 |
 |
| Plant Pathol J > Volume 40(1); 2024 > Article |
|
Abstract
The aim of this study was to isolate biocontrol bacteria that could antagonize brown rot of Dendrocalamus latiflorus, optimize the culture conditions, and develop an effective biocontrol preparation for brown rot of D. latiflorus. This study isolated a bacterium with an antagonistic effect on bamboo brown rot from healthy D. latiflorus rhizosphere soil. Morphology, molecular biology, and physiological biochemistry methods identified it as Bacillus siamensis. The following culturing media and conditions improved the inhibition effect of B. siamensis: the best culturing media were 2% sucrose, 1.5% yeast extract, and 0.7% potassium chloride; the optimal culturing time, temperature, pH, and inoculation amount were 48 h, 30┬░C, 6, and 20%. The optimum formula of the applying bacterial suspension was 14% sodium dodecyl benzene sulfonate emulsifier, 4% Na2HPO4┬Ę2H2O, 0.3% hydroxypropyl methylcellulose thickener, and 20% B. siamensis. The pot experiment results showed the control effect of applying bacterial suspension, diluted 1,000 times is still better than that of 24% fenbuconazole suspension. The applying bacterial suspension enables reliable control of brown rot in D. latiflorus.
Dendrocalamus latiflorus is a dual-purpose cluster bamboo of Dendrocalamus, a subfamily of Gramineae, and an excellent bamboo plant for ecological greening (Gao et al., 2011). In its growth and utilization, D. latiflorus is harmed by various diseases from the shooting stage to the bamboo forest cutting and utilization stage, resulting in a decline in shoot quality and yield. At present, the common diseases of D. latiflorus include shoot blight caused by Arthrinium phaeospermum (Yang et al., 2019), shoot rot caused by Fusarium spp. (Huang and Zhong, 2023), bituminous coal disease caused by Meliola sp. and Capnodium spp. (Li et al., 2009), and leaf rust caused by Puccinia spp. (Dey et al., 2023). According to reports, brown rot of Dendrocalamus latiflorus has occurred in both bamboo shoots and mature bamboo, with a disease incidence rate of over 70% found in a bamboo forest, leading to significant economic losses. The diseased D. latiflorus bamboo with brown rot first appears with light yellowish-brown disease spots at the base of the stem, and the disease spots rapidly expand upwards to form stripes, which gradually deepen in color and become purple-brown to black-brown (Wei et al., 2021). At the same time, the disease spots at the base of the stem also expand laterally. After surrounding the base of the stem, the diseased bamboos die. The section of diseased bamboo shows that the inner wall of the cavity and the septum become black, white mycelia grow, and there are black stripes in the bamboo flesh.
Bacillus is an important component of biocontrol microorganisms of plant diseases, which have significant biocontrol potential, can produce heat-resistant and stress-resistant spores, and are conducive to the production of biocontrol agents, preparation processing, survival, colonization, and reproduction in the environment (Chowdhury et al., 2015). The action mechanisms of Bacillus mainly include competition, antagonism, inducing plants to obtain systematic resistance, and promoting plant growth (Liu et al., 2013). Research shows that Bacillus can be used to control plant diseases, decompose crude oil, degrade compounds in soil, etc., and has broad application prospects (Liu et al., 2011). According to the report, B. siamensis widely exists in the natural environment. B. siamensis has a strong antagonistic ability to common disease fungi and has a high antagonistic effect on Pseudomonas solanacearum, Botryosphaeria dothidea, Botrytis cinerea, etc. (Wang et al., 2022). Feng et al. (2020) found that B. siamensis, as a biocontrol antagonist, has a significant effect on inhibiting diseases and improving the quality of fruits and vegetables. Xie et al. (2020) isolated a strain with antifungal activity against tobacco brown spot disease from 40 rhizosphere soil samples and identified it as B. siamensis. Ali et al. (2021) found tolerance of B. siamensis to salt stress in wheat.
Although chemical fungicides still play a leading role in the pesticide industry, the research, development, and application of biocontrol Bacillus fungicides meet the needs of modern society for agricultural production and integrated pest control, which is of great significance to the sustainable development of agriculture and shows strong market competitiveness (Mourou et al., 2022). However, in the process of implementing the applications, researchers have also encountered some difficulties. At present, most biocontrol agents are live bacteria. Under natural conditions, the ability of live bacteria to colonize stably and produce the appropriate effect is key to the spread of biological control agents (Bekele, 2022). The second factor is the stability of the control effect. Whether living bacteria can stabilize colonization and produce the corresponding effect is key to the promotion of biological control agents. Both environmental factors (temperature, pH) and biological factors (microbial competition within the plant) can affect the effectiveness of biological control and therefore control is not very stable (Pereira et al., 2022). Applying bacterial suspension is a good supplement to water-based and granulated green preparations and can be applied to pesticides with poor stability in water or difficult granulation. As vegetable oil is used as the carrier, it has good affinity to the target and can play a better role in drug efficacy; the production process is relatively simple and is basically the same as that of a water suspension agent. In application, it is not as demanding as water-based agents as far as auxiliaries, and it is basically unnecessary to add other synergists. It is especially suitable for a variety of spray preparations, such as low-volume spray preparations and ultralow-volume spray preparations. Ye et al. (2018) tested the control effect of Trichoderma Tr309T as an applying bacterial suspension on kiwifruit bacterial canker through liquid culturing and dosage form treatment and found that its disease index and control effect on kiwifruit plants were significantly higher than those of chemical treatment. Xie et al. (2017) developed a Trichoderma applying bacterial suspension: sodium lauroyl glutamate 2%, aluminum magnesium silicate 1%, soybean oil 77%, and highly concentrated Tr309T culturing solution 20%. The field test results showed that the ulcer tissue healing rate of kiwifruit disease plants treated with Trichoderma applying bacterial suspension and the recurrence rate of the next year were significantly higher and lower than other treatments, respectively. At present, there are very few various of vegetable applying bacterial suspension in China, which is not commensurate with their practical application value. Therefore, it is very beneficial to vigorously develop vegetable applying bacterial suspension, and the market prospects are quite broad.
This study aimed to separate and screen antagonistic bacteria with good inhibitory effects on pathogenic fungus from healthy leaves, rhizosphere soil, and stems of D. latiflorus, identify them, optimize the culturing medium and culturing conditions of antagonistic bacteria, screen carrier additives, and develop new biological agents. Combined with pot trials to determine the effectiveness of biological control, economic losses can be reduced. At the same time, it provides a reference for the biological control of brown rot of D. latiflorus and lays the foundation for further trials, which are important for the control of this disease.
The pathogen Diaporthe guangxinsis (ITS [internal transcribed spacer]: GenBank registration number: MW380383; CAL [calmodulin]: MW43318; TEF [translation elongation factor 1-╬▒] gene: MW43317; TUB [╬▓-tubulin]: MW43316) was obtained from the diseased tissue of D. latiflorus in Yibin City, Sichuan Province, China (28┬░45ŌĆ▓N, 104┬░72ŌĆ▓E) and purified by a single-spore isolation method (Wei et al., 2021). Healthy bamboo stumps, 4-year-old, 20-30 cm long seedlings, and 10 cm long bamboo branches, were planted in a shed at Sichuan Agricultural University, China (temperature: 20┬░C, relative humidity: 45%).
The nutrient agar (NA) culture medium consisted of 3 g beef extract, 10 g peptone, 5 g sodium chloride, 20 g agar, and 1,000 ml water; the nutritional broth (NB) medium consisted of 3 g beef extract, 10 g peptone, 5 g sodium chloride, 1,000 ml water; and the potato glucose agar (PDA) medium consisted of 200 g potato, 10 g glucose, 20 g agar, and 1,000 ml water. NA and NB media for the cultivation of antagonistic bacteria and PDA media for the cultivation of pathogenic fungi.
In the modern agricultural research and development base of Sichuan Agricultural University in Chongzhou, Sichuan Province (30┬░70ŌĆ▓N, 103┬░86ŌĆ▓E), fresh bamboo stalks (5 g), leaves (10 g), and rhizosphere soil (10 g) of healthy D. latiflorus were collected by using the five-point sampling method (Yang et al., 2020), and the samples were packed in plastic bags and brought to the lab for analysis.
Ten grams of rhizosphere soil was placed in a 250 ml conical flask filled with 90 ml sterile water and shaken it well to prepare a 10ŌłÆ1 diluent. Soil sample was diluted to 10ŌłÆ2, 10ŌłÆ3, 10ŌłÆ4, 10ŌłÆ5, 10ŌłÆ6, and 10ŌłÆ7, and 100 ╬╝l was aspirated from the above diluents. The dilutions were applied to NA medium and then incubated upside down in a 37┬░C incubator for 24 h (Slama et al., 2019). Each sample was repeated three times. A sterile inoculation loop was used to pick up a single colony with an obvious difference in colony morphology, and the purified strains were then isolated by plate streaking. The purified strain was inoculated on NA slant medium, and the strain was numbered, incubated at 30┬░C for 48 h and then stored in a 4┬░C refrigerator.
Five grams of the collected healthy leaves and stems, respectively, were sterilized in 75% absolute ethanol and 3% NaClO for 30 s, rinsed with sterile water three times, aspirated with sterile filter paper, ground in a mortar, placed into a 250 ml conical flask with 45 ml sterile water, and diluted to 10ŌłÆ1. The solution was diluted to 10ŌłÆ2, 10ŌłÆ3, 10ŌłÆ4, 10ŌłÆ5, 10ŌłÆ6, and 10ŌłÆ7, and 100 ╬╝l was drawn from the above, respectively. A sterilized coater was evenly adhered to the beef extract peptone agar medium plate, and placed upside down in 37┬░C incubators for cultivation for 24 h. This process was repeated 3 times per treatment. A sterilized inoculation loop was used to pick up a single colony with an obvious difference in colony morphology, and then a streak culture was made on NA medium to obtain the purified strain. The purified strain was inoculated on NA slant medium, and the strain was numbered, incubated at 30┬░C for 48 h, and then stored in a 4┬░C refrigerator.
The antagonistic bacteria were scraped with a sterile inoculation loop and incubated in NA medium for 48 h at 25┬░C.
PDA plate confrontation tests were used to screen the antagonistic bacteria (Boukaew et al., 2017). The pathogenic fungus cake was placed on one side of the PDA culture medium, a cultured single colony of antagonistic bacteria was picked up with a sterilized inoculation loop, a line was drawn on the other side of the PDA culture medium at a distance of 3 cm from the fungus cake, and a blank control was conducted as follows: only the pathogenic fungus was inoculated in the PDA culture medium and not the antagonistic bacteria. The inoculated PDA plates were incubated at 25┬░C for 7 days. It was observed and recorded whether there was an antifungal zone, the width of the antifungal zone (the distance from the center of the bacterial colony to the edge of pathogenic mycelium) in the culture medium was measured, and the strains with antagonistic effects were screened.
Morphological identification, physiological and biochemical characteristics analysis, and molecular biological identification were carried out (Claus, 1992). Strains of antagonistic bacteria were inoculated on NA medium and incubated at 28┬░C for 48 h. The cultural characteristics and macromorphological characteristics of the antagonistic bacteria were visually observed: the color of the bacteria and indication of whether there were wrinkles, texture, edge grooves, or biofilms. After Gram staining, the micromorphological characteristics of the antagonistic bacteria, such as the cell shape, size, and structure, were observed under a light microscope.
The stored bacteria were inoculated in NA culture medium and incubated at 28┬░C for 2 days. A DNA extraction kit was used to extract the DNA of the antagonistic bacteria according to the manufacturerŌĆÖs instructions. The extracted genomic DNA of the tested strain was used as the template, and the bacterial universal primers 16S rDNA (Nurhayati, 2019) and gyrB (Ili─Źi─ć et al., 2021) was used to amplify the genomic DNA of the tested strains. DNA markers and polymerase chain reaction (PCR) products (5 ╬╝l) were taken. Electrophoretic detection using 1% agarose gel, and the voltage, current and time for electrophoresis were set at 120 V, 120 mA, and 25 min, respectively. After electrophoresis, the gel was removed and placed in a gel imaging system to observe the size of the PCR product fragments by UV, and scan for an electrophoretogram. After agarose gel electrophoresis, the PCR products of the antagonistic bacteria were sent to Chengdu Qingke Biotechnology Co., Ltd. (Chengdu, China) for sequencing. The gene sequences were aligned in the National Center for Biotechnology Information (NCBI) database, and the phylogenetic tree was constructed using MEGA software was used to manually approve and delete the sequence as necessary, then PhyloSuite was used to jointly build a tree with multiple genes, and Figtree software was used to view and beautify the tree after generating a phylogenetic tree.
Gram staining and catalase (Thomas et al., 2006) tests were performed on the bacteria, including the gelatine hydrolysis test, citrate utilization test, and starch hydrolysis test (Smibert and Krieg, 1994).
OD600 was used to determine the antagonistic bacterial biomass. The biocontrol bacterial culturing solution was used, and sterile liquid culture medium was used as the control to detect the absorbance value at 600 nm, which was recorded as OD600 (Storek et al., 2019).
The Oxford cup method was used to test the antifungal effect of the antagonistic bacteria (He et al., 2018). On an ultraclean workbench, the Oxford cup was placed in a sterile PDA culture dish prepared in advance (Oxford cup diameter: 7 mm, culture dish diameter: 12 cm), and an adjustable pipette was used to transfer 120 ╬╝l of sample to the Oxford cup. At the same time, the center of the plate was inoculated with pathogenic fungi. Sterile water was added to the control group, the procedure was repeated three times, and the culture was conducted at 25┬░C. The diameter of the inhibition ring was measured when the mycelia of the control group were full of the culture medium.
To select the carbon source, NB medium was used as the initial medium with a 2% carbon source. The effects of seven common carbon sources (glucose, malt dust, sucrose, lactose, citric acid, and sodium acetate) on the bacterial biomass and inhibition rate were compared. The experiment was designed by shaking flask culturing. A 300 ml conical flask (including 100 ml culture medium) was selected, and the process was repeated three times for each carbon source. After 24 h of incubation at 28┬░C and 170 r/min, the inhibition rate and OD600 value were determined.
For nitrogen source screening, the carbon source was first fixed, and seven common nitrogen sources, namely, peptone, tryptone, ammonium sulfate, urea, beef paste, yeast extract, and ammonium chloride, were compared at 1% of the added amount. The inhibition rate and OD600 value were measured after shaking flask culturing, which was repeated three times for each nitrogen source.
Inorganic salt screening was performed. First, the carbon and nitrogen source were fixed after initial screening, and 8 common organic salts (KCl, NaCl, KH2PO4┬Ę3H2O, MgSO4┬Ę7H2O, CaCl2, ZnSO4┬Ę7H2O, Ca(NO3)2┬Ę4H2O, CaCO3) were added at 0.5% of the amount, and a group without inorganic salt was tested as the control group. After shaking the flask culturing, the inhibition rate and OD600 value were measured, and each inorganic salt was tested three times.
Taking the carbon source, nitrogen source, and inorganic salt preliminarily screened by the single-factor tests as factors, four levels were set, and an orthogonal table was designed to test the influence of each factor content on the antifungal activity of the antagonistic bacteria. An orthogonal test design (L16 (43)) was conducted for the initially determined carbon source, nitrogen source, and inorganic salt (Crijns et al., 2006).
The optimal pH values of the culturing solution of the biocontrol strain were adjusted to 4, 5, 6, 7, 8, 9, and 10 with 1 mol/l NaOH and 1 mol/l acetic acid. The original pH value was used as the control group to detect the OD600 values and calculate the inhibition rate. Each treatment was repeated three times to determine the optimal pH value.
In the optimal medium, 5% of the inoculum was cultivated in a shaker at 28┬░C and 170 r/min. The culturing solution was extracted every 6 h up to 72 h, the OD600 value was detected, and the inhibition rate was calculated. The treatment was repeated three times to determine the optimal culturing time.
After screening for the optimal culturing medium, the culturing solution was subjected to a 30-min water bath at temperatures of 20┬░C, 25┬░C, 30┬░C, 35┬░C, 40┬░C, 45┬░C, and 50┬░C. Untreated culturing solution was used as a control. The OD600 values were measured, and the antibacterial activity was calculated. Each treatment was repeated three times to select the optimal culturing temperature.
The biocontrol bacteria culturing solution was inoculated at 5%, 10%, 15%, 20%, 25%, 30%, 35%, 40%, 45%, and 50% into the culture medium and incubated in incubator shaker at 28┬░C and 170 rpm/min for 24 h. The OD600 values were detected, and the inhibition rate was calculated. The treatments were repeated three times each time to determine the best additional amount.
The selected culturing medium composition and culture conditions were used to optimize the culturing and culture of the strains. The original culturing conditions were used as the control to compare the size of the inhibition zone of the antifungal active substances against the bacteria.
The culturing liquid of antagonistic bacteria was concentrated as follows: the antagonistic bacteria was inoculated on NB medium, and shaken at 28┬░C at 170 r/min for 72 h to prepare a spore suspension of the antagonistic bacteria. The content of the spore suspensions was prepared (1 ├Ś 1012 cfu/ml). The culturing spore solution was then placed at 4┬░C and left for 60 h. The supernatant was discarded and 200 ml of concentrated precipitate was reserved.
The emulsifier with the best compatibility with the antagonistic bacteria was selected by testing the compatibility of five emulsifiers, namely, sodium dodecyl benzene sulfonate, sodium dodecyl sulfate, sodium lauroyl glutamate, alkyl phenol polyoxyethylene ether sodium carboxylate, cetyltrimethylammonium bromide at a mass percentage of 2%.
The selected reagents were added to NB, sterilized at 121┬░C for 30 min, cooled to the temperature, and poured into culture dishes, and the NB culture medium without additives was used as the blank control. The culturing liquid of the antagonistic bacteria was inoculated into the above culture media at a volume percentage of 5%. Each treatment had three parallels. After 24 h of culturing in a shake flask, the number of colonies was measured with the plate colony count method (Rizvi et al., 2014), and the inhibition rate was calculated according to the following formula.
Soybean oil as a solvent and emulsifier were mixed in accordance with the content of 2%, 3%, 4%, 5%, 6%. Then, 0.5 ml of the mixed solution was placed into a measuring cylinder with a stopper containing 250 ml of standard hard water, and its dispersion was observed. After shaking well, the emulsification was observed before placing it into a 30┬░C bath for 1 h. The emulsification dispersion stability observation was continued to select the optimal emulsifier and the optimal content.
The compatibility of 8 selected thickeners, including organic bentonite, attapulgite, magnesium aluminum silicate, sodium carboxymethyl cellulose, xanthan gum, hydroxypropyl methylcellulose, sodium polyacrylate, and J0602, was tested at 1% mass percentage, and the thickener with the best compatibility with antagonistic bacteria was selected. Then, 1%, 2%, 3%, 4%, and 5% of the selected thickeners were added to the oil suspension, a high-speed dispersion homogenizer was used to emulsify and disperse the samples for 40 min, and the dumping test and suspension rate detection were conducted after standing for 48 h to select the optimal thickener and the optimal content.
The compatibility of five dispersants, such as sodium lignosulfonate, MgSO4┬Ę7H2O, sodium sulfite, K2HPO4┬Ę3H2O, and NaH2PO4┬Ę2H2O, were tested at a 1% mass percentage, and the dispersant with the best compatibility with the antagonistic bacteria was selected. The solvent was mixed with the selected dispersants at 0.5%, 1%, 1.5%, 2%, and 2.5%, and 0.5 ml of the mixed solution was placed into a measuring cylinder with a stopper containing 250 ml of standard hard water, and its dispersibility was observed to select the optimal dispersant and the optimal content.
After mixing the selected best emulsifier, thickener, and dispersant with the solvent, the prepared concentrated settling liquid (1 ├Ś 1012 cfu/ml) was added, sealed in a bottle, placed at 0┬░C and stored in a 54 ┬▒ 2┬░C incubator for 14 days. The content of effective ingredients before and after storage was analyzed, and the analysis error was ┬▒1%. It was observed whether its appearance showed stratification, fluidity, or dispersion.
Referring to the standards issued by the Collaborative International Pesticides Analytical Council, the living bacteria rate, pH value (Collaborative International Pesticides Analytical Council, 1970a), persistent foaming (Collaborative International Pesticides Analytical Council, 1970b), dispersion stability (Collaborative International Pesticides Analytical Council, 1970c), suspension rate (Collaborative International Pesticides Analytical Council, 1970d), dumping property (Collaborative International Pesticides Analytical Council, 1970e), and storage stability (Collaborative International Pesticides Analytical Council, 1970f) of the oil suspension had been determined.
To determine the effect of antagonistic bacteria on suppression of brown rot, a pot planting control test was conducted in the greenhouse of the Forest Protection Laboratory of Sichuan Agricultural University. Dilutions of 50├Ś, 100├Ś, 200├Ś, 500├Ś, and 1,000├Ś of an applying bacterial suspension containing a suspension of pathogen spores (1 ├Ś 106 cfu/ml) were used as treatment groups, 500├Ś dilutions of a commercially available 24% nitrile phenenazole suspension (Yunnan Michelin Agricultural Technology Co., Ltd.) were used as chemical control and sterile water was used as a blank control for pot control experiments. Each treatment was repeated 10 times. A total of 210 healthy seedlings of D. latiflorus were selected and divided into three groups. The treatments were applied as follows: (1) First, the pathogen liquid was inoculated in the stems and trunks with the acupuncture method, 100 ml per plant; 15 days later, the applying bacterial suspension of different concentrations was sprayed in the same place. (2) Inoculate the oil suspension with different concentrations first, and then the pathogen suspension was applied 15 days later. (3) The pathogenic fungus and applying bacterial suspension of different concentrations were inoculated at the same time. After 30 days, the incidence was observed, and the incidence rate, disease index, and prevention and treatment effects were calculated.
A total of 36 isolates were isolated from healthy rhizosphere soil, leaves, and bamboo stems. The plate confrontation test was carried out on 36 isolates, only one isolate with an antagonistic effect was identified. Compared with the control, the mycelial growth of D. guangxinsis was significantly inhibited. The mycelia near the isolate grew sparsely, and there was an obvious inhibition zone, which was highly antagonistic to the pathogenic fungus. The diameter of the pathogen colony was 40.34 mm, and the inhibition rate was 30.6% (Supplementary Fig. 1A). Combined with the results of the screening test, the isolate had a good effect on D. guangxinsis and showed a strong antagonistic effect. Therefore, this isolate was selected as the target isolate for subsequent experiments, and was recorded as W1 (Supplementary Fig. 1A and B).
After 2 days of culture on NB medium, the isolate was round, white, dry, wrinkled, and generally yellow after aging (Supplementary Fig. 1C). Microscopic observation showed that the isolate was rod-shaped (Supplementary Fig. 1D). The spores varied from elongated or oval. There was no obvious swelling of the sporangia. The physiological and biochemical characteristics of isolate JKB05 are shown in Supplementary Table 1.
After PCR amplification, 1,017 bp (16S RNA) and 1,077 bp (gryB) bands (Supplementary Fig. 1E and F) were observed by 1% agarose gel electrophoresis, and their products were sequenced and identified. The obtained sequences were deposited in GenBank (16S rDNA, OP925891; gyrB, OP937001). The homology between strain JKB05 and Bacillus siamensis was 99% to 100% (Supplementary Fig. 2). Therefore, according to its morphological characteristics, physiological and biochemical characteristics, and sequence alignment of 16S rRNA and gyrB gene, strain W1 was identified as Bacillus siamensis.
As shown in Fig. 1A, sucrose had the highest inhibition rate (48.2%) and OD600 (1.32) values, which were significantly different from those of the other carbon sources. Therefore, sucrose was selected as the best carbon source. The OD600 results of beef paste and yeast extract were similar (1.53 and 1.57, respectively), but the inhibition rate of yeast extract (42.27%) was high, with a significant difference (Fig. 1B). Therefore, yeast extract was selected as the best nitrogen source. The inhibition rate of KCl (48.28%) was the highest compared with OD600 (1.479), and it was significantly different from other inorganic salts (Fig. 1C). Therefore, KCl was selected as the best inorganic salt. According to the above experimental results, sucrose, yeast extract, and KCl were selected for the orthogonal test (L16 (43)). According to the results in Table 1, the optimal culturing medium composition was determined to be 2% sucrose, 1.5% yeast extract, and 0.7% KCl.
As shown in Fig. 2, when the pH was 6, the inhibition rate (36.29%) and OD600 (1.87) reached to maximum values, and both the inhibition rate and OD600 gradually decreased with the increasing pH value, so the optimal pH value was 6. When the cultivation time reached 48 h, the maximum value was achieved (inhibition rate of 40.06%, OD600 of 2.56). Similarly, the maximum value was attained at a cultivation temperature of 30┬░C (inhibition rate of 45.49%, OD600 of 0.88). After 30┬░C, the inhibition rate gradually decreased as the temperature rose, and the OD600 value decreased. When the addition amount of antagonistic bacteria was 5-20%, the inhibition rate and OD600 gradually increased, reaching a stable value (38.59%, OD600 1.28) at 20% addition, and the inhibition rate and OD600 remained stable with increasing addition amount when the addition amount was 20-50%. The above experimental results showed that the optimal pH value was 6, the optimal culturing time was 48 h, the optimal culturing temperature was 30┬░C, and the optimal addition amount was 20%.
The results of the compatibility test between different emulsifiers and antagonistic bacteria are shown in Fig. 3. The inhibition rate (35.96%) and the bacterial content (33.33 ├Ś 107 cfu/ml) of sodium dodecyl benzene sulfonate were the highest. The other emulsifiers showed significantly lower inhibition of mycelial growth and bacterial content than sodium dodecyl benzene sulfonate. The results of the compatibility of different dispersants with the bacterium showed that the bacteriological content of disodium hydrogen phosphate dihydrate and potassium hydrogen phosphate trihydrate was significantly higher than the other dispersants when they were used as dispersants (3.87 ├Ś 107 cfu/ml and 3.6 ├Ś 107 cfu/ml, respectively). However, when disodium hydrogen phosphate dihydrate was used as dispersant, the inhibition rate of mycelial growth of pathogenic bacteria was 35.2%, which was significantly higher than that of potassium hydrogen phosphate trihydrate, therefore, disodium hydrogen phosphate dihydrate was the best dispersant. The compatibility test results of different thickeners and the antagonistic bacteria showed that the inhibition rate (25.8%) and bacterial content (8.5 ├Ś 108) of hydroxypropyl methylcellulose were the highest and were significantly different from other thickeners. From the above data, it can be concluded that the best emulsifier of the applying bacterial suspension is sodium dodecyl benzene sulfonate, the best dispersant is Na2HPO4┬Ę2H2O, and the best thickener is hydroxypropyl methylcellulose.
The results of the optimum content of the emulsifier are shown in Table 2. When the content of sodium dodecyl benzene sulfonate was 4-10%, the emulsification was poor, and there was an oil slick at room temperature. After holding at 30┬░C for 1 h, the emulsification was still poor and there was an oil slick, but with 10% emulsifier, the lotion turned white. When the emulsifier content was 12%, the lotion turned white with a small amount of oil slicks at a constant temperature. After holding at 30┬░C for 1 h, the lotion was white without an oil slick. When the content of the emulsifier was 14%, the emulsification effect was best at room temperature and after 1 h at 30┬░C, and the lotion was white without floating oil (Fig. 4A and C). Therefore, the optimum content of the emulsifier sodium dodecyl benzene sulfonate is 14%.
Meanwhile, the optimum content of dispersant is shown in Table 2. When the content of dispersant was 0.5-2.5%, the automatic dispersibility of the reagent was poor at room temperature and after 1 h at 30┬░C, and there was oil floating. When the content of the dispersant was 3%, the automatic dispersion of the reagent was good at room temperature, but there was a small amount of oil floating out. After holding at 30┬░C for 1 h, there was a large amount of oil floating. When the content of the dispersant was 4% under normal temperature and after 1 h at 30┬░C, the automatic dispersion of the reagent was good, and there was no floating oil (Fig. 4B and D). Therefore, the optimum content of dispersant Na2HPO4┬Ę2H2O is 4%.
Moreover, the optimum content of the thickener were shown in Table 2. When the content of thickener was 0.1-0.2%, the reagent had good fluidity without precipitation, but there was oil precipitation. When the content of thickener was 0.3-0.4%, the reagent had no precipitation, no oil strain, and good fluidity. When the content of the thickener was 0.5%, the reagent began to precipitate, and stratification occurred after standing (Fig. 4E). Based on the above results and considering the economic situation, the optimum content of the thickener hydroxypropyl methylcellulose is 0.3%.
All testing standards of applying bacterial suspension were in accordance with standards of the Collaborative International Pesticides Analytical Council. The test results are shown in Table 3. The viable rate of the applying bacterial suspension was 92.38%, the pH value was 5.5, the persistent foaming property was 2.98, the suspension rate was 91.197%, the residue after dumping in the dumping test was 2.78%, the residue after washing was 0.098%, the stability was qualified, and it met the standards of the Collaborative International Pesticides Analytical Council.
The pot experiment results presented in Table 4, the ability of the antagonistic bacteria to inhibit pathogens decreased with increasing dilution. The control effects of 50-fold, 100-fold, 500-fold, and 1,000-fold dilution were greater than 50%. The 50-fold dilution was the most effective for controlling brown rot in D. latiflorus, and the highest control amount observed was 94.23%. The control effect of the Bacillus siamensis oil suspension diluted 1,000├Ś was better than that of 24% nitrile phenenazole suspension diluted 500├Ś, but suspension diluted 2,000├Ś is less effective than 24% nitrile phenenazole suspension 500├Ś. Therefore, a concentration of 1,000 times is recommended to provide cost-effective control.
The application of biological control technology can significantly reduce the use of chemical pesticides, avoid threats to the development of agriculture and forestry due to serious pests and diseases, and ensure safe development. Paying attention to the development of biological control technology is a critical and urgent task to promote the development of agriculture and forestry. In the future development process, biological control technology is bound to become one of the main technologies of sustainable agricultural economic development and occupy a very important and irreplaceable position (Akutsu, 2006).
Because of the rich variety of microorganisms in the soil, it has become the first choice for researchers to isolate biocontrol microorganisms (Kurm et al., 2018). In addition to the isolation of antagonistic bacteria from soil, current research shows that there are also antagonistic bacteria with strong vitality in plants (Dong et al., 2019). In this study, antagonistic bacteria were isolated from the rhizosphere soil, leaves, and stems of healthy D. latiflorus, and only one Bacillus strain was isolated from the soil, which had an antagonistic effect on the brown rot in D. latiflorus, proving that the rhizosphere soil of D. latiflorus was an important source of biocontrol microorganisms against the disease.
Yuan (2022) found that the endophytic antagonistic bacteria of Phyllostachys pubescens have antagonistic effects on three kinds of edible fungi. Zhang et al. (2022) found that saponin culturing had a field control effect of 77.86% on the disease of Dictyophora rubescens, which could effectively control the disease of D. rubescens and promote the growth of D. rubescens eggs. The previous studies on biocontrol bacteria for bamboo diseases are different from B. siamensis obtained in this study, probably because the control objects and screening sources are different. Furthermore, B. siamensis can also effectively control melon fusarium wilt, postharvest pear ring rot and soft rot, peanut crown rot, and peanut white silk disease. Jiang et al. (2022) found that B. siamensis treatment may act to induce disease resistance in mango fruit by affecting multiple pathways. Yoo et al. (2020) found that Chryseobacterium soldanellicola T16E-39 and B. siamensis T20E-257 play an important role in inhibiting two kinds of soil-borne diseases and inducing growth. Both can be used as biological control agents and biofertilizers in sustainable agriculture. Combined with the results of this study, it can be seen that B. siamensis has good application prospects in plant disease control.
In modern bacterial classification, 16S rRNA gene sequence analysis is a standard method to distinguish bacterial species, which can effectively distinguish populations with distant genetic relationships. However, its detection in related species has limitations, which makes it difficult to distinguish related species of some populations. At the same time, a series of protein-coding genes, such as gyrB, have higher mutation rates, which can make up for the shortcomings of using 16S rRNA gene identification alone. Thus, bacterial taxa were successfully distinguished (Yamamoto and Haramaya, 1995), consistent with the results of this study. In this study, homology comparison of the 16S rRNA sequences showed that the percentage of similarity between strain W1 and the four kinds of Bacillus was more than 99%, and the strains to be tested could only be temporarily identified to genera, but could not be effectively identified at the species level. On this basis, the homology comparison of the gyrB gene sequence showed that strain W1 only had a similarity rate of more than 99% with B. siamensis, and had a closer genetic relationship.
On the basis of a single-factor experiment, the culturing medium and culturing conditions of B. siamensis were optimized. Gu et al. (2022) determined that the best medium group for B. amyloliquefaciens is sucrose (19.65 g/l), tryptone (2.73 g/l), NaCl (20.71 g/l), and yeast extract (13.00 g/l), with a pH value of 6-8. The optimal culturing conditions were a temperature of 25┬░C, inoculation amount of 2%, rotational speed of 150 r/min, and liquid volume 75 ml. These differ from the culturing conditions determined in this study, possibly because the biological characteristics of different bacterial species vary. An applying bacterial suspension is a highly competitive pesticide formulation with stable performance and good adhesion, which facilitates targeting, and has no dust or pollution in the production and uses low processing cost, moderate price, and broad application prospects (Qin et al., 2010). Previous studies on the formulation of applying bacterial suspension are different, and most of them examine chemical reagents. Wang et al. (2021) used a 30% propiconazole dispersible applying bacterial suspension to control wheat scab by more than 90%. There are only a few studies on applying bacterial suspension with beneficial microorganisms as active ingredients. Mbarga et al. (2014) found that a T. echinococcus soybean oil-based suspension agent can effectively inhibit the spread of disease. In this experiment, the biocompatibility and inhibition rate of pathogenic bacteria were used as screening indicators to screen the applying bacterial suspension additives and determine the best formula for the applying bacterial suspension. All detection indicators met the relevant international standards. The prepared suspension agent of B. siamensis oil is used for biological control, which can reduce environmental pollution and effectively reduce the amount of residual toxin and is safe for people and livestock.
The use of biocontrol bacteria to control plant diseases is an effective measure for plant disease control. However, there are few studies on microbial pesticides, and the formulation of microbial pesticides is the key to the successful commercialization of microbial pesticides. This study found that B. siamensis could be used to effectively control brown rot in D. latiflorus, and the control effect of the disease was significantly improved by optimizing the culturing medium and culturing conditions. A pot experiment showed that the control effect of B. siamensis applying bacterial suspension on brown rot in D. latiflorus was better than that of the control group (24%).
Acknowledgments
This research was supported by the National Natural Science Foundation of China (grant number 32171795) and the Sichuan Natural Science Foundation for Distinguished Young Scholar (grant number 2023NSFSC1936), and the Young Talents of Sichuan Tianfu Qingcheng Program.
Electronic Supplementary Material
Supplementary materials are available at The Plant Pathology Journal website (http://www.ppjonline.org/).
Fig.┬Ā1
Optimization of the culturing media of Bacillus siamensis W1 strain. (A) Screening results of carbon sources. (B) Screening results of nitrogen sources. (C) Screening results of inorganic salts. Different lowercase letters represent significant differences at 0.05 level; the error bar represents the uncertainty of the measured value. AC, ammonium chloride; AS, ammonium sulfate; CA, citric acid; SA, sodium acetate; YE, yeast extract.

Fig.┬Ā2
Optimization of the culturing conditions of Bacillus siamensis W1 strain. (A) Screening results of pH. (B) Screening results of temperature (┬░C). (C) Screening results of the vaccination time (h). (D) Screening results of the inoculation amount. Different lowercase letters represent significant differences at the 0.05 level; the error bar represents the uncertainty of the measured value.
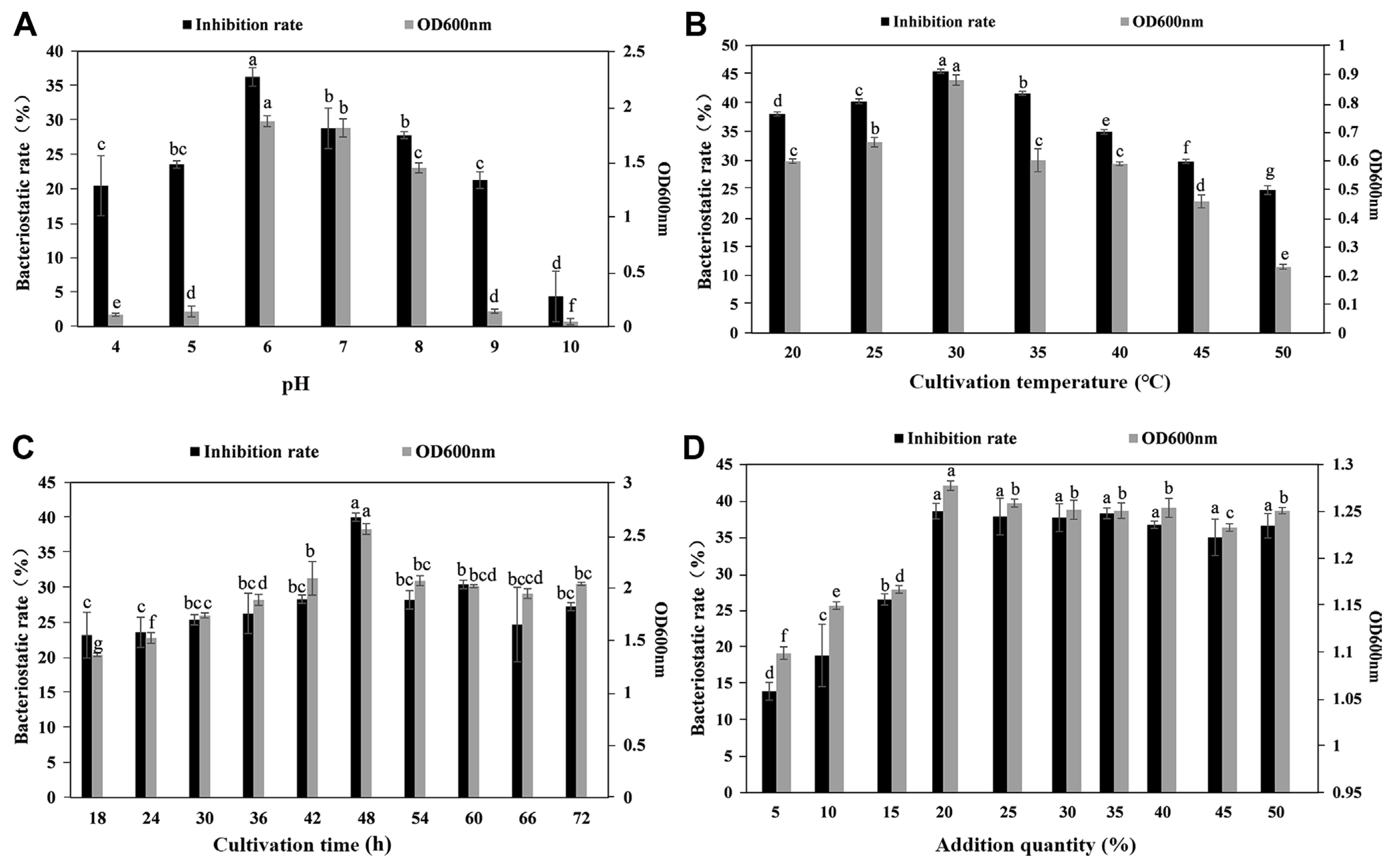
Fig.┬Ā3
Optimization of the additives of Bacillus siamensis W1 strain. (A) Screening results of emulsifiers. (B) Screening results of dispersants. (C) Screening results of thickeners. Different lowercase letters represent significant differences at the 0.05 level; the error bar represents the uncertainty of the measured value. APEC-Na, alkyl phenol polyoxyethylene ether sodium carboxylate; CB, cetyltrimethylammonium bromide; HMC, hydroxypropyl methylcellulose; MAS, magnesium aluminum silicate; OB, organic bentonite; SCC, sodium carboxymethyl cellulose; SDBS, sodium dodecyl benzene sulfonate; SDS, sodium dodecyl sulfate; SI, sodium lignosulfonate; SLG, sodium lauroyl glutamate; SS, sodium sulfite; SP, sodium polyacrylate; XG, xanthan gum.

Fig.┬Ā4
Forms of the optimum additivesŌĆÖ contents of Bacillus siamensis W1 strain. (A) Reagent state with 14% emulsifier content at room temperature. (B) Reagent state with 4% dispersant content at room temperature. (C) Reagent state with 14% emulsifier content after holding at 30┬░C for 1 h. (D) Reagent state with 4% dispersant content after holding at 30┬░C for 1 h. (E) Reagent state with 0.3% thickener content at room temperature.

Table┬Ā1
Orthogonal test of the culturing medium of Bacillus siamensis W1 strain (L16 (43))
| No. | Experimental factor | OD600 | Inhibition rate (%) | ||
|---|---|---|---|---|---|
|
|
|||||
| Sucrose (%) | Yeast extract (%) | KCl (%) | |||
| 1 | 0.50 | 0.50 | 0.10 | 1.64 ┬▒ 0.02 ga | 15.49 ┬▒ 1.01 c |
| 2 | 0.50 | 1.00 | 0.30 | 1.34 ┬▒ 0.04 j | 21.12 ┬▒ 1.66 c |
| 3 | 0.50 | 1.50 | 0.50 | 1.38 ┬▒ 0.51 i | 23.90 ┬▒ 0.21 c |
| 4 | 0.50 | 2.00 | 0.70 | 1.32 ┬▒ 0.01 j | 25.39 ┬▒ 0.63 c |
| 5 | 1.00 | 0.50 | 0.70 | 1.45 ┬▒ 0.01 h | 26.00 ┬▒ 1.60 c |
| 6 | 1.00 | 1.00 | 0.10 | 1.33 ┬▒ 0.02 j | 25.21 ┬▒ 0.41 c |
| 7 | 1.00 | 1.50 | 0.30 | 1.69 ┬▒ 0.01 f | 28.85 ┬▒ 1.34 b |
| 8 | 1.00 | 2.00 | 0.50 | 1.64 ┬▒ 0.01 g | 29.34 ┬▒ 0.87 b |
| 9 | 1.50 | 0.50 | 0.50 | 2.00 ┬▒ 0.01 e | 27.13 ┬▒ 0.98 b |
| 10 | 1.50 | 1.00 | 0.70 | 2.08 ┬▒ 0.01 d | 26.89 ┬▒ 1.36 b |
| 11 | 1.50 | 1.50 | 0.10 | 2.14 ┬▒ 0.01 b | 28.19 ┬▒ 1.36 b |
| 12 | 1.50 | 2.00 | 0.30 | 1.99 ┬▒ 0.01 e | 27.48 ┬▒ 1.46 b |
| 13 | 2.00 | 0.50 | 0.30 | 1.98 ┬▒ 0.01 e | 18.81 ┬▒ 0.67 c |
| 14 | 2.00 | 1.00 | 0.50 | 2.12 ┬▒ 0.01 bc | 27.60 ┬▒ 2.42 b |
| 15 | 2.00 | 1.50 | 0.70 | 2.38 ┬▒ 0.01 a | 32.91 ┬▒ 1.12 a |
| 16 | 2.00 | 2.00 | 0.10 | 2.09 ┬▒ 0.01 cd | 32.62 ┬▒ 1.53 a |
Table┬Ā2
Screening of the optimum additivesŌĆÖ contents of Bacillus siamensis W1 strain
Table┬Ā3
Quality inspection of Bacillus siamensis W1 applying bacterial suspension
Table┬Ā4
Control effects of Bacillus siamensis W1 applying bacterial suspension on brown rot in Dendrocalamus latiflorus
| Treatmenta | Pathogenic fungus first | Biocontrol bacteria first | Simultaneous inoculation | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| IR (%) | DI | CE (%) | IR (%) | DI | CE (%) | IR (%) | DI | CE (%) | |
| 50 | 26.67 ┬▒ 4.71 db | 20 ┬▒ 2.72 d | 70.54 ┬▒ 4 a | 6.67 ┬▒ 4.71 e | 3.33 ┬▒ 2.72 e | 94.23 ┬▒ 4.71 a | 13. 33 ┬▒ 4.71 e | 8.89 ┬▒ 1.56 d | 86.20 ┬▒ 2.44 a |
| 100 | 30 ┬▒ 8.16 cd | 21.11 ┬▒ 1.57 d | 68.91 ┬▒ 2.31 a | 13.33 ┬▒ 4.71 de | 6.67 ┬▒ 2.72 e | 88.46 ┬▒ 4.71 a | 16. 67 ┬▒ 4.71 de | 12.22 ┬▒ 3.14 d | 81.03 ┬▒ 4.88 a |
| 200 | 33.33 ┬▒ 4.71 bcd | 22.22 ┬▒ 4.16 d | 67.27 ┬▒ 6.12 a | 20 ┬▒ 8.16 cd | 11.11 ┬▒ 3.13 de | 80.77 ┬▒ 5.43 ab | 20 ┬▒ 8.16 cde | 15.56 ┬▒ 4.16 cd | 75.86 ┬▒ 6.45 ab |
| 500 | 36.67 ┬▒ 4.71 bcd | 32.22 ┬▒ 1.57 c | 52.55 ┬▒ 2.31 b | 26.67 ┬▒ 4.71 bc | 18.89 ┬▒ 3.13 cd | 67.30 ┬▒ 5.43 bc | 23.33 ┬▒ 4.71 cde | 22.22 ┬▒ 5.66 bc | 65.51 ┬▒ 8.78 bc |
| 1,000 | 40 ┬▒ 8.16 bcd | 36.67 ┬▒ 3.14 c | 54.18 ┬▒ 4.62 b | 30 ┬▒ 0 bc | 23.33 ┬▒ 2.72 bc | 59.61 ┬▒ 4.73 cd | 26.67 ┬▒ 4.71 cd | 23.33 ┬▒ 2.72 bc | 63.79 ┬▒ 4.23 bc |
| 2,000 | 43.33 ┬▒ 4.71 b | 40 ┬▒ 2.72 b | 41.09 ┬▒ 4 c | 36.67 ┬▒ 4.71 b | 26.67 ┬▒ 7.2 b | 48.07 ┬▒ 12.5 cd | 40 ┬▒ 8.16 b | 28.89 ┬▒ 4.15 b | 51.18 ┬▒ 6.45 c |
| CK | 96.33 ┬▒ 4.71 a | 67.9 ┬▒ 3.24 a | - | 86.67 ┬▒ 4.71 a | 57.77 ┬▒ 4.16 a | - | 90 ┬▒ 0 a | 64.44 ┬▒ 4.15 a | - |
| G | 46.67 ┬▒ 4.71 bc | 31.11 ┬▒ 2.72 bc | 46 ┬▒ 4.61 c | 33.33 ┬▒ 4.71 b | 30 ┬▒ 5.44 bc | 53.84 ┬▒ 9.42 d | 33.33 ┬▒ 0 bc | 27.78 ┬▒ 4.16 b | 56.9 ┬▒ 4.45 c |
References
Akutsu, K. 2006. Studies into biocontrol technology and development of genetic control technology for plant diseases. J. Gen. Plant Pathol. 72:396-399.


Ali, J., Ali, F., Ahmad, I., Rafique, M., Munis, M. F. H., Hassan, S. W., Sultan, T., Iftikhar, M. and Chaudhary, H. J. 2021. Mechanistic elucidation of germination potential and growth of Sesbania sesban seedlings with Bacillus anthracis PM21 under heavy metals stress: an in vitro study. Ecotoxicol. Environ. Saf. 208:111769.


Bekele, K. B. 2022. Potential biocontrol agents diversity for coffee leaf rust Hemileia vastatrix from Southwesten Ethiopia. Am. J. Life Sci. 10:45-52.
Boukaew, S., Prasertsan, P., Troulet, C. and Bardin, M. 2017. Biological control of tomato gray mold caused by Botrytis cinerea by using Streptomyces spp. BioControl 62:793-803.


Chowdhury, S. P., Hartmann, A., Gao, X. and Borriss, R. 2015. Biocontrol mechanism by root-associated Bacillus amyloliquefaciens FZB42: a review. Front. Microbiol. 6:780.



Collaborative International Pesticides Analytical Council 1970a MT 75 - Determination of pH Values (revised method) URL https://www.cipac.org/index.php/component/content/article/329-mt-75-determination-of-ph-values?catid=2:uncategorised&Itemid=210 [30 June 2023].
Collaborative International Pesticides Analytical Council 1970b MT 47 - Persistent foaming URL https://www.cipac.org/index.php/component/content/article/274-mt-47-persistent-foaming?catid=2:uncategorised&Itemid=210 [30 June 2023].
Collaborative International Pesticides Analytical Council 1970c MT 160 - Spontaneity of dispersion of suspension concentrates URL https://www.cipac.org/index.php/component/content/article/150-mt-160-spontaneity-of-dispersion-of-suspension-concentrates?catid=2:uncategorised&Itemid=210 [30 June 2023].
Collaborative International Pesticides Analytical Council 1970d MT 161 - Suspemsibility of aqueous suspension concentrates URL https://www.cipac.org/index.php/component/content/article/137-mt-161-suspemsibility-of-aqueous-suspension-concentrates?catid=2:uncategorised&Itemid=210 [30 June 2023].
Collaborative International Pesticides Analytical Council 1970e MT 148 - Pourability of suspensionconcentrates: MT 148.1 - Pourability of suspension concentrates (revised method) URL https://www.cipac.org/index.php/component/content/article/190-mt-148-pourability-of-suspension-concentrates?catid=2:uncategorised&Itemid=210 [30 June 2023].
Collaborative International Pesticides Analytical Council 1970f MT 46.3 - Accelerated storage procedure URL https://www.cipac.org/index.php/component/content/article/37-mt-46-4-accelerated-storage-procedure?catid=2:uncategorised&Itemid=210 [30 June 2023].
Crijns, A. P. G., Gerbens, F., Plantinga, A. E. D., Meersma, G. J., de Jong, S., Hofstra, R. M. W., de Vries, E. G. E., van der Zee, A. G. J., de Bock, G. H. and te Meerman, G. J. 2006. A biological question and a balanced (orthogonal) design: the ingredients to efficiently analyze two-color microarrays with confirmatory factor analysis. BMC Genomics 7:232.




Dey, S., Biswas, S., Kundu, A. and Pal, A. 2023. Current understanding on major bamboo diseases, pathogenicity, and resistance genes. In: Genetics, genomics and breeding of bamboos, eds. by M. Das, L. Ma, A. Pal and C. Kole, pp. 256-278. CRC Press, Boca Raton, FL, USA.

Dong, Y., Li, H., Rong, S., Xu, H., Guan, Y., Zhao, L., Chen, W., He, X., Gao, X., Chen, R., Li, L. and Xu, Z. 2019. Isolation and evaluation of Bacillus amyloliquefaciens Rdx5 as a potential biocontrol agent against Magnaporthe oryzae. Biotechnol. Biotechnol. Equip. 33:408-418.


Feng, F., Chen, X., Wang, Q., Xu, W., Long, L., EL-Masry, G. N., Wan, Q., Yan, H., Cheng, J. and Yu, X. 2020. Use of Bacillus siamensis inoculated biochar to decrease uptake of dibutyl phthalate in leafy vegetables. J. Environ. Manag. 253:109636.

Gao, Z. M., Li, C. L. and Peng, Z. H. 2011. Generation and analysis of expressed sequence tags from a normalized cDNA library of young leaf from Ma bamboo (Dendrocalamus latiflorus Munro). Plant Cell Rep. 30:2045-2057.



Gu, X., Zheng, L., Zhai, Q., Sun, J., He, H., Tang, Y., Liang, S. and Zhang, H. 2022. Optimization of fermentation medium for biocontrol strain Pantoea jilinensis D25 and preparation of its microcapsules. Process Biochem. 121:216-227.

He, Y., Jin, Y., Wang, X., Yao, S., Li, Y., Wu, Q., Ma, G., Cui, F. and Liu, H. 2018. An antimicrobial peptide-loaded gelatin/chitosan nanofibrous membrane fabricated by sequential layer-by-layer electrospinning and electrospraying techniques. Nanomaterials 8:327.



Huang, L. H. and Zhong, H. 2023. Control technology of main diseases and insect pests of bamboos in Liucheng County. South China Agric. 17:149-151 (in Chinese).
Ili─Źi─ć, R., Jelu┼Īi─ć, A., Markovi─ć, S., Bara─ć, G., Bagi, F. and Popovi─ć, T. 2021. Pseudomonas cerasi, the new wild cherry pathogen in Serbia and the potential use of recG helicase in bacterial identification. Ann. Appl. Biol. 180:140-150.
Jiang, Z., Li, R., Tang, Y., Cheng, Z., Qian, M., Li, W. and Shao, Y. 2022. Transcriptome analysis reveals the inducing effect of Bacillus siamensis on disease resistance in postharvest mango fruit. Foods 11:107.



Kurm, V., van den Putten, W. H., Pineda, A. and Hol, W. H. G. 2018. Soil microbial species loss affects plant biomass and survival of an introduced bacterial strain, but not inducible plant defences. Ann. Bot. 2:311-319.



Li, R. J., Zhou, P. Y., Lu, Z. G., Cai, C. and Wu, J. R. 2009. Investigation on diseases and insects of Dendrocalamus latiflorus and methods for its prevention and control in Longchuan County. For. Inventory Plan. 34:95-97 (in Chinese).
Liu, G.-H., Lin, Y.-Z., Lin, N.-Q. and Liu, B. 2011. Study on genus Bacillus classification. Fujian J. Agric. Sci. 26:911-917.
Liu, J., Sui, Y., Wisniewski, M., Droby, S. and Liu, Y. 2013. Review: utilization of antagonistic yeasts to manage postharvest fungal diseases of fruit. Int. J. Food Microbiol. 167:153-160.


Mbarga, J. B., Begoude, B. A. D., Ambang, Z., Meboma, M., Kuate, J., Schiffers, B., Ewbank, W., Dedieu, L. and ten Hoopen, G. M. 2014. A new oil-based formulation of Trichoderma asperellum for the biological control of cacao black pod disease caused by Phytophthora megakarya. Biol. Control 77:15-22.

Mourou, M., Hanani, A., DŌĆÖOnghia, A. M., Davino, S. W., Balestra, G. M. and Valentini, F. 2022. Antagonism and antimicrobial capacity of epiphytic and endophytic bacteria against the phytopathogen Xylella fastidiosa. Agronomy 12:1266.

Nurhayati, 2019. The amylase production on cassava starchand identification of bacteria by 16S rRNA. IOP Conf. Ser. Mater. Sci. Eng. 509:012106.


Pereira, P. M., Santana, F. M. and Van der Sand, S. 2022. Evaluation of Streptomyces spp. strains as potential biocontrol agents for Pyrenophora tritici-repentis. Biocontrol Sci. Technol. 32:1095-1106.

Qin, K., Keeney, F. N., Oliver, M. P., Lindsay, A. D., Zollinger, R. and Dean, S. W. 2010. Assessment of oil dispersion pesticide formulations using rheology and near infrared centrifugation techniques. J. ASTM Int. 7:102884.

Rizvi, R. Z., Wahab, A. and Pirzada, Z. A. 2014. Screening and identification of aquatic bacteriocinogenic Bacillus strains inhibiting clinical methicillin resistant Staphylococcus aureus and vancomycin resistant enterococcus from Pakistan. Asian J. Pharm. Clin. Res. 7:53-56.
Slama, H. B., Cherif-Silini, H., Bouket, A. C., Qader, M., Silini, A., Yahiaoui, B., Alenezi, F. N., Luptakova, L., Triki, M. A., Vallat, A., Oszako, T., Rateb, M. E. and Belbahri, L. 2019. Screening for Fusarium antagonistic bacteria from contrasting niches designated the endophyte Bacillus halotolerans as plant warden against. Fusarium. Front. Microbiol. 9:3236.
Smibert, R. M. and Krieg, N. R. 1994. Phenotypic characterization. In: Methods for general and molecular bacteriology, eds. by P. Gerhardt, R. G. E. Murray, W. A. Wood and N. R. Krieg, pp. 611-654. American Society for Microbiology, Washington, DC, USA.
Storek, K. M., Chan, J., Vij, R., Chiang, N., Lin, Z., Bevers, J., Koth, C. M., Vernes, J.-M., Meng, Y. G., Yin, J., Wallweber, H., Dalmas, O., Shriver, S., Tam, C., Schneider, K., Seshasayee, D., Nakamura, G., Smith, P. A., Payandeh, J., Koerber, J. T., Comps-Agrar, L. and Rutherford, S. T. 2019. Massive antibody discovery used to probe structure-function relationships of the essential outer membrane protein LptD. eLife 8:e46258.


Thomas, V., Herrera-Rimann, K., Blanc, D. S. and Greub, G. 2006. Biodiversity of amoebae and amoeba-resisting bacteria in a hospital water network. Appl. Environ. Microbiol. 72:2428-2438.




Wang, D., Li, Y., Yuan, Y., Chu, D., Cao, J., Sun, G., Ai, Y., Cui, Z., Zhang, Y., Wang, F. and Wang, X. 2022. Identification of non-volatile and volatile organic compounds produced by Bacillus siamensis LZ88 and their antifungal activity against Alternaria alternata. Biol. Control 169:104901.

Wang, D. Y., Xiong, G. Y., Shen, Y. H., Zhang, T. F. and Zhu, Y. C. 2021. The preparation of prothioconazole 30% oil dispersion. World Pestic. 11:26-29 (in Chinese).
Wei, W., Zhang, H., Xie, L., Liu, H., Luo, F., Zhu, T., Han, S., Qiao, T., Liu, Y., Yang, C., Lin, T. and Li, S. 2021. Brown culm rot of Dendrocalamus latiflorus caused by Diaporthe guangxiensis in Sichuan Province, China. Plant Dis. 105:4163.

Xie, J., Ajila, A. M. A., Jin, X. X., Zhang, X. W. and Chen, J. 2017. Development of oil applying bacterial suspensions with Trichoderma sp. Agrochemicals 56:18-22 (in Chinese).
Xie, Z., Li, M., Wang, D., Wang, F., Shen, H., Sun, G., Feng, C., Wang, X., Chen, D. and Sun, X. 2020. Biocontrol efficacy of Bacillus siamensis LZ88 against brown spot disease of tobacco caused by Alternaria alternata. Biol. Control 154:104508.

Yamamoto, S. and Haramaya, S. 1995. PCR amplification and direct sequencing of gyrB genes with universal primers and their application to the detection and taxonomic analysis of Pseudomonas putida strains. Appl. Environ. Microbiol. 61:1104-1109.




Yang, C. L., Xu, X. L. and Liu, Y. G. 2019. First report of bamboo blight disease caused by Arthrinium pseudoparenchymaticum on Dendrocalamus latiflorus in Sichuan, China. Plant Dis. 103:1411-1411.

Yang, L., Gao, W., Wang, W., Zhang, C. and Wang, Y. 2020. First report of stem and root rot of coriander caused by Fusarium equiseti in China. Plant Dis. 105:220.

Ye, N. W., Wang, C. F., Geng, K., Zhang, X. W. and Mao, W. L. 2018. Study on combined application methods of an oil-based suspension with Trichoderma spp. on controlling bacterial canker of kiwi. J. Anhui Agric. Sci. 46:161-164 (in Chinese).
Yoo, S-J, Weon, H-Y, Song, J. and Sang, M. K. 2020. Effects of Chryseobacterium soldanellicola T16E-39 and Bacillus siamensis T20E-257 on biocontrol against phytophthora blight and bacterial wilt and growth promotion in tomato plants. Int. J. Agric. Biol. 23:534-540.
Yuan, Z. S. 2022. The biological control mechanism of endophytic antagonistic bacteria in Phyllostachys edulis on edible fungi diseases. J. Anhui Agric. Sci. 8:126-128 (in Chinese).
Zhang, X., Zhang, X., Hu, X., Xu, H. and Tian, Y. 2022. Effects of Gleditsia sinensis culturing product on disease control and main soil microbial community of Dictyophora rubrovolvata. Mycosystema 41:618-629 (in Chinese).
- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 822 View
- 125 Download
- ORCID iDs
-
Shujiang Li

https://orcid.org/0000-0003-3328-5332 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Supplement1
Supplement1 Print
Print



