Weather Conditions Drive the Damage Area Caused by Armillaria Root Disease in Coniferous Forests across Poland
Article information
Abstract
Armillaria root disease affects forests around the world. It occurs in many habitats and causes losses in the infested stands. Weather conditions are important factors for growth and development of Armillaria species. Yet, the relation between occurrence of damage caused by Armillaria disease and weather variables are still poorly understood. Thus, we used generalized linear mixed models to determine the relationship between weather conditions of current and previous year (temperature, precipitation and their deviation from long-term averages, air humidity and soil temperature) and the incidence of Armillaria-induced damage in young (up to 20 years old) and older (over 20 years old) coniferous stands in selected forest districts across Poland. We used unique data, gathered over the course of 23 years (1987–2009) on tree damage incidence from Armillaria root disease and meteorological parameters from the 24-year period (1986–2009) to reflect the dynamics of damage occurrence and weather conditions. Weather parameters were better predictors of damage caused by Armillaria disease in younger stands than in older ones. The strongest predictor was soil temperature, especially that of the previous year growing season and the current year spring. We found that temperature and precipitation of different seasons in previous year had more pronounced effect on the young stand area affected by Armillaria. Each stand’s age class was characterized by a different set of meteorological parameters that explained the area of disease occurrence. Moreover, forest district was included in all models and thus, was an important variable in explaining the stand area affected by Armillaria.
Armillaria root disease is one of the major damaging factors in forests worldwide (Coetzee et al., 2018; Guillaumin et al., 1985; Kile and Watling, 1983; Lockman and Kearns, 2016; Pavlov, 2015; Shaw and Kile, 1991). As a result of root systems colonization by pathogens of the genus Armillaria, a decline in stand productivity has been reported as tree resources are used for defence responses rather than growth (Franceschi et al., 2005) as well as increased tree mortality (Bendz Hellgren and Stenlid, 1995; Bloomberg and Morrison, 1989; Cruickshank, 2011; Cruickshank et al., 2011; Filip et al., 2010; Kile et al., 1991; Shaw and Toes, 1977). Economic losses occur, reaching a value of up to 1000 Euros/ha/y in the most heavily infested stands (Kaliszewski et al., 2007; Smith, 1984). Armillaria root disease is also a serious problem for commercial forests in Poland. It causes damages to forest stands of different age categories on an area of tens to hundreds of thousands of hectares annually, peaking at over 250,000 hectares in 2002 (Sierota et al., 2003). Among the fungi of the genus Armillaria identified in Poland, Armillaria ostoyae (Romagn.) Herink has the greatest economic importance. It colonizes many native tree species, including all major tree species such as Scots pine, Norway spruce, European fir, oaks, common beech, silver birch, and European larch (Żółciak, 2003). It prefers conifers, especially pine, spruce, and fir, but occurs in most habitats, in coniferous, deciduous, and mixed stands of all age classes (Żółciak, 1999). However, it causes the greatest damage in the spruce forests of the Carpathians (Kaliszewski et al., 2007; Lech, 2003; Lech and Żółciak, 2006a).
The conditions for spore germination, growth, and development of Armillaria spp. are characterized by a wide temperature range. However, the thermal requirements of the individual species are very different. Moreover, the habitats of the different species differ in terms of their distribution - A. ostoyae occurs between latitude 61° (southern Finland, central Sweden) and latitude 40° (Calabria, southern Italy), A. borealis between latitude 69° (Lapland, northern Scandinavia) or latitude 55° in Scotland and latitude 49° in France and 46° in Austria and Slovenia, and A. cepistipes between latitude 66° (Arctic Circle) and latitude 42° (Italy - Tuscany, Pyrenees) (Guillaumin et al., 1993; Kile et al., 1994). The mycelium of Armillaria grows at temperatures above 6–8°C (Lesowskij and Martyszeczkina, 1978; Rishbeth, 1978), and the rhizomorphs, the infecting organs of the pathogen, at 5–28°C (Rishbeth, 1968). The optimal temperature for growth of the mycelium and rhizomorphs in situ was 20–22°C, while no growth occurred at a temperature of 30°C (Keca, 2005; Rishbeth, 1968, 1978). Similarly, temperatures below 10°C restricted the growth of rhizomorphs, which was confirmed by in vitro experiments (Pearce and Malajczuk, 1990). Nutrient, temperature and light conditions are key environmental factors for formation of fungal fruiting body (Sakamoto, 2018). A July temperature 4°C below the long-term average accelerated the fruiting body emergence in the northwestern, European part of the former USSR by more than two weeks - from the late of August to the beginning of this month (Szubin, 1976). Substrate moisture, in turn, determines the activity of the apical meristem, which is responsible for the growth of the rhizomorphs. It has been demonstrated that it can only take place when the tip of the rhizomorph is covered with a thin layer of water (Smith and Griffin, 1971). Mihail et al. (2002) found that the rhizomorphs of A. tabescens can only form in soil of high moisture. Such conditions and elevated CO2 up to 1000 ppm in the air stimulated the growth of rhizomorphs of A. ostoyae in the gas chamber experiment (Lech and Żółciak, 2006b), while elevated CO2 alone did not (Lech and Żółciak, 2017). On the other hand, drought as a predisposing factor has been indicated to promote Armillaria root disease incidence in many tree species (Goheen and Otrosina, 1998; Kliejunas, 2011; La Porta et al., 2008; Livingstone et al., 1982; Murray and Leslie, 2021; Szubin, 1976), however the field evidence is not clear and rather speculative (Kolb et al., 2016; Roy et al., 2004; Sturrock et al., 2011).
According to the concept of the disease triangle (Duggar, 1909; Gäumann, 1950; Stevens, 1960), the disease process develops as a result of the interaction between host, pathogen and environment. However, in a several-year perspective and in the reciprocal relationship between a given tree and a specimen of a pathogenic fungus, it can be assumed that of all the environmental variables contributing to this interaction, only weather conditions are subject to dynamic changes. Thus, they are factors that determine the resistance/susceptibility of the host tree on the one hand and the infection potential of the pathogen on the other in the short term. Based on this assumption, the aim of the study was defined as to determine the intensity and character of relationship between the damage area caused by Armillaria disease in conifer stands of different ages (younger vs. older) and the weather conditions from the previous and current years by using the set of generalized linear mixed models (GLMMs) with a year as a random factor.
We asked the following research questions:
(1) In what type of stands (younger versus older) have weather conditions more pronounced effect on damage area caused by Armillaria root disease?
(2) What set of weather variables explained the best the area of disease occurrence?
(3) Do weather variables of the previous year have more pronounced effect on the damage area caused by Armillaria root disease compared to weather variables of current year?
Materials and Methods
Data collection on the area of conifer stands damaged by Armillaria root disease
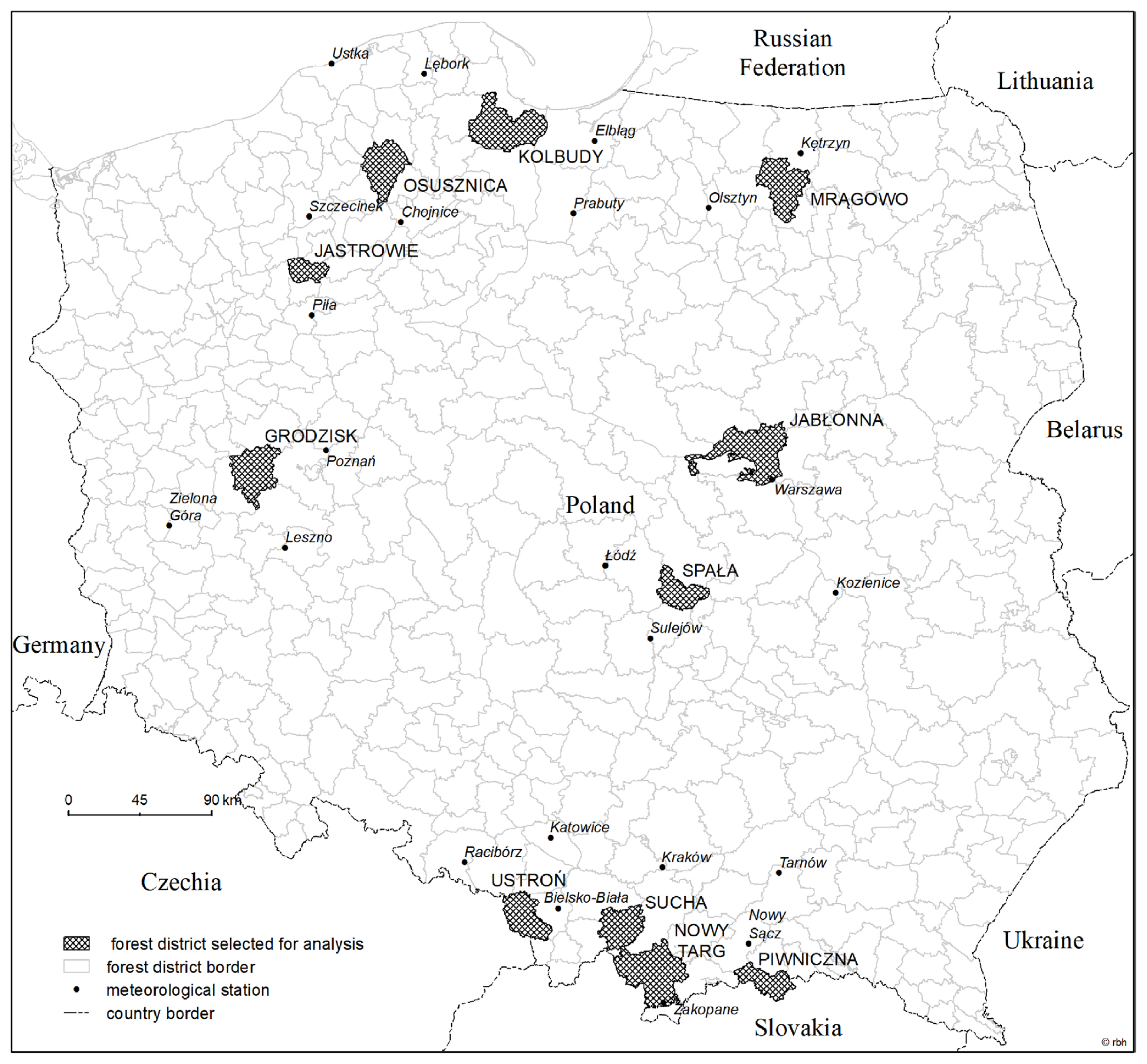
As forests in Poland are dominated by coniferous species (mainly Scots pine and Norway spruce) and these stands are most affected by Armillaria root rot (Żółciak, 2003), our study was limited exclusively to coniferous stands. We used data on the area of stands damaged by Armillaria root disease from 11 forest districts (out of about 430 in the country) of the State Forest Enterprise (SFE) during 1987–2009. The selection of forest districts was made on the following criteria: (1) no change in forest district boundaries as a prerequisite for comparability of data from subsequent years; (2) continuity of Armillaria root rot occurrence in most years of the 1987–2009 period; (3) different levels of risk from the disease, i.e., high (average annual occurrence of damages caused by Armillaria root rot of 40–62% of the total area of conifer stands in the forest district during the whole period), medium (10–25%) and low (less than 1%) (Table 1).

Forest districts chosen for the analysis of the relationship between the area of coniferous stands damaged by Armillaria root disease and weather conditions
Taking the above into account, the following forest districts were selected: Ustroń, Sucha, Nowy Targ, Jabłonna, Kolbudy, Piwniczna, Mrągowo, Spała, Grodzisk, Jastrowie and Osusznica (Fig. 1). These forest districts are in different parts of the country with various physiographic and natural conditions. We assumed that this variability caused the risk of forest damage from Armillaria root disease to vary among forest districts but had no significant effect on the variation in damage occurrence between years during the period analyzed, since at each site all these natural conditions except weather were constant throughout the study period.
The Forest Condition Information System was developed for stands managed by the SFE, and data on pest and fungal disease damage are registered annually at the forest district level throughout the country. This has allowed to create of a unique database on the occurrence of fungal diseases in forests, including Armillaria root disease. This database was compiled based on information provided by local forest administration and verified by the SFE Forest Protection Service. Data collection related to forest protection issues (i.e., the incidence of damage caused by fungal diseases or insect pests) is a routine task of the forest rangers in each forest district and is done annually by inspecting stands accordingly to the Guide to Forest Protection (1988). The reports on this inspection are summarized and published annually (Collective Work, 1988–2010). The data was divided into two subcategories depending on the age of the stands: incidence of Armillaria root rot damage in plantations and thickets (young stands up to 20 years old) and in older stands (over 20 years old).
Weather condition data
We used meteorological data from 1986–2009 provided by the Institute of Meteorology and Water Management and published in the Bulletin of the State Hydrological and Meteorological Service (1986–2009). The weather elements were registered monthly and included average air temperature, average soil temperature at 5 cm depth, precipitation total, average humidity. In the case of meteorological stations located outside a given forest district, which concerned 10 out of 11 districts, weather parameters were determined by interpolating the values of these parameters measured at 2 to 4 meteorological stations located in the vicinity of the forest district concerned, at a distance ranging from a few to about 85 kilometers. For this purpose, the inverse distance method was used (Meijerink et al., 1994), which assumes that the distance is a weighting factor for the influence of the value of the weather parameter measured at a particular meteorological station for the value of this parameter at the calculation point (forest district). This means that the further away the measuring point is from the calculation point, the smaller its influence on the interpolated value of the weather parameter.
Based on the data collected and interpolated to the area of the forest districts, the following values were calculated for the years 1986–2009: seasonal average air temperature (°C), seasonal sum of precipitation (mm), seasonal average air humidity (%) and seasonal average soil temperature at 5 cm depth (°C). We considered winter from December of the previous year till February of the current year, spring from March till May, summer from June till August, autumn from September till November and the growing season from March till September in the analyses of air temperature, precipitation, and soil temperature. We also calculated the absolute deviation from the long-term average temperature (±°C) and the relative deviation from the long-term average sum of precipitation (%) for each season (winter, spring, summer, autumn) and growing season. Seasonal average air humidity (%) was calculated for spring (April–May), summer (June–August), autumn (September–October), and growing season (April–September). We did not have humidity data for the months from November till March. Therefore, we had to shorten a period of spring and autumn and considered only two months instead of three.
Statistical analyses
We included the following weather parameters as explanatory variables in our models: (1) average air temperature (°C), (2) sum of precipitation (mm), (3) soil temperature at a depth of 5 cm (°C), (4) absolute deviation from long-term average temperature (±°C), (5) relative deviation from long-term average sum of precipitation (%), and (6) average air humidity (%). After checking the correlations between variables, we grouped them into three main models in which the variables were not strongly correlated with each other: (1) temperature–precipitation model, (2) deviation from the long-term average temperature–deviation from the long-term average sum of precipitation (dT–dP) model, (3) soil temperature–air humidity model. Therefore, we constructed a set of GLMMs with Gamma distribution and log link to analyze the effects of current year average air temperature, current year sum of precipitation and site (forest district) on the area of damage caused by Armillaria root disease. We conducted our analyses for each season (winter, spring, summer, autumn, growing season) and stand type (young stands up to 20 years old and older stands over 20 years old). Similarly, we constructed the models with the average air temperature in the previous year, the sum of precipitation in the previous year and the site (forest district) for each season and stand type separately, as we considered that the weather conditions of the previous year could have an impact on the stand area damaged by Armillaria root disease. We also included the interaction term of temperature and precipitation in all models. The year was added to all models as a random effect to account for the unknown effect of year-to-year variability. In total, we obtained 20 models. In addition, we built another 10 models with the absolute deviation from the long-term average temperature in the current year, the relative deviation from the long-term average sum of precipitation in the current year and the site (forest district) as explanatory variables and another 10 models with absolute deviation from the long-term average of temperature in the previous year, the relative deviation from the long-term average sum of precipitation in the previous year and the site for each season and type of stands separately. In these models, the year was also used as a random factor and the interaction between weather variables was included. The average humidity in the current year, soil temperature in the current year and site (forest district) were used as explanatory variables in another 8 models for each season (spring, summer, autumn, and growing season) and stand type (young stands and older stands), and the average humidity in the previous year, soil temperature in the previous year and site (forest district) were used as explanatory variables in another 8 models for the above mentioned seasons and stand types. In these models, the year was a random factor and we also included the interaction term between humidity and soil temperature.
The best models were selected based on the Akaike information criterion (AIC) by backward stepwise selection for each model. Any patterns in the residuals for model validation were examined by plotting the residuals against the fitted values. We used R software, version 4.3.0 (R Core Team, 2023) with the packages lme4 (Bates et al., 2015), ggplot2 (Wickham, 2016), sjPlot (Lüdecke, 2021) and gridExtra (Auguie, 2017).
Results
Temperature–precipitation models
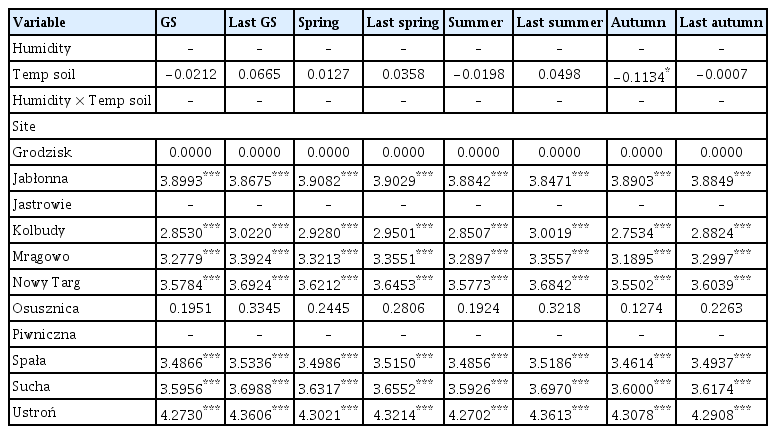
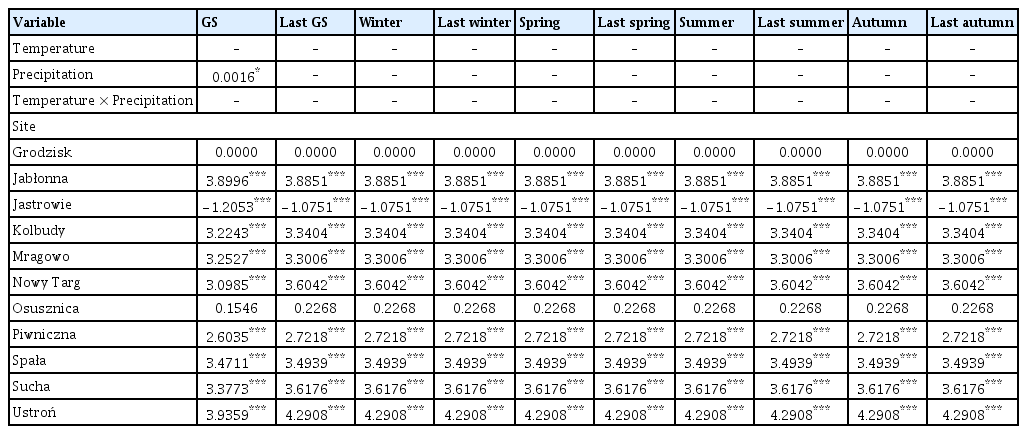
Out of the 20 models analyzed, only 9 models included temperature/precipitation (or both) in the best model selected on the basis of the lowest AIC value (Tables 2 and 3, Supplementary Table 1). Temperature and precipitation were weak predictors of area affected by Armillaria root disease in older stands, as only growing season precipitation was included in the best model (Table 3). In other models for older stands, only site (forest district) was chosen as an explanatory variable for the best model. On the other hand, the area affected by Armillaria root disease in younger stands was well explained by precipitation and temperature (Table 2). Precipitation was chosen for the best model for the following seasons: growing season, previous year winter and summer, while precipitation and temperature together were chosen for the winter model and temperature for the previous year spring and previous year autumn models (Supplementary Table 1). Temperature, precipitation, and the interaction term between temperature and precipitation were chosen to the previous year growing season and previous year summer models. The site (forest district) was included in all models (Supplementary Table 1).

The coefficient values of predictor variables selected for best ‘temperature-precipitation’ models of the area infected by Armillaria root rot in younger stands
The coefficient values of predictor variables selected for best ‘temperature-precipitation’ models of the area infected by Armillaria root rot in older stands
The precipitation totals of the growing season, last winter and summer had a positive effect on the area damaged by Armillaria root disease in younger stands, i.e., the more rainfall, the larger the area with symptoms of damage (Table 2, Fig. 2A, D and E). Similarly, last year spring and last year autumn temperatures also had a positive influence on the area affected by Armillaria root disease in younger stands (Fig. 2G–H). Interestingly, the model with winter precipitation and temperature showed that higher winter precipitation resulted in a larger area of younger stands affected by Armillaria root disease that year. However, this effect was even stronger when the average winter temperature was low compared to the mild temperatures during this season (Fig. 2C). Our models showed that greater rainfall in combination with high temperature in the previous year growing season and in the previous year summer resulted in a larger area of younger stands showing damage symptoms of Armillaria root disease. However, when temperature was average or low in these two seasons, we did not observe this relationship (Fig. 2B and F).
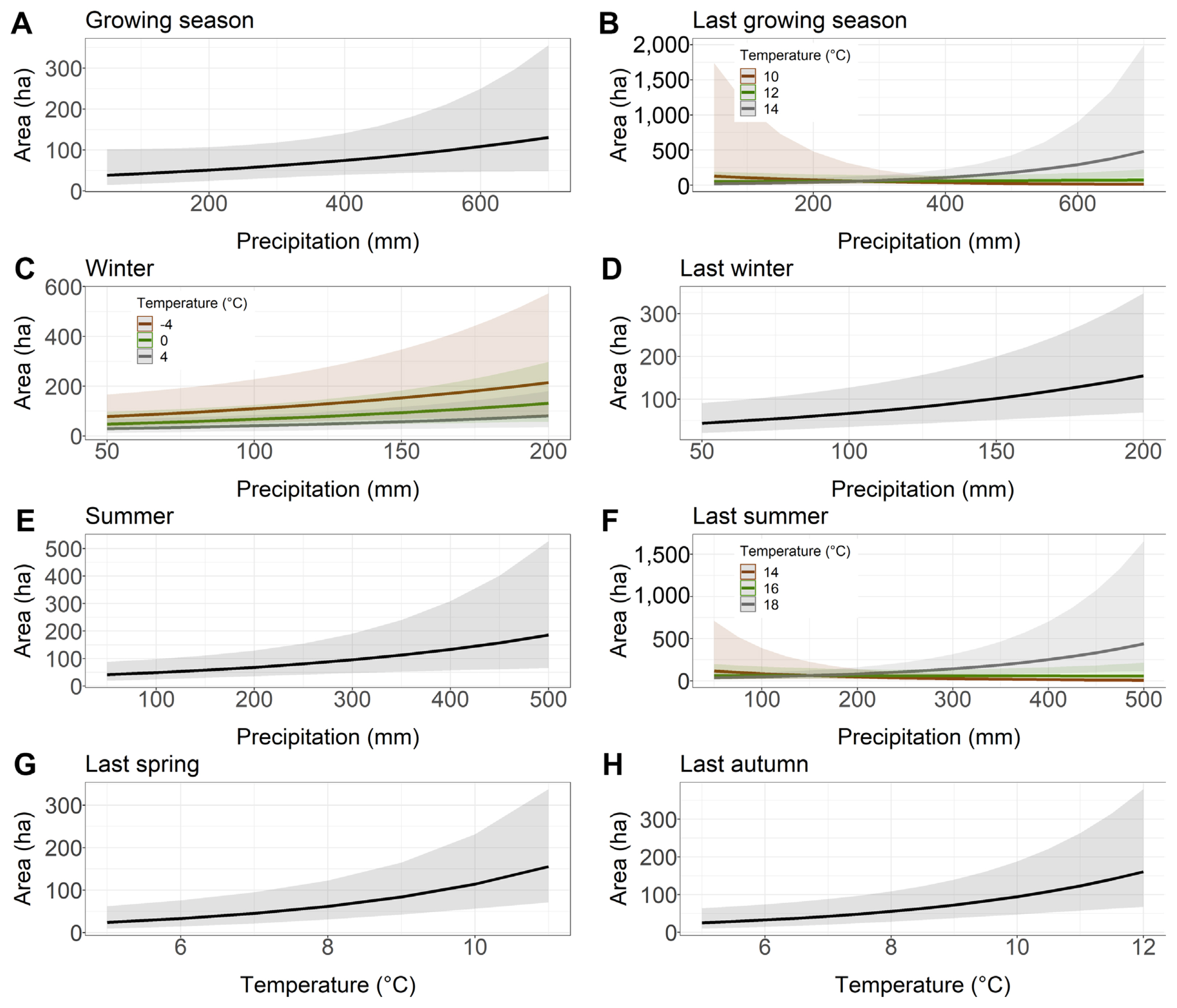
Relationship between the younger stand area with Armillaria root rot (ha) and the precipitation and temperature chosen to the best models based on Akaike information criterion. (A) Growing season. (B) Last growing season. (C) Winter. (D) Last winter. (E) Summer. (F) Last summer. (G) Last spring. (H) Last autumn.
Deviation from the long-term average of temperature/deviation from the long-term average sum of precipitation (dT–dP) model
dT and dP were average predictors of the occurrence of damages caused by Armillaria root disease in younger and older stands, as they were selected based on the lowest AIC in only 10 out of 20 models (Tables 4 and 5, Supplementary Table 2).
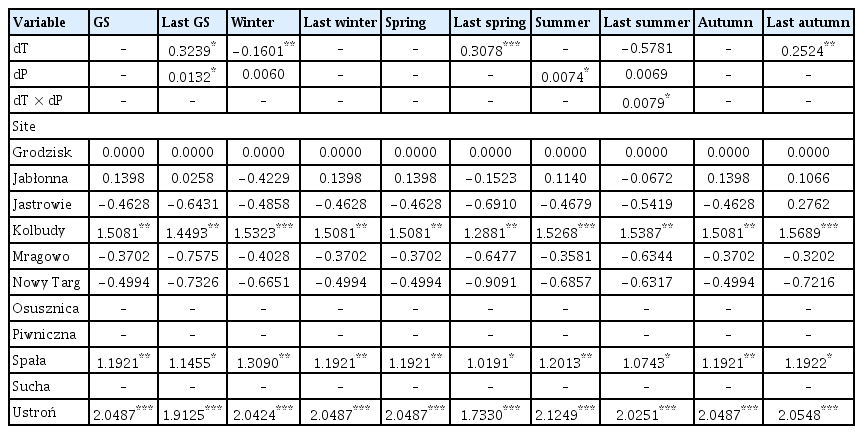
The coefficient values of predictor variables selected for best ‘deviation from the long-term average of temperature and the deviation from the long-term average sum of precipitation’ models of the area infected by Armillaria root rot in younger stands

The coefficient values of predictor variables selected for best ‘deviation from the long-term average of temperature and the deviation from the long-term average sum of precipitation’ models of the area infected by Armillaria root rot in older stands
dT of previous year spring and previous year autumn had a positive effect on the area affected by Armillaria root disease in younger stands, i.e., if the temperature in last spring or last autumn was higher than the long-term average, the model showed that the area with Armillaria root disease damage symptoms was larger (Table 4, Fig. 3C and D). Similarly, summer dP had a positive effect on the area of young stands showing symptoms of Armillaria root disease damage (Table 4, Fig. 3E). Our model showed that warmer temperatures in the previous year growing season, compared to the long-term average, together with a higher precipitation total than the long-term average, resulted in an expansion of the area where the Armillaria root disease symptoms were observed (Fig. 3A). Interestingly, we found that colder winter temperatures compared to its long-term average, together with a higher rainfall sum compared to the long-term average of this parameter, also increased the area with Armillaria root disease symptoms in young stands (Fig. 3B). Furthermore, our model showed that with a higher precipitation sum in summer compared to the long-term average, Armillaria-related symptoms of injury area in young stands increased rapidly when the temperature was 1°C or more above the long-term average (Fig. 3F).
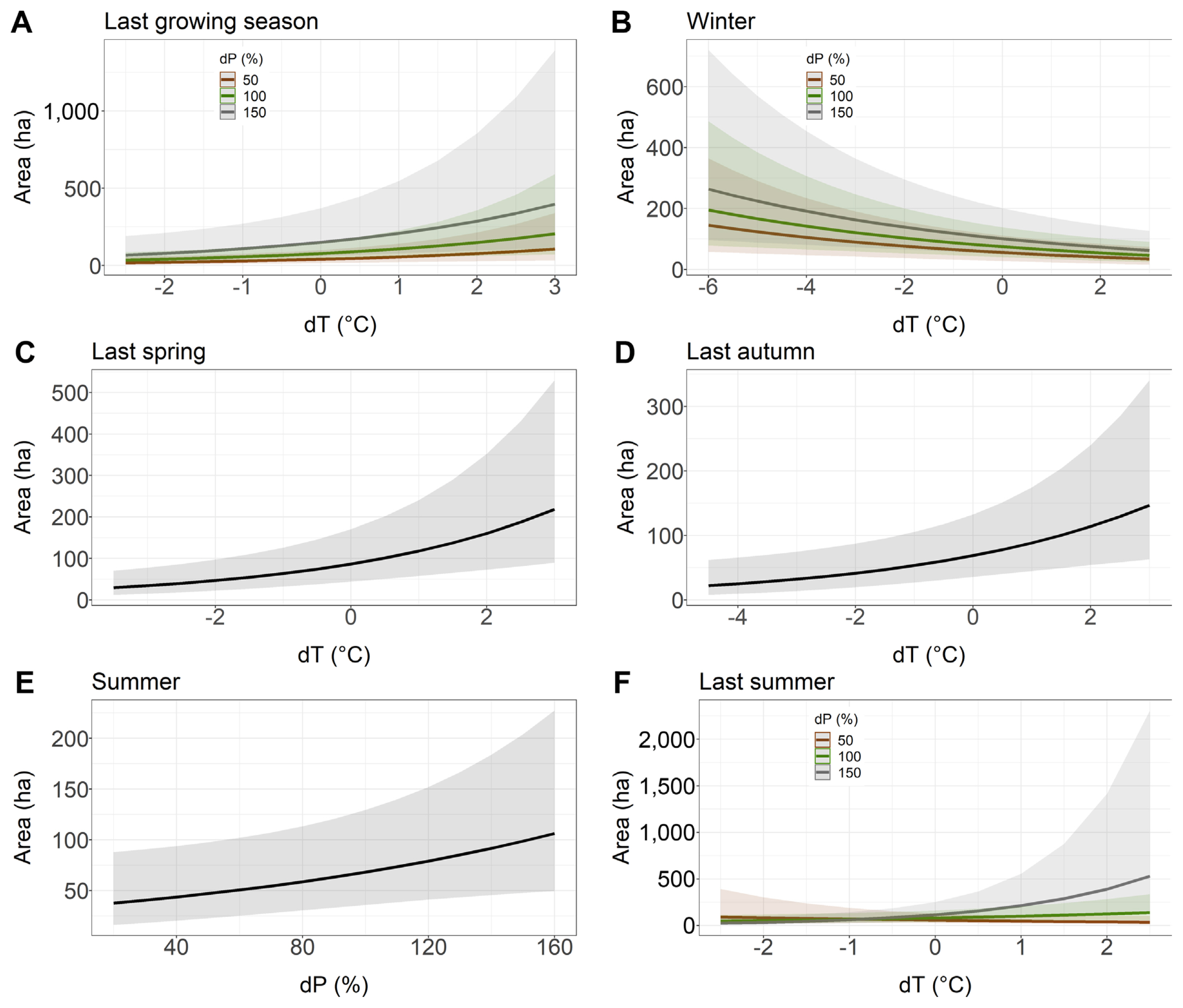
Relationship between the younger stand area with Armillaria root rot (ha) and the deviation from the long-term average of temperature (dT) and the deviation from the long-term average sum of precipitation (dP) chosen to the best models based on Akaike information criterion. (A) Last growing season. (B) Winter. (C) Last spring. (D) Last autumn. (E) Summer. (F) Last summer.
In older stands, it was found that the dP of the growing season and summer had a positive influence on the area affected by Armillaria root disease (Table 5, Fig. 4A and B). On the other hand, previous year autumn’s dP had a negative influence on the area of older stands with Armillaria root disease symptoms, i.e., a lower rainfall total compared to the long-term average resulted in a larger area with Armillaria root disease damage (Table 5, Fig. 4D). Similarly, autumn dT also negatively affected the area of older stands with damage caused by Armillaria root disease (Table 5, Fig. 4C). The site (forest district) was included in all best models (Supplementary Table 2).
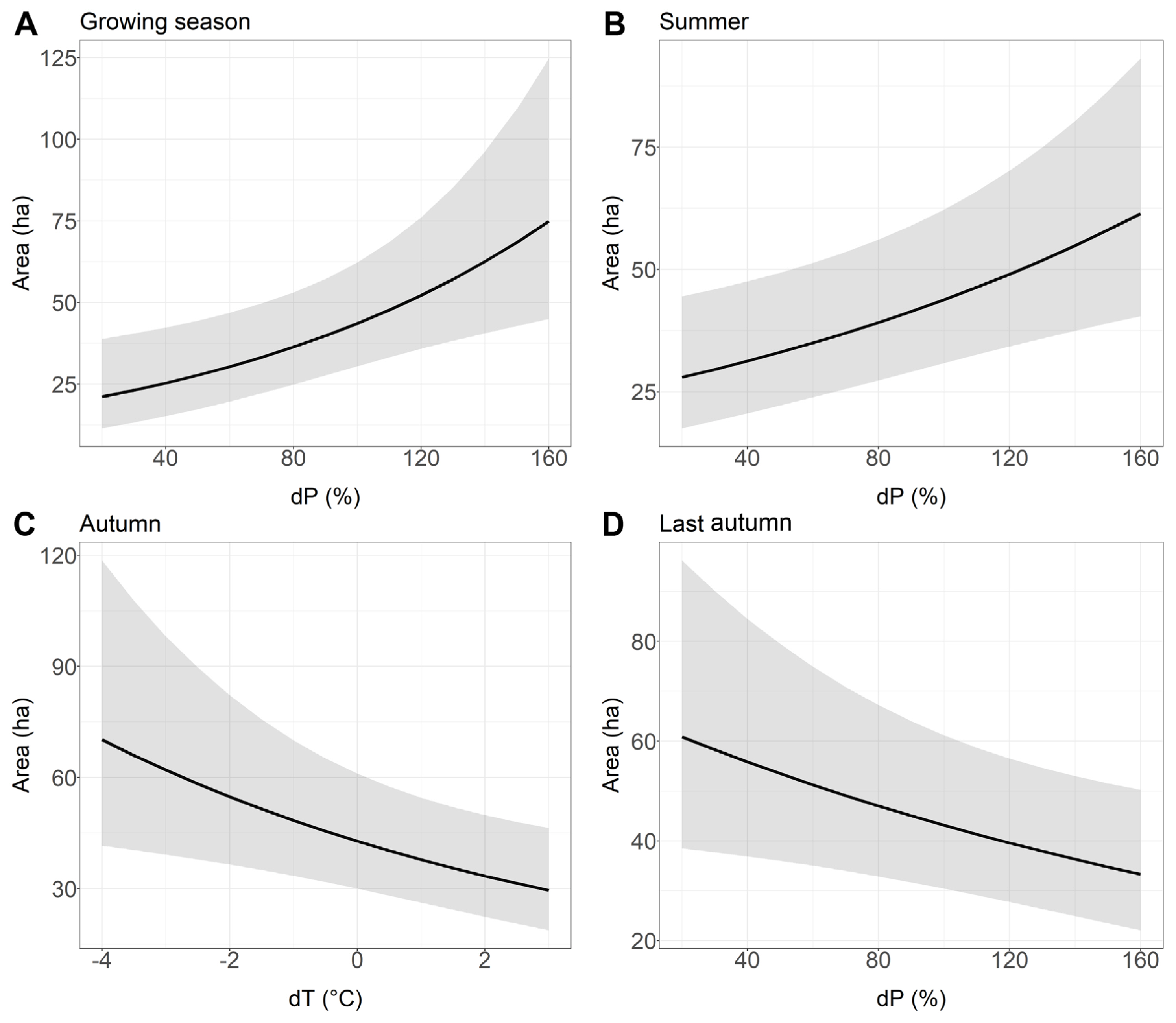
Relationship between the older stand area with Armillaria root rot (ha) and the deviation from the long-term average of temperature (dT) and the deviation from the long-term average sum of precipitation (dP) chosen to the best models based on Akaike information criterion. (A) Growing season. (B) Summer. (C) Autumn. (D) Last autumn.
Soil temperature–air humidity models
Soil temperature was a very good predictor of area affected by Armillaria root disease in both younger and older stands, as this variable was included in all models (Supplementary Table 3). Air humidity, on the other hand, was a rather weak predictor and was included in 2 out of 16 models. Soil temperature in the previous year growing season, previous year spring, previous year autumn, and current year autumn had a positive influence on the area of young stand damaged by Armillaria root rot (Table 6, Fig. 5B, D, G, and H). Our results showed that soil temperature in summer and previous year summer had a negative influence on the area of young stand damaged by Armillaria root disease (Table 6, Fig. 5E and F). We found that soil temperature, humidity during the growing season and spring, and the interaction of both terms were included in the best model to describe the young stand area damaged by Armillaria root rot (Supplementary Table 3). Higher humidity combined with lower soil temperature during the growing season resulted in a larger area affected by Armillaria root disease (Fig. 5A). Similarly, our model showed that higher humidity and lower soil temperature in spring also resulted in a larger area in the young stand with Armillaria root rot damage (Fig. 5C). However, when soil temperature was higher and humidity was lower in spring, the same area in the young stand was infested with Armillaria as when soil temperature was higher and humidity was lower in spring.
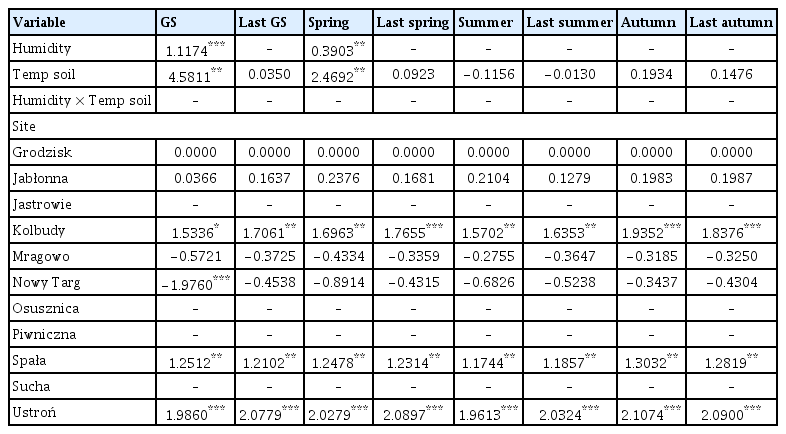
The coefficient values of predictor variables selected for best ‘soil temperature-air humidity’ models of the area infected by Armillaria root rot in younger stands

Relationship between the younger stand area with Armillaria root rot (ha) and soil temperature and humidity chosen to the best models based on Akaike information criterion. (A) Growing season. (B) Last growing season. (C) Spring. (D) Last spring. (E) Summer. (F) Last summer. (G) Autumn. (H) Last autumn.
We found that soil temperature of the growing season, summer, autumn, and previous year autumn had a negative effect on the area of older stands damaged by Armillaria root disease (Fig. 6A, E, G, and H). However, the effects were rather weak, except for soil temperature in autumn (Table 7). Conversely, our results showed positive but rather weak effects of soil temperature in the previous year growing season, spring, previous year spring, and previous year summer on the area of older stands affected by Armillaria root disease (Fig. 6B–D and F).

Relationship between the older stand area with Armillaria root rot (ha) and soil temperature chosen to the best models based on Akaike information criterion. (A) Growing season. (B) Last growing season. (C) Spring. (D) Last spring. (E) Summer. (F) Last summer. (G) Autumn. (H) Last autumn.
Discussion
Our models have proved useful in determining the relationship between weather conditions and the incidence of Armillaria root disease damage in coniferous forests. The method seems to be particularly suitable to study the relationship between the dynamics of disease and pest occurrence and different environmental variables or in disease prediction, as it has been applied in other works (Choi, 2003; Coakley et al., 1988; Landau et al., 2000; Sharma-Poudyal and Chen, 2011; Wu et al., 2005).
According to the results, the incidence of Armillaria root disease damage was strongly associated with soil temperature, air temperature, and precipitation of the previous year. Similar results were obtained in earlier studies on the relationship between weather conditions and the occurrence of root diseases in entire Poland or in selected regional directorates of State Forests (Kolk et al., 1996; Mychayliv, 2011; Mychayliv and Małecka, 2012).
We also found that location (forest district) was a good predictor of the area of damage caused by Armillaria root disease in all models. We could observe the large difference in the damage area caused by Armillaria between forest districts and it was also shown by our models. We can speculate whether the reason for this lies in the specific ecological and environmental circumstances at each site, which form a “disease triangle”. The complexity of forest ecosystems and the elements involved in Armillaria pathogenesis, whose interplay is further complicated by climate change and its long-term effects (Kubiak et al., 2017), could further support this opinion.
Young coniferous stands
Soil temperature proved to be the best predictor of disease-affected area compared to other meteorological parameters. In young conifer stands, soil temperature in the previous year’s growing season, spring and autumn had a positive influence on the damage area, while in the previous and in current year summer - negative. This means that relatively high soil temperatures in the early and late months of the previous year’s growing season, leading to a temporal prolongation of conditions suitable for the growth of rhizomorphs and mycelium, promote the infection potential and spread of Armillaria root disease in the next year, while high soil temperatures in the summer months inhibit the spread of the disease in young stands. The area affected by the disease could also be well explained by precipitation (during the growing season of the current year, winter and summer of the previous year), temperature (spring and autumn of the previous year) or a combination of both. Similar results were presented by Mychayliv (2011). This is also consistent with the work of Rishbeth (1968, 1978), Pearce and Malajczuk (1990), and Keca (2005), who found in in vitro experiments that temperatures below 10oC and above 28oC restrict the growth of the pathogen’s infectious organs, and with the observations of Lesowskij and Martyszeczkina (1978) on the minimum temperature that allows rhizomorph growth.
Precipitation, air temperature, and humidity (in spring and in the growing season of the current year) had a positive influence on the area damaged by Armillaria root disease in young stands. Previous studies found that the damaged area was positively influenced by high rainfall in the previous year’s growing season (Kolk et al., 1996; Mychayliv, 2011; Mychayliv and Małecka, 2012). These observations are justified by the dependence of rhizomorph growth and the resulting infection potential on high soil moisture (Lech and Żółciak, 2006b; Mihail et al., 2002), which is the prerequisite for the activity of the rhizomorph apical meristem (Smith and Griffin, 1971). In younger stands warmer temperatures combined with higher rainfall in the previous year growing season than the long-term averages also resulted in a larger area damaged by the disease. The explanation for this could be the same as described above - warm temperatures (especially in the spring and autumn months of the previous year’s growing season) and high precipitation create suitable conditions for the pathogen’s infectious potential to build up, leading to the spread of the disease and an increase in the damaged area the following year. It was also found that the deviation of temperature and precipitation from long-term averages had a smaller influence on the area of damage caused by Armillaria root disease than soil temperature. This could be due to the fact that the soil is the environment where the spread of the rhizomorphs, the infection organs of the honey fungus, takes place. Therefore, the conditions there, such as soil temperature, directly affect the possibility of their growth, while the conditions in the atmosphere (air temperature, precipitation) affect this growth only indirectly (through the soil) and therefore have a lesser impact.
Stands older than 20 years
We found that in older stands the dependence of the damage area on meteorological parameters was much weaker and occurred much less frequently in the models than in younger stands. This statement applies to all three distinguished groups of models: temperature-precipitation, temperature deviation from long-term average-precipitation deviation from long-term average, and soil temperature-air humidity. This can be explained by the generally lower susceptibility of older trees of many coniferous species to the effects of Armillaria root disease, which in turn translates into a weaker relationship with weather conditions. Risbeth (1972) indicated that mortality of conifers (including Scots pines) decreases with age, often leading to stabilization of disease outbreaks. Robinson and Morrison (2001) found that infection of 6–8-year-old western larch and Douglas-fir progressed unimpeded and overcame any host resistance, followed by moderate defence responses (lesion limited by necrophilous periderm with multiple bands of phellem) in 18- and 19-year-old trees and a more severe response in 85- to 95-year-old trees. Similarly, Cruickshank (2020) indicated a stronger defence response in older Douglas-fir, both from plantings and natural regeneration. The greater resistance of older pines in Poland was also reported by Żółciak (1999).
In the best model, only the precipitation of the growing season was considered. In the case of precipitation deviation from the long-term average, the situation was unclear - the dPs of the current year growing season and summer had a positive influence on damage area caused by Armillaria root disease, while the dPs of the previous year and current year autumn had a negative influence. The literature on the relationship between atmospheric precipitation and the occurrence of Armillaria root disease is also ambiguous. On the one hand, it is said that the prerequisite for the growth of Armillaria rhizomorphs and thus their ability to colonize new spaces is the presence of water at the rhizomorph tip (Smith and Griffin, 1971) and high soil moisture (Mihail et al., 2002). On the other hand, drought has also been cited as a predisposing and stress-inducing factor favoring infection of trees by the pathogen (Kliejunas, 2011; La Porta et al., 2008; Livingstone et al., 1982). However, the results of our analyses have not confirmed such an effect of moisture deficiency.
A similar ambiguity concerns soil temperature as a predictor of the area affected by the disease. Although this parameter was included in several models, its effects were inconsistent. Soil temperatures of the current year’s growing season, summer, autumn and previous year’s autumn had a negative impact on the area damaged by Armillaria root rot, while soil temperatures of the previous year’s growing season, spring, summer, and spring of the current year had a positive impact. These results indicate once again that the weather conditions of the previous year, which are suitable for the development of the disease, have the greatest influence on the damage occurring the next year. A similar conclusion was also reported by Kolk et al. (1996) and Mychayliv and Małecka (2012).
Based on the results of the conducted analysis, we can draw the following conclusions:
(1) The importance of weather conditions for the occurrence of damage caused by Armillaria root disease in coniferous forests was confirmed for both young and older stands. However, in older stands the meteorological parameters occurred much less frequently in the models than in younger stands and their influence was not as clear.
(2) The most influential meteorological parameter that had a positive effect on the area of damage caused by the disease was soil temperature, especially that of the spring and the growing season of the year before the damage occurred.
(3) Temperature and precipitation, as well as their deviations from long-term averages, were weaker predictors of the area affected by Armillaria root disease than soil temperature, especially in older stands where an unclear influence of different meteorological parameters was found. However, it should be noted that in young stands, warm temperatures combined with high rainfall in the previous growing season, spring, summer, or fall, resulted in a larger area affected by the disease.
(4) The incidence of Armillaria root disease was more strongly associated with the weather of the year before the damage occurred than with the weather of the current year. This was particularly evident for meteorological parameters such as soil temperature, precipitation, air temperature, and their deviation from long-term averages in younger stands and for soil temperatures in the growing season, spring, and summer in older stands.
(5) We recommend monitoring weather variables, especially soil temperature, in stands at high risk for occurrence of Armillaria root disease. This should make it possible to predict the occurrence of the risk in advance and prepare protective measures to limit the damage. It should also be noted that permanent reduction of the negative impact of Armillaria root disease on stands can only be achieved through complex hylotechnical measures that limit the spread of the pathogen in the forest environment.
(6) GLMMs have proven useful in determining the relationship between weather conditions and the incidence of Armillaria root rot damage in coniferous forests.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Acknowledgments
The study described in the article was performed under project titled “Support Platform of Operational Decision Making Depending on Weather Conditions (PROZA)”, co-financed by the European Union from the European Regional Development Fund through the Innovative Economy Operational Program (grant no. UDA=POIG.01.03.01-00-140/08-00).
Electronic Supplementary Material
Supplementary materials are available at The Plant Pathology Journal website (http://www.ppjonline.org/).