 |
 |
| Plant Pathol J > Volume 39(6); 2023 > Article |
|
Abstract
Plant pathogenic bacteria colonize plant surfaces and inner tissues to acquire essential nutrients. Nonstructural sugars hold paramount significance among these nutrients, as they serve as pivotal carbon sources for bacterial sustenance. They obtain sugar from their host by diverting nonstructural carbohydrates en route to the sink or enzymatic breakdown of structural carbohydrates within plant tissues. Despite the prevalence of research in this domain, the area of sugar selectivity and preferences exhibited by plant pathogenic bacteria remains inadequately explored. Within this expository framework, our present review endeavors to elucidate the intricate variations characterizing the distribution of simple sugars within diverse plant tissues, thus influencing the virulence dynamics of plant pathogenic bacteria. Subsequently, we illustrate the apparent significance of comprehending the bacterial preference for specific sugars and sugar alcohols, postulating this insight as a promising avenue to deepen our comprehension of bacterial pathogenicity. This enriched understanding, in turn, stands to catalyze the development of more efficacious strategies for the mitigation of plant diseases instigated by bacterial pathogens.
Plants produce carbohydrates in the leaves that are translocated to various plant segments via the phloem tissues. This translocated photosynthate either undergoes direct cellular assimilation and metabolic processing for energy generation or is stored within plant tissues in varied molecular arrangements. Although sucrose is the most common and abundant photoassimilate translocated across plant tissues, a plethora of alternative sugars including raffinose, sorbitol, mannitol galacticol, and mannoheptulose exist as primary photosynthetic derivatives across diverse plant species (Carmi et al., 2003; Moing, 2000). The members of the Rosaceae family, for instance, have the sugar alcohol sorbitol while the plantaginaceae family harbors sorbitol and mannitol as the primary photosynthetic product (Fatima and Senthil-Kumar, 2015; Moing, 2000). In another study, a comparative analysis of sugar profiles in leaves and fruits of strawberries and blueberries revealed a distinction in sugar prominence with glucose, fructose, and sucrose more prominent in strawberries whereas glucose, fructose, and galactose dominated in blueberries (Ak┼Īi─ć et al., 2019) (Fig. 1A). Dominant nonstructural carbohydrates as found in some plants is presented in Table 1.
Furthermore, the abundance and diversity of these sugars demonstrated marked variations within a given plant. Soluble sugar content is considerably higher in leaves compared to roots and stems, a pattern observed across divergent plant species (Mart├Łnez-Vilalta et al., 2016). The diversity also manifests intraplant, as exemplified by differential distributions of polyphenolic and sugar constituents in various segments of the buckwheat plant. Each of the analyzed samples contained an appreciable amount of carbohydrates though the leaves had the highest total sugars. Xylose, arabinose, sucrose isomaltose, melibiose, and maltotriose predominate in the leaf; fructose, panose, and sorbitol were highest on the stem; glucose and mannitol on the flowers; glycerol and erythritol on the grains; maltose, melezitose, and raffinose on the pollen (Ne┼Īovi─ć et al., 2021) (Fig. 1B). Moreover, galactitol, melibiose, and gentiobiose were the key sugars that split out strawberry fruits and leaves (Fig. 1B).
Environmental conditions exert influence on the quantity of certain sugars as manifested by the accumulation of raffinose during drought and cold exposure proving the adaptability of sugar profiles to abiotic stresses (Hannah et al., 2006). This substantiates the notion that the typology of plant sugars can exhibit plasticity in response to environmental perturbations. Leveraging on this, we postulate that sugar variability may pose a constraint to pathogenic bacteria limiting their propagation within specific plant species, tissues, or growth stages. As such, it becomes imperative to examine the reaction of plant sugars to biotic stressors, inclusive of bacterial infections, for a comprehensive understanding of these complex interactions.
Plant surfaces, particularly leaves and roots, serve as habitats for a substantial bacterial population that relies on the host plant as a carbon source to fuel their energy generation. This dependence on plant-derived sugars and other nutrients sustain the proliferation of diverse microbial community (Mercier and Lindow, 2000). The leaf is a specialized organ for photosynthesis that features an epidermal layer that regulates gas exchange, water regulation, and secretion of secondary metabolites (Glover, 2000). Water and solute are continuously secreted through the hydathodes while sugars, protein, and various exudates are released through the glandular trichomes (Schlechter et al., 2019). This dynamic condition provides an ideal niche for the colonization of insects and microorganisms (Zhao and Chen, 2016). Notably, the leaf surface harbors a predominant microbial presence, including epiphytic bacteria, some of which have been reported to enhance plant growth and confer protection against pathogenic agents that also inhabit plant surfaces (Mercier and Lindow, 2000). While several plant diseases are attributed to endophytic bacteria, a substantial portion of the documented diseases emanate from epiphytic bacterial populations (Beattie and Lindow, 1995) (Fig. 2A).
Plant roots enrich the soil by releasing up to 20% of the sugar produced during photosynthesis into the rhizosphere thereby attracting a diverse array of microbial inhabitants (Knights et al., 2021). These root exudates can also stimulate the germination and proliferation of dormant spores of pathogenic microorganisms (Pascale et al., 2020). Plant microbes colonize the root either as epiphytes or endophytes, and both categories may be parasitic or symbiotic with the host plant (Knights et al., 2021). The presence of root-associated microbiota significantly influences plant health and productivity through various mechanisms such as nutrient acquisition, priming of plant defense, and suppression of pathogenic microorganisms (Berendsen et al., 2012). In the rhizosphere, dominant microbes are selected based on the type and nature of exuding sugars, which determine disease-causing capabilities (Sasse et al., 2018) (Fig. 2A).
Despite the availability of diverse sugars, epiphytic bacteria exhibit a marked capacity to metabolize various sugars, albeit with some degree of preference for specific sugars over others (Ammar et al., 2018). In the absence of limitations imposed by the physical environment on plant tissues, the growth of epiphytic bacteria is governed by the availability of primary carbon-rich compounds, prominently sugars (Schlechter et al., 2019). Epiphytic bacteria face antagonism from other bacteria through mechanisms such as the production of biosurfactants, pyocyanin, antibiotics, bacteriocins, volatile organic compounds synthesis, siderophores, and competition for space and nutrients. (Karthikeyan et al., 2021). In this context, sugars as valuable nutrient resources indirectly influence the activity of pathogenic bacteria. This suggests that favorable growth conditions that encourage the growth of the dominant epiphytic bacteria including the presence of suitable utilizable sugars, can effectively prevent the growth and establishment of pathogenic bacterial populations.
Plants have a complex system for transporting sugar throughout their tissues. Sugar transport within plants encompasses long-distance conveyance from leaves to other tissues, facilitated by phloem sieve cells. The phloem sap mainly consists of a complex mixture of sugars, sugar alcohols, organic acids, amino acids, and minerals, primarily move through a sequential process involving loading into companion cells from the apoplast and subsequent transportation into the phloem sap of sieve tube elements (Fatima and Senthil-Kumar, 2015).
In contrast, short-distance transport of sugar consists of symplastic movement through plasmodesmata and apoplastic transport facilitated by specific monosaccharide or disaccharide transport proteins. Apoplastic sugar plays a significant role in plant disease development as the apoplast represents a major site of bacteria colonization (Yamada et al., 2016). The apoplast offers a more favorable environment and protection for pathogenic bacteria from the adverse environmental factors associated with plant surfaces including ultraviolet radiations and low water availability (Beattie and Lindow, 1995). Within the apoplastic context, biotrophic bacteria reside and divert the plant apoplastic sugar for their metabolic sustenance. In response, the plant employs diverse strategies to thwart pathogen proliferation within the extracellular compartment by different mechanisms which include regulation of the sugar transport protein thus modulating the availability of sugars. Notably, studies emphasize that the dynamics of various plant-pathogen interaction systems are shaped by the coordinated regulation of cell wall invertase and sugar transporter proteins (Toruno et al., 2016) (Fig. 2B).
Additionally, several studies highlighted the ability of pathogenic bacteria to manipulate the regulatory apparatus of Sugar Will Eventually be Exported Transporters (SWEET) and hexose transporters, to acquire sugar from the apoplast or xylem (Fig. 2B). Example of this phenomenon can be demonstrated by a report that shows Xanthomonas oryzae pv. oryzae pathogen up-regulates rice OsSWEET11, OsSWEET12, or OsSWEET14 genes, prompting the efflux of sucrose from host cells into the apoplast for pathogen uptake. Consequently, interference with OsSWEET11 confers resistance, as pathogen growth within the apoplast and xylem is hindered due to constrained sugar availability. Similarly, Pseudomonas syringae pv. tomato strain DC3000 infection also highly induces the up-regulation of AtSWEET4, AtSWEET5, AtSWEET7, AtSWEET8, AtSWEET10, AtSWEET12, and AtSWEET15 in Arabidopsis leaves (Chen et al., 2010). These findings highlighted the multifaceted interplay between plant sugar transporters and pathogenic exploitation strategies within the apoplastic environment.
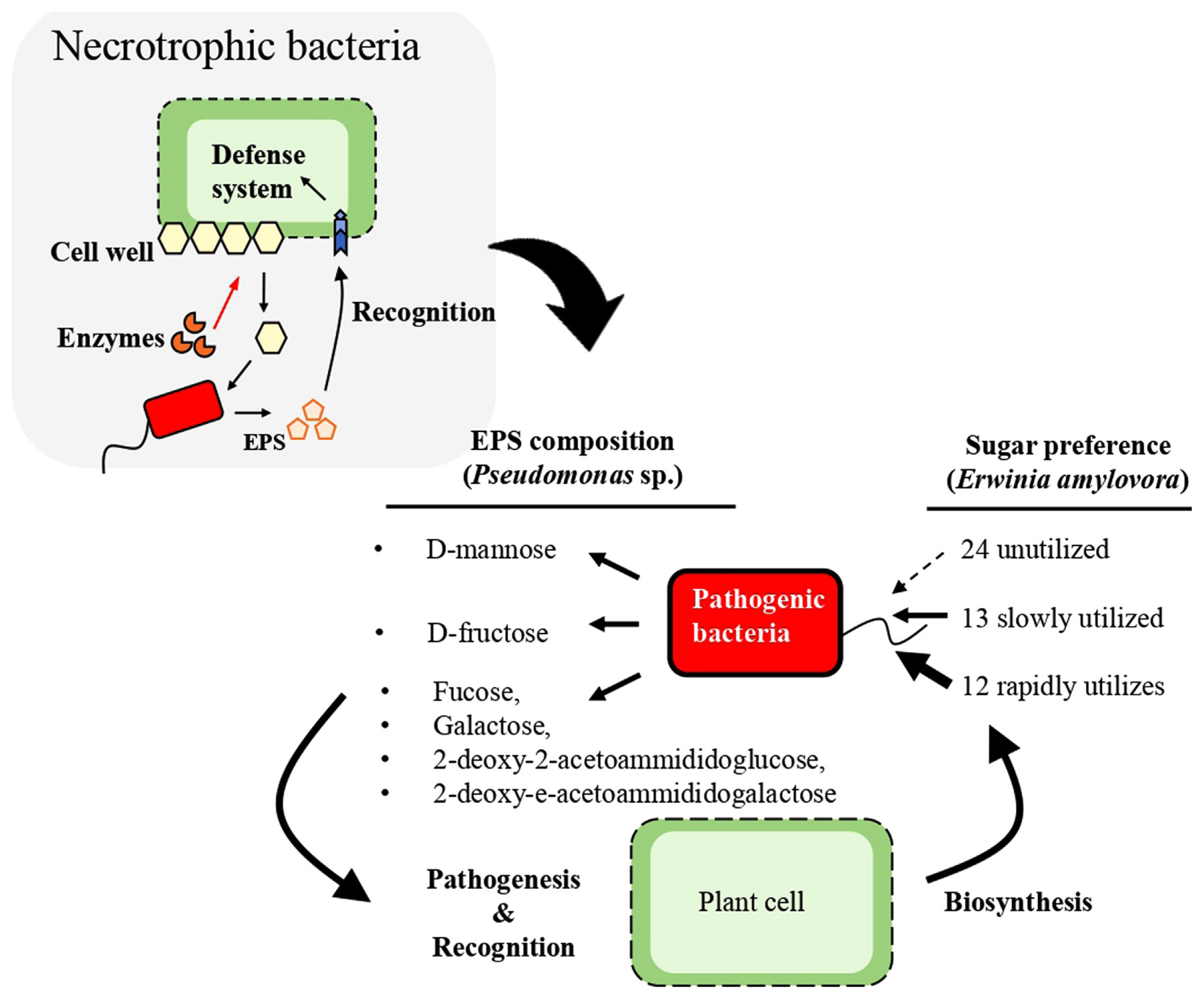
The catabolic breakdown of carbohydrates by pathogenic bacteria results in the generation of distinct monomeric units, including hexose and pentose sugars contingent upon the polysaccharide substrate undergoing degradation. Among the monomers, notable examples include D-glucose, D-fructose, D-ribose, D-mannose, D-arabinose, D-maltose, D-trehalose, D-mannitol, and D-sorbitol (Milkovska-Stamenova et al., 2015). While bacteria exhibit the capacity to utilize a spectrum of such molecules as a source of carbon, they manifest preference when presented with multiple options (Ammar et al., 2018). It has also been reported that some bacteria are not able to metabolize certain carbohydrates. Nevertheless, instances are documented where certain bacteria exhibit an inability to metabolize certain carbohydrates. This was highlighted when in a study that examine the utilization of 49 carbohydrates by Erwinia amylovora revealing that 24 carbohydrates remained unutilized, while 13 were slowly utilized and 12 were rapidly and completely utilized (Havesi et al., 2004). This observation aligns with the report of Aldridge et al., 1997 where a study of phloem sap showed that pathogenic bacteria such as E. amylovora that infect plants belonging to the Rosaceae family use sorbitol as the preferred carbon source (Aldridge et al., 1997). Moreover, it has been reported that in bacterial growth scenarios involving diverse sugar mixtures, a phenomenon known as catabolite repression ensues wherein the genes associated with the metabolism of one sugar repress the genes implicated in the metabolism of alternative sugars. This sequential sugar consumption subject to unique sequences has been reported across different bacterial species. Investigations focusing on Escherichia coli grown on a mixture of lactose, arabinose, and xylose have elucidated that lactose exerts inhibitory effects on the consumption of arabinose and xylose, concomitantly reducing the transcription of their corresponding metabolic genes (Aidelberg et al., 2014; Ammar et al., 2018).
As heterotrophic entities, pathogenic bacteria exploit plant-derived sugars as carbon substrates, ultimately jeopardizing the host plantŌĆÖs well-being. Following the entry into plant tissues through wounds or natural openings, necrotrophic bacteria deploy enzymatic cascades to degrade cellulose and pectin into soluble disaccharides, subsequently metabolized to monosaccharides (Jeckelmann and Erni, 2020). This enzymatic activity leads to the decay of affected plant tissue, as cellulose and pectin constitute pivotal constituents of the cell wall responsible for the structural integrity of plant cells (Abbott and Boraston, 2008). The bacteriaŌĆÖs regulatory response involves the transcription of carbohydrate utilization genes and virulence factor expression, adapting to changes in environmental conditions encountered during the infection process. Research focusing on group A Streptococcus bacteria has demonstrated significant variations in colony morphology and expression of virulence genes upon cultivation on media containing different compositions of carbohydrates (Shelburne et al., 2008). It can thus be inferred that different carbohydrates play distinct roles in modulating the virulence attributes of pathogenic bacteria.
Furthermore, exopolysaccharides (EPS) produced by numerous Gram-negative bacteria have been implicated in virulence manifestation, with notable heterogeneity observed in the chemical composition across bacterial species. P. syringae pv. ciccaronei for instance produces an EPS dominated by a homo-polymer composed of D-mannose, while P. savastanoi pv. neriiŌĆÖs EPS exhibits a complex hetero-polymeric structure mainly encompassing four different monosaccharides (fucose, galactose, 2-deoxy-2-acetoammidoglucose and 2-deoxy-2-acetoammidogalactose). In contrast, P. caryophylli produces an EPS that constitutes a homo-polymer of D-fructose (Corsaro et al. 2001). Consequently, we hypothesize that plant recognition of pathogenic bacteria will pivot on the compatibility between predominant plant carbohydrates and the carbohydrate constituents of the bacteriaŌĆÖs EPS (Fig. 3).
Bacteria regulate their gene expression in response to fluctuations in population density through quorum sensing affording bacteria the ability for intercellular communication and synchronize communal gene expression and behavior. This coordination extends to the modulation of essential physiological processes, including biofilm formation, antibiotic synthesis, and virulence. There is evidence that autoinducers of quorum sensing are regulated by substrate availability particularly reducing sugars that exerts regulatory influence over virulence-associated genes (Ha et al., 2018). A study showed that glucose induces biofilm formation and quorum sensing in Bacillus thuringiensis (Jha et al., 2021). Further investigation reveals that glucose concentration above 0.05% regulates the dynamics of biofilm and quorum sensing mechanisms in Aeromonas hydrophila (Jahid et al., 2013). The molecules involved in quorum sensing namely N-acyl homoserine have a profound effect on the host plant. Notably, Shrestha et al. (2020) demonstrated that quorum-sensing molecules produced on Arabidopsis thaliana infected with P. syringae pv. tomato influenced plant growth and elicit induced resistance. Moreover, the genetic transcription during quorum sensing was shown to be passed down from parents to offspring as demonstrated in a study where a delayed switch to quorum sensing mode in Ralstonia solanacearum resulted in reduced pathogenicity of ancestral strain (Tang et al., 2020). In light of these revelations, we postulate that the sequential activation of resistance genes by sugar-mediated quorum sensing can be epigenetically inherited potentially nurturing a more virulent pathogen. Evidently, an exploration into the influence of diverse sugar molecules on quorum sensing dynamics bears a promising prospect of disease management by manipulating plant-microbe interactions.
The intricate relationship between the sugar status of plants and their susceptibility to pathogenic infections, commonly referred to as ŌĆ£high sugar resistance,ŌĆØ has long been recognized. Pioneering work by Horsfall and Dimond (1957) illuminated how diminished sugar content in plant tissue renders it more vulnerable to certain diseases while concurrently diminishing susceptibility to others. Subsequent investigations have unveiled a spectrum of plant responses to pathogen challenges, encompassing alterations in apoplastic sugar levels, source-to-sink transitions, enhanced cell-wall invertase activity, and modifications in the sucrose/hexose ratio. Such responses are essential in inducing defensive mechanisms (Bolouri-Moghaddam and Van Den Ende, 2012; Herbers et al., 1996; Kocal et al., 2008; Schaarschmidt et al., 2007).
Recent studies have highlighted the efficacy of rare sugars, an assembly of monosaccharides and their derivatives infrequently encountered in nature, as tools for managing plant diseases. Treatment of rice plants with synthesized rare sugars D-psicose and D-allose conferred resistance to bacterial blight (Kano et al., 2011). Similarly, the application of naturally occurring rare sugar D-tagatose was reported to suppress the incidence of powdery mildews and downy mildews on cucumber and rice by inhibiting the growth of mycelia and conidia (Mochizuki et al., 2020). Consequent to their efficacy in eliciting induced resistance, commercial formulations featuring sugars like D-psicose, D-allose, D-altrose, and L-galactose have emerged as biocides, stimulating systemic acquired resistance, regulating plant growth, and inhibiting the growth of pathogens.
A variety of other commonly occurring sugars in nature showed promising results in controlling plant diseases. Strategies involving root drenching with L-arabinose at a concentration of 0.5% led to an 85% reduction in the severity of tomato bacterial wilt (Fu et al., 2020). Furthermore, xylose has emerged as a prominently investigated sugar in plant disease contexts. Studies have showcased its potential to enhance the management of cucumber wilt disease (Xu et al., 2019) and bolster colonization by the plant growth-promoting rhizobacterium B. velezensis, thereby mitigating wilt symptoms (Bauer et al., 2023). Intriguingly, xylose was also found to have contrasting effects, serving both as a nutrient and a virulence enhancer for X. citri (Alexandrino et al., 2023). These divergent outcomes underscore the necessity for a systematic exploration of carbohydrate variability in plant families concerning defense induction.
Manipulation of sugar transporter genes, notably those governing SWEET, offers a promising avenue for engineering plant resistance to bacterial diseases. Genetic down-regulation of SWEET genes may deprive pathogenic bacteria of the requisite sugars for sustained pathogenicity.
In disease management strategies, promoting an environment hostile to pathogens and advantageous to antagonistic organisms holds significance. Notably, certain rhizobacteria have demonstrated the capability to hinder disease initiation or reduce its severity. Investigation into the carbohydrate preferences of these bacteria will enhance the success of biological control approaches, as the viability of antagonistic organisms and synthetic microbial communitiesŌĆÖ hinges on the availability of utilizable carbohydrates. Furthermore, understanding the favored carbohydrates of pathogenic bacteria is indispensable for devising suitable synthetic growth media for in vitro cultivation.
In summary, nonstructural sugars wield an important role in shaping the growth and pathogenicity of plant pathogenic bacteria. Sugars, manifesting as exudates on roots or plant surfaces or as internal photosynthetic products, critically influence the susceptibility of plants to bacterial invasion and subsequent disease development. The diverse roles played by different sugars in bacterial pathogenicity necessitate focused investigations into their effects on the activation or suppression of virulence genes. These insights, along with an exploration of sugarsŌĆÖ impact on soil microbiomes, whether maintaining native populations or triggering dysbiosis, hold the promise of advancing our understanding of plant-microbe interactions and aiding disease management strategies.
Fig.┬Ā1
The major nonstructural carbohydrates in strawberries and blueberries differed. Glucose and fructose are common in both plants; while sucrose is a critical sugar in strawberries, galactose is in blackberry (A). Different sugars were reported on different plant parts of buckwheat and strawberry. The leaf of buckwheat has more sugar varieties than other parts. The major sugars on strawberry leaves are different from the fruits (B).
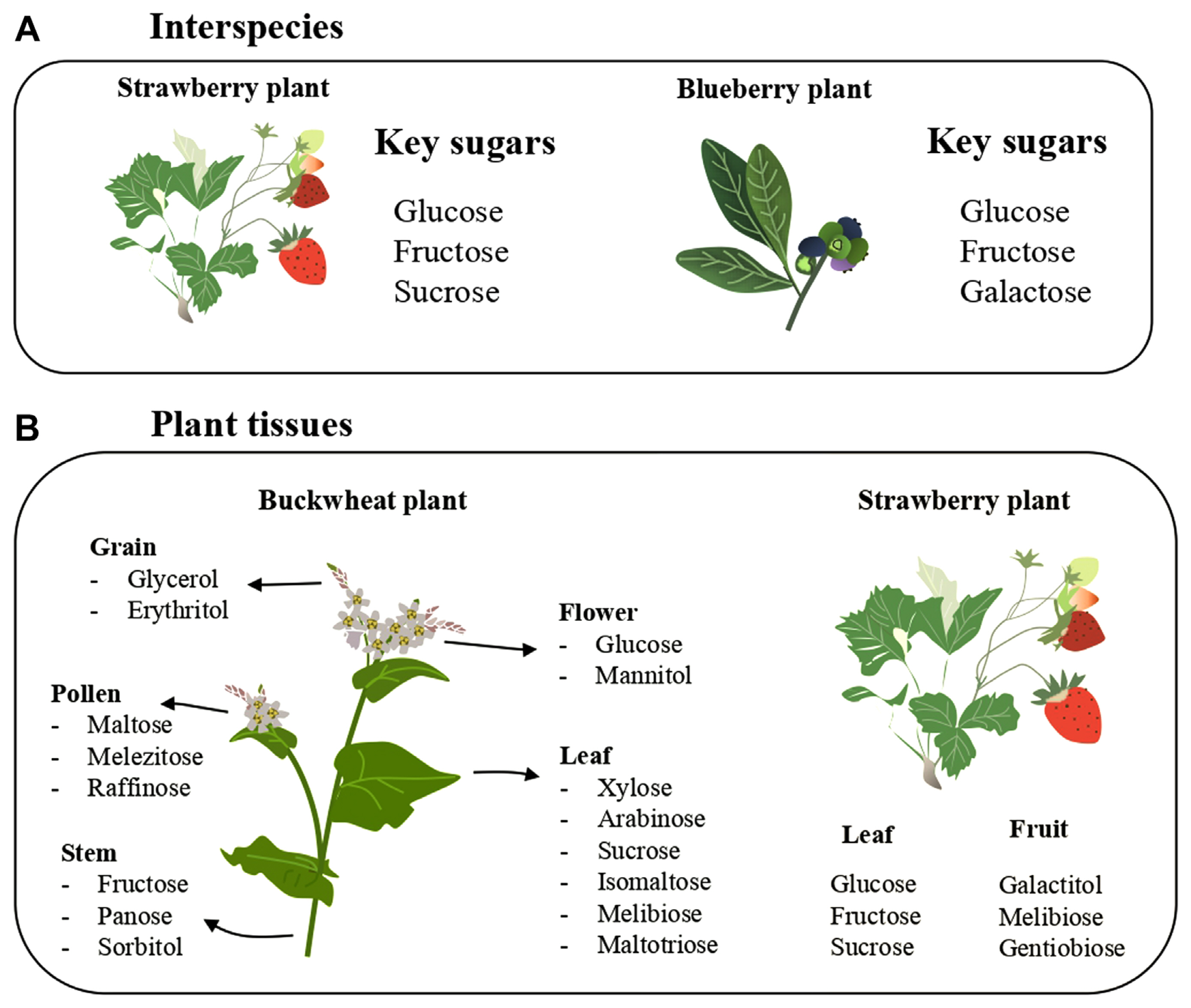
Fig.┬Ā2
Diagrammatic depiction of bacteria competition for sugar in the phyllosphere and rhizosphere. Bacteria on plant surfaces and around plant roots use the sugar exudates as an energy source (A), sugar being transported through the apoplast or phloem cells gets intercepted by pathogenic bacteria (B).

Fig.┬Ā3
Diagrammatic presentation of bacteria exopolysaccharide (EPS) recognition by plants. Recognition depends on the type of EPS produced and the type of sugar that the pathogenic bacteria can utilize. Also, the bacteriaŌĆÖs sugar preference will determine the EPSŌĆÖs sugar component.
Table┬Ā1
Dominant nonstructural carbohydrates reported on crops
| Plant | Dominant carbohydrate | Reference |
|---|---|---|
| Cotton (Gossypium hirsitum) | Glucose at mid-season, xylose at pre-defoliation stage | He et al. (2020) |
| Soybean (Glycine max) | Glucose, Trehalose during drought stress | Hlahla et al. (2022) |
| Tomato (Solanum lycopersicum) fruits | Fructose | Zhao et al. (2016) |
| Peach (Prunus persica) | Sorbitol | Morandi et al. (2008) |
| Rice (Oryza sativa) | Glucose | Mohan et al. (2010) |
| Carrot (Daucus carota) | Sucrose | Soria et al. (2009) |
| Apple (Malus domestica) | Sorbitol | Breen et al. (2020) |
References
Abbot, D. W. and Boraston, A. B. 2008. Structural biology of pectin degradation by Enterobacteriaceae. Microbiol. Mol. Biol. Rev. 72:301-316.



Aidelberg, G., Towbin, B. D., Rothschild, D., Dekel, E., Bren, A. and Alon, U. 2014. Hierarchy of non-glucose sugars in Escherichia coli. BMC Syst. Biol. 8:133.




Ak┼Īi─ć, M. F., Tosti, T., Sredojevi─ć, M., Milivojevi─ć, J., Meland, M. and Nati─ć, M. 2019. Comparison of sugar profile between leaves and fruits of blueberry and strawberry cultivars grown in organic and integrated production system. Plants 8:205.



Aldridge, P., Metzger, M. and Geider, K. 1997. Genetics of sorbitol metabolism in Erwinia amylovora and its influence on bacterial virulence. Mol. Gen. Genet. 256:611-619.



Alexandrino, A. V., Prieto, E. L., Nicolela, N. C. S., da Silva Marin, T. G., dos Santos, T. A., de Oliveira da Silva, J. P. M., da Cunha, A. F., Behlau, F. and Novo-Mansur, M. T. M. 2023. Xylose isomerase depletion enhances virulence of Xanthomonas citri subsp. citri in Citrus aurantifolia. Int. J. Mol. Sci. 24:11491.



Ammar, E. M., Wang, X. and Rao, C. V. 2018. Regulation of metabolism in Escherichia coli during growth on mixtures of the non-glucose sugars: arabinose, lactose, and xylose. Sci. Rep. 8:609.




Bauer, K., Nayem, S., Lehmann, M., Wenig, M., Shu, L.-J., Ranf, S., Geigenberger, P. and Vlot, A. C. 2023. ╬▓-D-XYLOSIDASE 4 modulates systemic immune signaling in Arabidopsis thaliana. Front. Plant Sci. 13:1096800.



Beattie, G. A. and Lindow, S. E. 1995. The secret life of foliar bacterial pathogens on leaves. Annu. Rev. Phytopathol. 33:145-172.


Berendsen, R. L., Pieterse, C. M. J. and Bakker, P. A. H. M. 2012. The rhizosphere microbiome and plant health. Trends Plant Sci. 17:478-486.


Bolouri Moghaddam, M. R. and Van den Ende, W. 2012. Sugars and plant innate immunity. J. Exp. Bot. 63:3989-3998.


Breen, K., Tustin, S., Palmer, J., Boldingh, H. and Close, D. 2020. Revisiting the role of carbohydrate reserves in fruit set and early-season growth of apple. Sci. Hortic. 261:109034.

Carmi, N., Zhang, G., Petreikov, M., Gao, Z., Eyal, Y., Granot, D. and Schaffer, A. A. 2003. Cloning and functional expression of alkaline alpha-galactosidase from melon fruit: similarity to plant SIP proteins uncovers a novel family of plant glycosyl hydrolases. Plant J. 33:97-106.


Chen, L.-Q., Hou, B.-H., Lalonde, S., Takanaga, H., Hartung, M. L., Qu, X.-Q., Guo, W.-J., Kim, J.-G., Underwood, W., Chaudhuri, B., Chermak, D., Antony, G., White, F. F., Somerville, S. C., Mudgett, M. B. and Frommer, W. B. 2010. Sugar transporters for intercellular exchange and nutrition of pathogens. Nature 468:527-532.




Corsaro, M. M., Evidente, A., Lanzetta, R., Lavermicocca, P. and Molinaro, A. 2001. Structural determination of the phytotoxic mannan exopolysaccharide from Pseudomonas syringae pv. ciccaronei. Carbohydr. Res. 330:271-277.


Fatima, U. and Senthil-Kumar, M. 2015. Plant and pathogen nutrient acquisition strategies. Front. Plant Sci. 6:750.



Fu, H.-Z., Marian, M., Enomoto, T., Suga, H. and Shimizu, M. 2020. Potential use of L-arabinose for the control of tomato bacterial wilt. Microbes Environ. 35:ME20106.



Ha, J.-H., Hauk, P., Cho, K., Eo, Y., Ma, X., Stephens, K., Cha, S., Jeong, M., Suh, J.-Y., Sintim, H. O., Bentley, W. E. and Ryu, K.-S. 2018. Evidence of link between quorum sensing and sugar metabolism in Escherichia coli revealed via cocrystal structures of LsrK and HPr. Sci. Adv. 4:eaar7063.



Hannah, M. A., Zuther, E., Buchel, K. and Heyer, A. G. 2006. Transportation and metabolism of raffinose family oligosaccharides in transgenic potato. J. Exp. Bot. 57:3801-3811.


Havesi, M., Farkas, A., Kasa, K. and Oros-Kovacs, Zs. 2004. Carbohydrate utilization of Erwinia amylovora in vitro. Int. J. Hortic. Sci. 10:31-34.
He, Z., Olk, D. C., Tewolde, H., Zhang, H. and Shankle, M. 2020. Carbohydrate and amino acid profiles of cotton plant biomass products. Agriculture 10:2.

Herbers, K., Meuwly, P., Frommer, W. B., Metraux, J. P. and Sonnewald, U. 1996. Systemic acquired resistance mediated by the ectopic expression of invertase: possible hexose sensing in the secretory pathway. Plant Cell 8:793-803.



Hlahla, J. M., Mafa, M. S., van der Merwe, R., Alexander, O., Duvenhage, M.-M., Kemp, G. and Moloi, M. J. 2022. The photosynthetic efficiency and carbohydrates responses of six edamame (Glycine max L. Merrill) cultivars under drought stress. Plants 11:394.



Horsfall, J. G. and Dimond, A. E. 1957. Interactions of tissue sugar, growth substances, and disease susceptibility. Z. Pflanzenkr. Pflanzenschutz 64:415-421.
Jahid, I. K., Lee, N.-Y., Kim, A. and Ha, S.-D. 2013. Influence of glucose concentrations on biofilm formation, motility, exoprotease production, and quorum sensing in Aeromonas hydrophila. J. Food Prot. 76:239-247.



Jeckelmann, J.-M. and Erni, B. 2020. Transporters of glucose and other carbohydrates in bacteria. Pflugers Arch. 472:1129-1153.



Jha, S., Bhadani, N. K., Kumar, A. and Sengupta, T. K. 2021. Glucose-induced biofilm formation in Bacillus thuringiensis KPWP1 is associated with increased cell surface hydrophobicity and increased production of exopolymeric substances. Curr. Microbiol. 79:24.



Kano, A., Hosotani, K., Gomi, K., Yamasaki-Kokudo, Y., Shirakawa, C., Fukumoto, T., Ohtani, K., Tajima, S., Izumori, K., Tanaka, K., Ishida, Y., Nishizawa, Y., Ichimura, K., Tada, Y. and Akimitsu, K. 2011. D-Psicose induces upregulation of defense-related genes and resistance in rice against bacterial blight. J. Plant Physiol. 168:1852-1857.


Karthikeyan, S., Orellana, L. H., Johnston, E. R., Hatt, J. K., L├Čffler, F. E., Ayala-Del-R├Ło, H. L., Gonz├Īlez, G. and Konstantinidis, K. T. 2021. Metagenomic characterization of soil microbial communities in the Luquillo Experimental Forest (Puerto Rico) and implications for nitrogen cycling. Appl. Environ. Microbiol. 87:e0054621.



Knights, H. E., Jorrin, B., Haskett, T. L. and Poole, P. S. 2021. Deciphering bacterial mechanisms of root colonization. Environ. Microbiol. Rep. 13:428-444.




Kocal, N., Sonnewald, U. and Sonnewald, S. 2008. Cell wall-bound invertase limits sucrose export and is involved in symptom development and inhibition of photosynthesis during compatible interaction between tomato and Xanthomonas campestris pv vesicatoria. Plant Physiol. 148:1523-1536.




Mart├Łnez-Vilalta, J., Sala, A., Asensio, D., Galiano, L., Hoch, G., Palacio, S., Piper, F. I. and Lloret, F. 2016. Dynamics of non-structural carbohydrates in terrestrial plants: a global synthesis. Ecol. Monogr. 86:495-516.

Mercier, J. and Lindow, S. E. 2000. Role of leaf surface sugars in the colonization of plants by bacterial epiphytes. Appl. Environ. Microbiol. 66:369-374.




Milkovska-Stamenova, S., Schmidt, R., Frolov, A. and Birkemeyer, C. 2015. GC-MS method for the quantitation of carbohydrate intermediates in glycation systems. J. Agric. Food Chem. 63:5911-5919.


Mochizuki, S., Fukumoto, T., Ohara, T., Ohtani, K., Yoshihara, A., Shigematsu, Y., Tanaka, K., Ebihara, K., Tajima, S., Gomi, K., Ichimura, K., Izumori, K. and Akimitsu, K. 2020. The rare sugar D-tagatose protects plants from downy mildews and is a safe fungicidal agrochemical. Commun. Biol. 3:423.




Mohan, B. H., Malleshi, N. G. and Koseki, T. 2010. Physico-chemical characteristics and non-starch polysaccharide contents of Indica and Japonica brown rice and their malts. LWT - Food Sci. Technol. 43:784-791.

Moing, A. 2000. Sugar alcohols as carbohydrate reserves in some higher plants. Dev. Crop Sci. 26:337-358.

Morandi, B., Corelli Grappadelli, L., Rieger, M. and Lo Bianco, R. 2008. Carbohydrate availability affects growth and metabolism in peach fruit. Physiol. Plant. 133:229-241.


Ne┼Īovi─ć, M., Ga┼Īi─ć, U., Tosti, T., Horvacki, N., Nedi─ć, N., Sredojevi─ć, M., Blagojevi─ć, S., Ignjatovi─ć, L. and Te┼Īi─ć, ┼Į. 2021. Distribution of polyphenolic and sugar compounds in different buckwheat plant parts. RSC Adv. 11:25816-25829.



Pascale, A., Proietti, S., Pantelides, I. S. and Stringlis, I. A. 2020. Modulation of the root microbiome by plant molecules: the basis for targeted disease suppression and plant growth promotion. Front. Plant Sci. 10:1741.



Sasse, J., Martinoia, E. and Northen, T. 2018. Feed your friends: do plant exudates shape the root microbiome? Trends Plant Sci. 23:25-41.


Schaarschmidt, S., Kopka, J., Ludwig-M├╝ller, J. and Hause, B. 2007. Regulation of arbuscular mycorrhization by apoplastic invertases: enhanced invertase activity in the leaf apoplast affects the symbiotic interaction. Plant J. 51:390-405.


Schlechter, R. O., Miebach, M. and Remus-Emsermann, M. N. P. 2019. Driving factors of epiphytic bacterial communities: a review. J. Adv. Res. 19:57-65.



Shelburne, S. A., Keith, D., Horstmann, N., Sumby, P., Davenport, M. T., Graviss, E. A., Brennan, R. G. and Musser, J. M. 2008. A direct link between carbohydrate utilization and virulence in the major human pathogen group A Streptococcus. Proc. Natl. Acad. Sci. U. S. A 105:1698-1703.



Shrestha, A., Grimm, M., Ojiro, I., Krumwiede, J. and Schikora, A. 2020. Impact of quorum sensing molecules on plant growth and immune system. Front. Microbiol. 11:1545.



Soria, A. C., Sanz, M. L. and Villamiel, M. 2009. Determination of minor carbohydrates in carrot (Daucus carota L.) by GC-MS. Food Chem. 114:758-762.

Tang, M., Bouchez, O., Cruveiller, S., Masson-Boivin, C. and Capela, D. 2020. Modulation of quorum sensing as an adaptation to nodule cell infection during experimental evolution of legume symbionts. mBio 11:e03129-19.




Toruno, T. Y., Stergiopoulos, I. and Coaker, G. 2016. Plant-pathogen effectors: cellular probes interfering with plant defenses in spatial and temporal manners. Annu. Rev. Phytopathol. 54:419-441.



Xu, Z., Xie, J., Zhang, H., Wang, D., Shen, Q. and Zhang, R. 2019. Enhanced control of plant wilt disease by a xylose-inducible degQ gene engineered into Bacillus velezensis strain SQR9XYQ. Phytopathology 109:36-43.


Yamada, K., Saijo, Y., Nakagami, H. and Takano, Y. 2016. Regulation of sugar transporter activity for antibacterial defense in Arabidopsis. Science 354:1427-1430.


- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 1,009 View
- 161 Download
- Related articles
-
The Interaction of Human Enteric Pathogens with Plants2014 June;30(2)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



