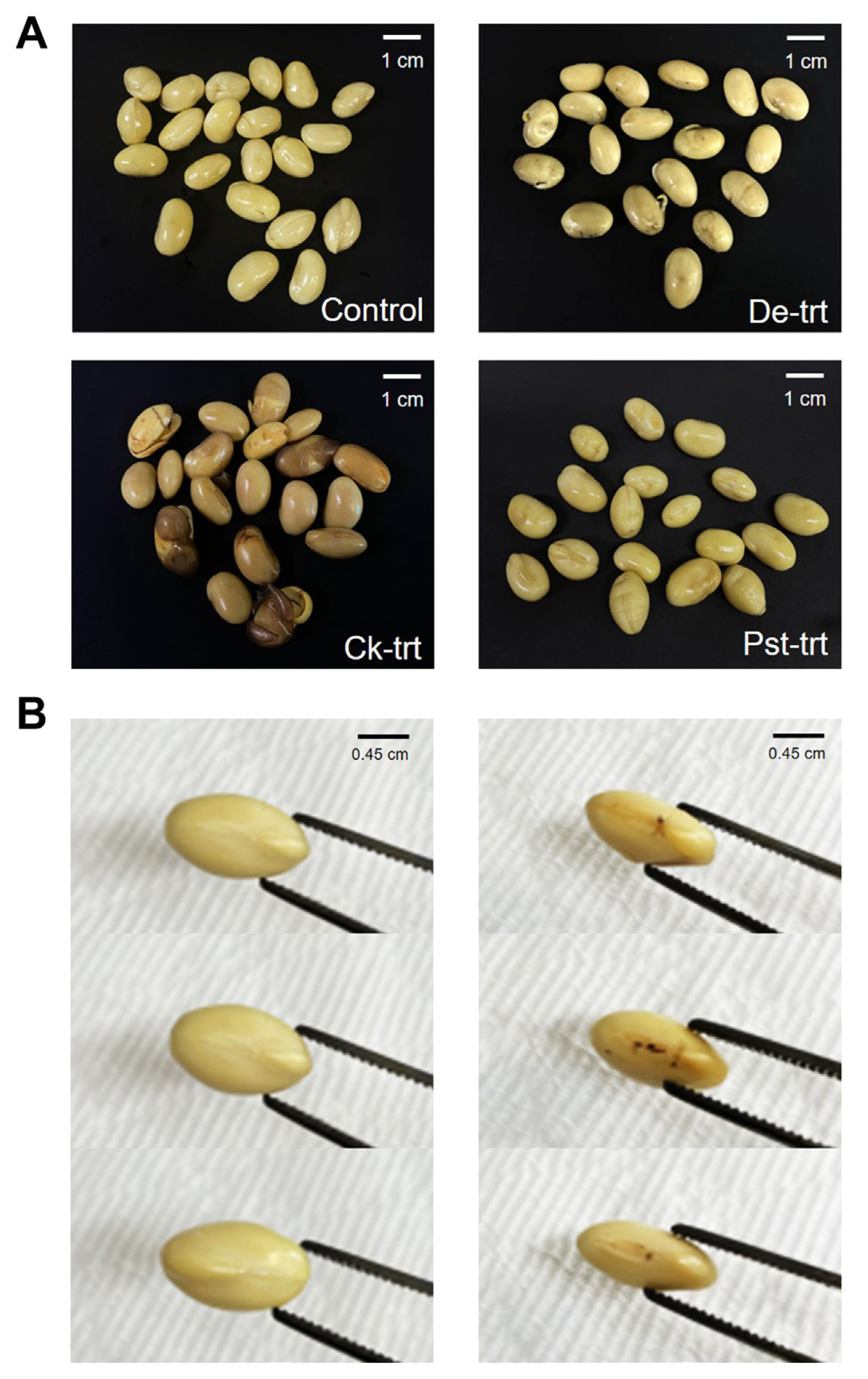
Fig. 3
Soybean plants stimulated by treatment with endophytic isolates and pathogens. Soybean seeds were inoculated with endophytic isolates (three strains of bacteria; Bacillus sp. DWB21155, Pseudomonas koreensis DWB21122, and Stenotrophomonas maltophilia DWB21134 and three strains of fungi; Alternaria hungarica DWF21130, Cladosporium pseudocladosporioidies DWF21127, and Penicillium steckii DWF21107). The images represent soybean plants inoculated with the six isolates and seed-borne pathogens (Pst, Xag, De, Sg, Cs, and Ck). HC indicates healthy control with non-microbial inoculation. Pst, Pseudomonas syringae pv. syringae; Xag, Xanthomonas axonopodis pv. glycines; De, Diaporthe eres; Sg, Septoria glycine; Cs, Cercospora sojina; Ck, Cercospora kikuchii. Non-Trt, non-microbial treatment; Trt(P), treatment of pathogen suspension; Trt(E+P), treatment of endophytes and pathogen suspension.
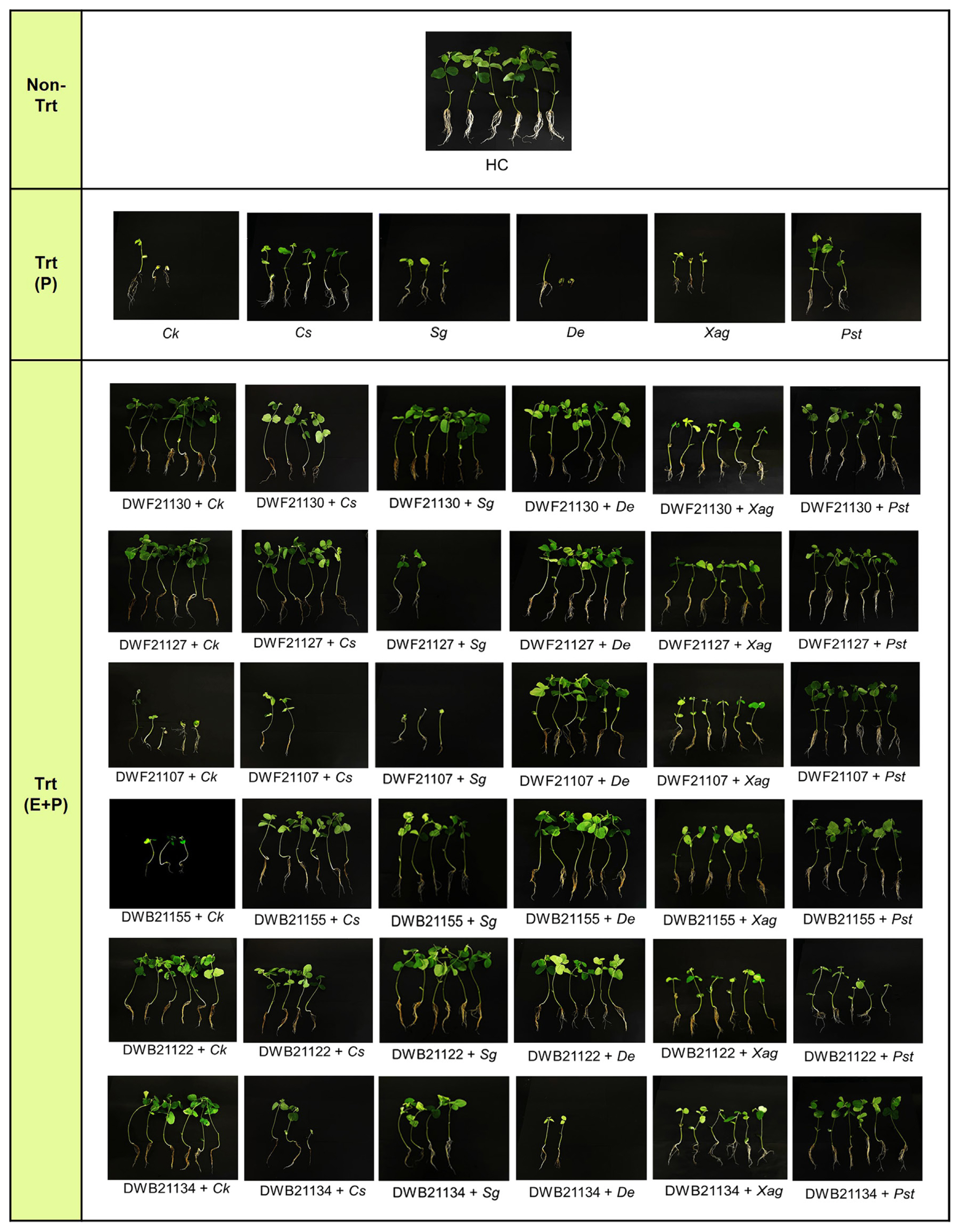
Fig. 4
Heatmap showing log(p-value) from statistical analysis of comparison among control and treatment groups. Log(p-value) is displayed as gradient of green color. Treatments were made with endophyte isolates and seed-borne pathogen. Soybean seeds were inoculated with six isolated strains (three strains of bacteria; Bacillus sp. DWB21155, Pseudomonas koreensis DWB21122, and Stenotrophomona maltophilia DWB21134 and three strains of fungi; Alternaria hungarica DWF21130, Cladosporium pseudocladosporioidies DWF21127, and Penicillium steckii DWF21107). Each row represents the comparison of traits associated with the development of soybean plants inoculated with endophytic isolates and seed-borne pathogens (Pst, Xag, De, Sg, Cs, and Ck, in (A), (B), (C), (D), (E), and (F), respectively). HC indicates healthy control with non-microbial inoculation. Pst, Pseudomonas syringae pv. tabaci; Xag, Xanthomonas axonopodis pv. glycines; De, Diaporthe eres; Sg, Septoria glycine; Cs, Cercospora sojina; Ck, Cercospora kikuchii. SL, shoot length; RL, root length; NOL, number of leaves; FLW, fresh leaves weight; FSW, fresh stem weight; FRW, fresh root weight; DLW, dry leaves weight; DSW, dry stem weight; DRW, dry root weight.
References
Allen, T. W., Bradley, C. A., Sisson, A. J., Byamukama, E., Chilvers, M. I., Coker, C. M., Collins, A. A., Damicone, J. P., Dorrance, A. E., Dufault, N. S., Esker, P. D., Faske, T. R., Giesler, L. J., Grybauskas, A. P., Hershman, D. E., Hollier, C. A., Isakeit, T., Jardine, D. J., Kelley, H. M., Kemerait, R. C., Kleczewski, N. M., Koenning, S. R., Kurle, J. E., Malvick, D. K., Markell, S. G., Mehl, H. L., Mueller, D. S., Mueller, J. D., Mulrooney, R. P., Nelson, B. D., Newman, M. A., Osborne, L., Overstreet, C., Padgett, G. B., Phipps, P. M., Price, P. P., Sikora, E. J., Smith, D. L., Spurlock, T. N., Tande, C. A., Tenuta, A. U., Wise, K. A. and Wrather, J. A. 2017. Soybean yield loss estimates due to diseases in the United States and Ontario, Canada, from 2010 to 2014. Plant Health Prog 18:19-27.

Ayesha, M. S., Suryanarayanan, T. S., Nataraja, K. N., Prasad, S. R. and Shaanker, R. U. 2021. Seed treatment with systemic fungicides: time for review. Front. Plant Sci 12:654512.



Card, S. D., Hume, D. E., Roodi, D., McGill, C. R., Millner, J. P. and Johnson, R. D. 2015. Beneficial endophytic microorganisms of Brassica: a review. Biol. Control 90:102-112.

Chaudhary, P., Agri, U., Chaudhary, A., Kumar, A. and Kumar, G. 2022. Endophytes and their potential in biotic stress management and crop production. Front. Microbiol 13:933017.



Cruz, C. D., Mills, D., Paul, P. A. and Dorrance, A. E. 2010. Impact of brown spot caused by Septoria glycines on soybean in Ohio. Plant Dis 94:820-826.


Debray, R., Herbert, R. A., Jaffe, A. L., Crits-Christoph, A., Power, M. E. and Koskella, B. 2022. Priority effects in microbiome assembly. Nat. Rev. Microbiol 20:109-121.



Gomes, R. R., Glienke, C., Videira, S. I. R., Lombard, L., Groenewald, J. Z. and Crous, P. W. 2013. Diaporthe: a genus of endophytic, saprobic and plant pathogenic fungi. Persoonia 31:1-41.


Harman, G., Khadka, R., Doni, F. and Uphoff, N. 2021. Benefits to plant health and productivity from enhancing plant microbial symbionts. Front. Plant. Sci 11:10065.

Hong, J. K., Sung, C. H., Kim, D. K., Yun, H.-T., Jung, W. and Kim, K. D. 2012. Differential effect of delayed planting on soybean cultivars varying in susceptibility to bacterial pustule and wildfire in Korea. Crop Prot 42:244-249.

Javed, T., Afzal, I., Shabbir, R., Ikram, K., Zaheer, M. S., Faheem, M., Ali, H. H. and Iqbal, J. 2022. Seed coating technology: an innovative and sustainable approach for improving seed quality and crop performance. J. Saudi Soc. Agri. Sci 21:536-545.

Kang, I. J., Shim, H. K., Shin, D. B., Roh, J. H., Goh, J. and Heu, S. 2015. Assessing frogeye leaf spot resistance on recommended soybean cultivars. Res. Plant Dis 21:243-249 (in Korean).

Karges, K., Bellingrath-Kimura, S., Watson, C. A., Stoddard, F. L., Halwani, M. and Reckling, M. 2022. Agro-economic prospects for expanding soybean production beyond its current northerly limit in Europe. Eur. J. Agron 133:126415.

Kim, J., Roy, M., Ahn, S.-H., Shanmugam, G., Yang, J. S., Jung, H. W. and Jeon, J. 2022. Culturable endophytes associated with soybean seeds and their potential for suppressing seed-borne pathogens. Plant Pathol. J 38:313-322.




Li, H., Parmar, S., Sharma, V. K. and White, J. F. 2019a. Seed endophytes and their potential applications. In: Seed endophytes: biology and biotechnology, eds. by S. K. Verma and J. F. White, pp. 35-54. Springer International Publishing, Cham, Switzerland.

Li, S., Sciumbato, G., Boykin, D., Shannon, G. and Chen, P. 2019b. Evaluation of soybean genotypes for reaction to natural field infection by Cercospora species causing purple seed stain. PLoS ONE 14:e0222673.


Lugtenberg, B. J. J., Caradus, J. R. and Johnson, L. J. 2016. Fungal endophytes for sustainable crop production. FEMS Microbiol. Ecol 92:fiw194.


Petrović, K., Vidić, M., Riccioni, L., Đorđević, V. and Rajković, D. 2015. First report of Diaporthe eres species complex causing seed decay of soybean in Serbia. Plant Dis 99:1186.

Soares, A. P. G., Guillin, E. A., Borges, L. L., da Silva, A. C. T., de Almeida, A. M. R., Grijalba, P. E., Gottlieb, A. M., Bluhm, B. H. and de Oliveira, L. O. 2015. More Cercospora species infect soybeans across the Americas than meets the eye. PLoS ONE 10:e0133495.



Vasanthakumari, M. M., Shridhar, J., Madhura, R. J., Nandhitha, M., Kasthuri, C., Janardhana, B., Nataraja, K. N., Ravikanth, G. and Shaanker, R. U. 2019. Role of endophytes in early seedling growth of plants: a test using systemic fungicide seed treatment. Plant Physiol. Rep 24:86-95.


Verma, P. 2019. Seed endophytes in crop plants: metagenomic approaches to study the functional roles and interactions. In: Seed endophytes: biology and biotechnology, eds. by S. K. Verma and J. F. White, pp. 483-507. Springer International Publishing, Cham, Switzerland.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Supplement
Supplement Print
Print




