Re-identification of Colletotrichum gloeosporioides Species Complex Isolates in Korea and Their Host Plants
Article information
Abstract
The Colletotrichum gloeosporioides species complex includes many phytopathogenic species, causing anthracnose disease on a wide range of host plants and appearing to be globally distributed. Seventy-one Colletotrichum isolates in the complex from different plants and geographic regions in Korea were preserved in the Korean Agricultural Culture Collection (KACC). Most of them had been identified based on hosts and morphological features, this could lead to inaccurate species names. Therefore, the KACC isolates were re-identified using DNA sequence analyses of six loci, comprising internal transcribed spacer, gapdh, chs-1, his3, act, and tub2 in this study. Based on the combined phylogenetic analysis, KACC strains were assigned to 12 known species and three new species candidates. The detected species are C. siamense (n = 20), C. fructicola (n = 19), C. gloeosporioides (n = 9), C. aenigma (n = 5), C. camelliae (n = 3), C. temperatum (n = 3), C. musae (n = 2), C. theobromicola (n = 2), C. viniferum (n = 2), C. alatae (n = 1), C. jiangxiense (n = 1), and C. yulongense (n = 1). Of these, C. jiangxiense, C. temperatum, C. theobromicola and C. yulongense are unrecorded species in Korea. Host plant comparisons showed that 27 fungus-host associations are newly reported in the country. However, plant-fungus interactions need to be investigated by pathogenicity tests.
The Colletotrichum gloeosporioides species complex is the most diverse group in the genus Colletotrichum, causing anthracnose disease in many economically important crops worldwide and also occurring as asymptomatic endophytes in a broad range of host plants (Hyde et al., 2009; Liu et al., 2022; Lu et al., 2004). In Korea, the species in this complex have been reported as destructive diseases on widely cultivated crops such as C. siamense and C. fructicola on apple (Kim et al., 2018a; Park et al., 2018), peach (Lee et al., 2020a, 2020b) and strawberry (Nam et al., 2013, 2022), and C. gloeosporioides on citrus, apple, grape, persimmon and strawberry (Korean Society of Plant Pathology, 2022).
C. gloeosporioides was first introduced by Penzig (1882), and then became a common phytopathogenic fungus with a global distribution. Prior to the availability of DNA sequence data, C. gloeosporioides and other taxa in the genus Colletotrichum were identified based on host species and morphological characteristics. However, micro-morphological features such as the size and shape of conidia, appressoria and setae or teleomorph characters overlapped in many species and these features could change under different growing conditions (Weir et al., 2012). For instance, cylindrical conidia with round ends were typical characteristics of species of the C. gloeosporioides species complex, but these characteristics were also observed in many members in the C. dracaenophilum, C. magnum, and C. orchidearum species complexes, or in some singleton species (Damm et al., 2019; Liu et al., 2022). Cultural characteristics of different strains of a species in the C. gloeosporioides species complex were often significant variations (Weir et al., 2012). On the other hand, most members of the C. gloeosporioides species complex have a broad host range and a plant could be associated with more than one Colletotrichum species (Jayawardena et al., 2021; Johnston, 2000; Johnston et al., 2005). Therefore, the host reference and morphology are insufficient to distinguish species members in this species complex.
A large number of Colletotrichum species have been re-identified based on DNA sequence analyses of multiple loci such as the nuclear ribosomal internal transcribed spacer (ITS) region, glyceraldehyde-3-phosphate dehydrogenase (gapdh), chitin synthase 1 (chs-1), histone-3 (his3), actin (act), and beta-tubulin 2 (tub2) (Cannon et al., 2012; Jayawardena et al., 2016; Liu et al., 2022; Weir et al., 2012). To date, at least 61 accepted species have been introduced in the C. gloeosporioides species complex (Liu et al., 2022; Zhang et al., 2023; Zheng et al., 2022). They shared high similarity of ITS sequences but could separate using multi-locus analyses of informative loci, especially gapdh and tub2 genes (Weir et al., 2012).
Isolates of the C. gloeosporioides species complex have been collected in Korea since the 1990s and stored in the Korean Agricultural Culture Collection (KACC), National Institute of Agricultural Sciences. However, these isolates had been identified based mainly on the host species and morphological characteristics by depositors. Hence, this study aims to (1) reassess the identification of the C. gloeosporioides species complex isolates in KACC using multi-locus analyses of six loci (ITS, gapdh, chs-1, his3, act, and tub2); (2) re-arrange the combination between host plants and fungal species.
Materials and Methods
Fungal isolates
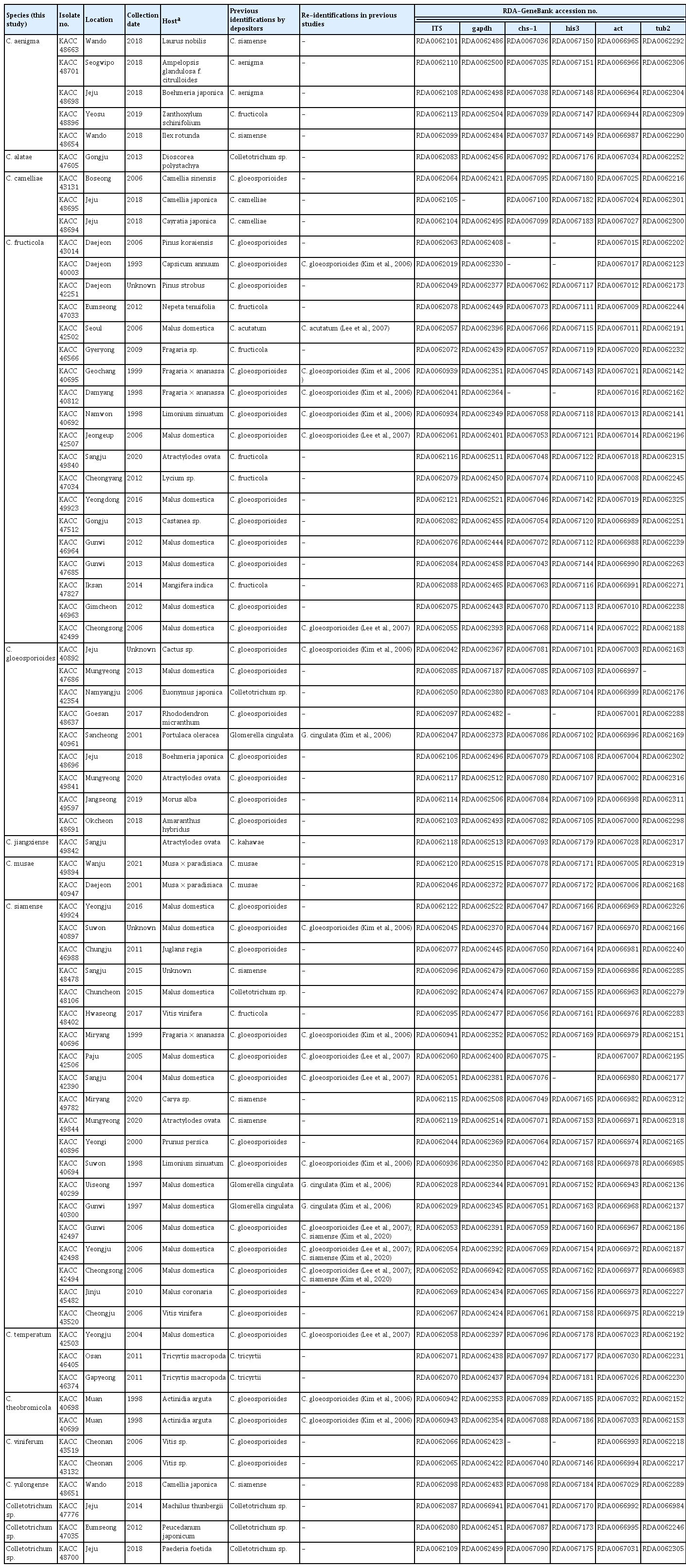
Seventy-one isolates in the C. gloeosporioides species complex that originated in Korea and stored in KACC were used in this study. Details of the isolates are listed in Table 1. The living cultures preserved in liquid nitrogen were retrieved on potato dextrose agar (PDA; Difco Laboratories, Detroit, MI, USA) and were used for DNA extraction and phylogenetic analyses.
DNA extraction, polymerase chain reaction amplification, and sequencing
The mycelium of each isolate was taken from a 5-day-old culture on a PDA medium. Genomic DNA extraction was performed with the DNeasy plant mini kit (Qiagen, Hilden, Germany), following the manufacturer’s instructions. Six loci of Colletotrichum isolates, including ITS, gapdh, chs-1, his3, act, and tub2 were amplified with the primer pairs ITS1/ITS4 (White et al., 1990), GDF1/GDR1 (Guerber et al., 2003), CHS-79F/CHS-345R (Carbone and Kohn, 1999), CYLH3F/CYLH3R (Crous et al., 2004), ACT-512F/ACT-783R (Carbone and Kohn, 1999), and T1/BT2b (Glass and Donaldson, 1995; O’Donnell and Cigelnik, 1997), respectively. Each polymerase chain reaction (PCR) was performed in a volume of 25 μl containing 12.5 μl PCR Master Mix (2×), 8.5 μl nuclease-free water, 1 μl (4.5 pMol) of each primer, and 2 μl DNA template (100 ng/μl). PCR conditions of six loci were set up as described by Thao et al. (2023). PCR amplifications were carried out using a MJ Research PTC-200 Thermal Cycler (MJ Research, Ramsey, MN, USA). PCR products were purified with the QIAquick PCR Purification Kit (Qiagen) and checked by gel electrophoresis before being sent to the Macrogen Company (Seoul, Korea) for sequencing with the amplification primers.
Phylogenetic analysis
Raw sequences generated from forward and reverse primers in this study were trimmed and paired by MEGA 11 (Tamura et al., 2021). Assemble sequences were deposited to RDA-GeneBank (http://genebank.rda.go.kr) with accession numbers in Table 1. The closest reference sequences in GenBank were obtained by BLASTN search and were used for phylogenetic analyses with sequences from 71 KACC isolates and a species in the different species complex as an outgroup. The dataset of each locus was separately aligned using the multiple alignment program MAFFT version 7 (https://mafft.cbrc.jp/alignment/server/) with the G-INS-1 option. The poor alignments at both ends were cut and the alignments were improved by visual inspection in MEGA 11 and concatenated afterward in this software. A maximum likelihood (ML) phylogenetic tree was inferred based on the combined six datasets (ITS, gapdh, chs-1, his3, act, and tub2), using IQ-TREE with the best-fit model “TN+F+G4” and 1,000 ultrafast bootstrap replicates. The substitution model options were auto-evaluated according to the provided alignment files. The phylogenetic trees were viewed in MEGA11 and depicted in Adobe Illustrator.
Results
The phylogenetic analysis included 71 KACC isolates that originated in Korea (Table 1), 58 reference strains from 56 previously accepted species and C. arecacearum (MH0003) as the outgroup (Supplementary Table 1). The concatenated dataset alignment consisted of 2,504 characters (1,419 constant, 1,002 variable, and 502 parsimony-informative characters), including gaps. Of these, 565 characters were from ITS, 282 characters from gapdh, 251 characters from chs-1, 376 characters from his3, 264 characters from act, and 766 characters from tub2.
The multi-locus phylogenetic analysis resolved all KACC isolates into 15 clades. Each of the 12 clades was well clustered with an ex-type or holotype strain of the previously described species, supported by 86% to 100% ML bootstrap values (Fig. 1). Of which, 20 isolates clustered with C. siamense, 19 isolates with C. fructicola, nine isolates with C. gloeosporioides sensu stricto, five isolates with C. aenigma, three isolates with C. camelliae, three isolates with C. temperatum, two isolates with C. musae, two isolates with C. theobromicola, two isolates with C. viniferum, one isolate with C. alatae, one isolate with C. jiangxiense and one isolate with C. yulongense. The isolate KACC 47776 formed a sister clade to C. makassarense and C. tropicale. However, this isolate was distinguished from C. makassarense at gapdh (93.97% identity), act (98.76% identity), chs-1 (98.8% identity), and ITS (98.97% identity), and differentiated from C. tropicale by gapdh (94.03% identity), act (98.77% identity), and chs-1 (97.61% identity). The isolate KACC 47035 was phylogenetically related to C. endophyticum and C. artocarpicola, but shared low sequence similarity at gapdh (92.13% and 90.75%, respectively), act (97.93% and 98.35%), his3 (97.2% with C. endophyticum), chs-1 (98.01% with both species), and tub2 (98.34% with both species). Exception of ITS, all other loci of KAC 48700 shared low sequence similarity with the most closely related species, consisting of gapdh (91.3% identity shared with C. siamense), his3 (92.2% identity with C. siamense), act (95.45% identity with C. conoides, C. aenigma and C. siamense), tub2 (95,71% identity with C. endophyticum), and chs-1 (97.61% identity with C. siamense). According to phylogenetic analysis and BLASTN search results, each of the isolates, KACC 47776, KACC 47035, and KACC 48700, were genetically distinct from the other taxa.
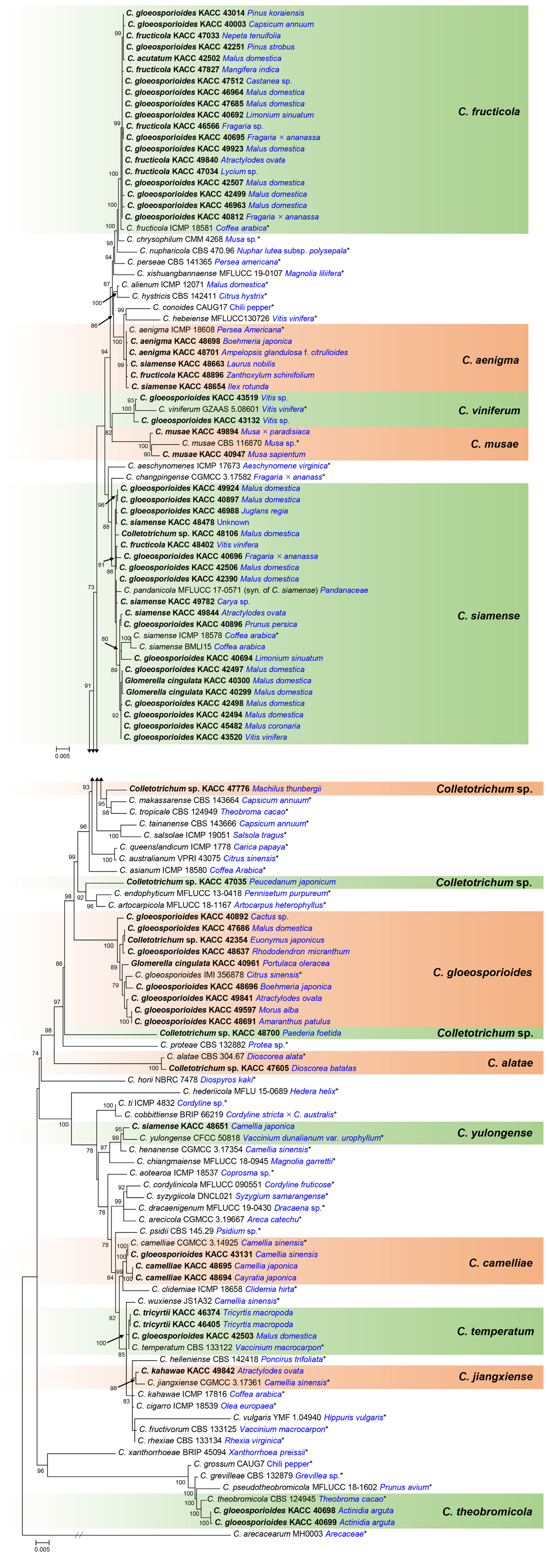
Maximum likelihood tree based on multi-locus sequences of ITS, tub2, his3, gapdh, chs-1, and act. Species names are followed by strain accession numbers and their hosts (blue). Isolates in this study are in bold and listed by the original names. Bootstrap values below 70% are not present. Ex-type strains are emphasised by asterisks (*). The tree is rooted to Colletotrichum arecacearum (MH003).
Host plants of fungal species in this study
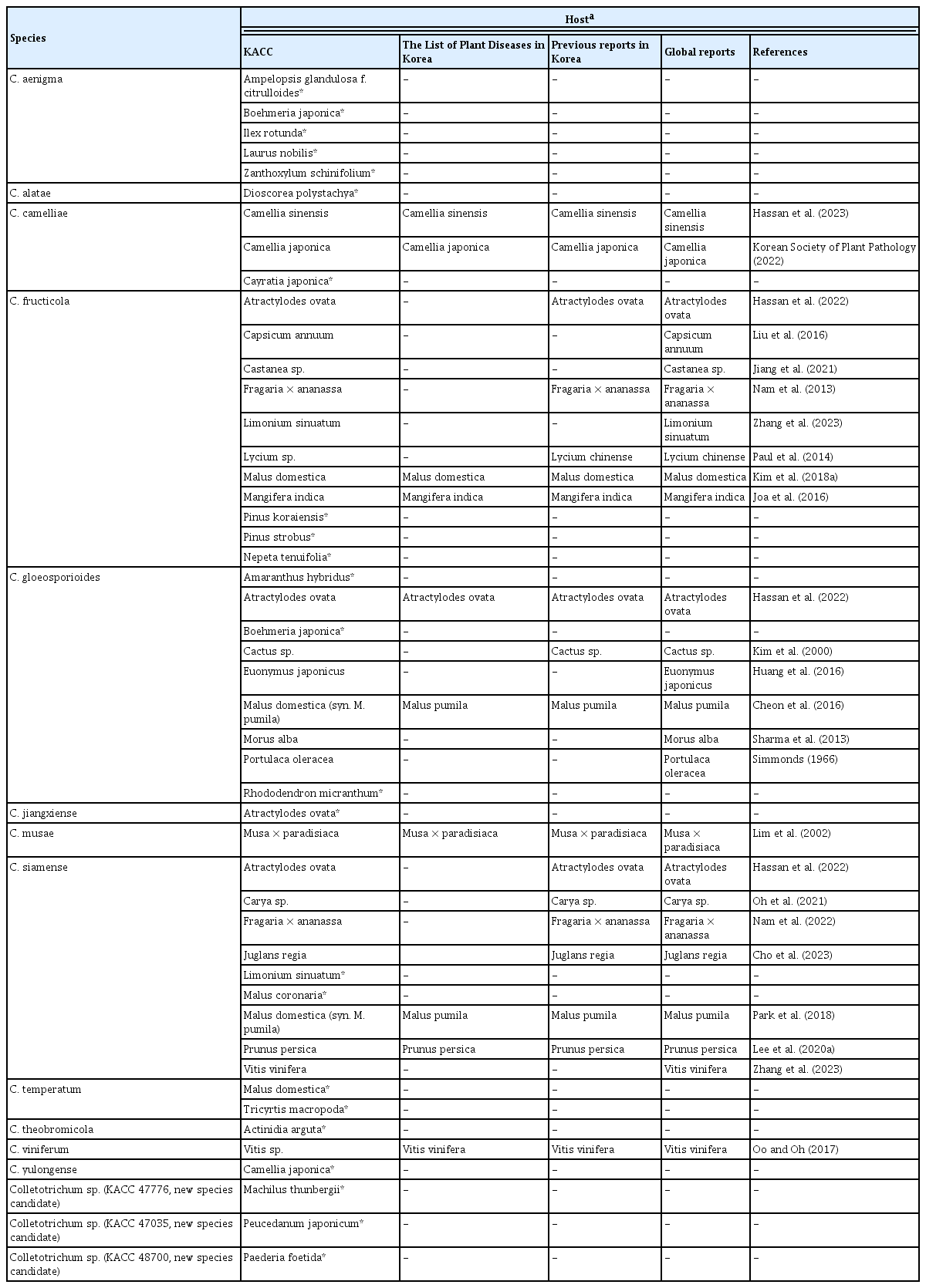
Twenty combinations between hosts and accepted fungal species found in this study have not been reported in any countries. However, the fungi were just isolated from the hosts and their pathogenicity was not confirmed. They are C. aenigma on Ampelopsis glandulosa f. citrulloides, Boehmeria japonica, Ilex rotunda, Laurus nobilis, and Zanthoxylum schinifolium; C. alatae on Dioscorea polystachya; C. camelliae on Cayratia japonica; C. fructicola on Pinus koraiensis, Pinus strobus, and Nepeta tenuifolia; C. gloeosporioides on Amaranthus hybridus, Boehmeria japonica, and Rhododendron micranthum; C. jiangxiense on Atractylodes ovata; C. siamense on Limonium sinuatum and Malus coronaria; C. temperatum on Malus domestica and Tricyrtis macropoda; C. theobromicola on Actinidia arguta; and C. yulongense on Camellia japonica. Seven other combinations, including C. fructicola on Capsicum annuum, Castanea sp., and Limonium sinuatum; C. gloeosporioides on Euonymus japonicus, Morus alba, and Portulaca oleracea; and C. siamense on Vitis vinifera are first reported in Korea (Table 2).
Discussion
Based on DNA sequence analyses of six loci, 43 KACC isolates were renamed, three isolates were newly identified at the species level, 21 strains remained as original names and three isolates were considered as three new species candidates. Nineteen isolates in this study had previously been identified as C. gloeosporioides sensu lato by Kim et al. (2006) and one isolate had been identified as C. acutatum by Lee et al. (2007) using morphological features, ITS and tub2 sequence analyses. However, these isolates were resolved by Kim et al. (2020) and by the present study into four different species, C. siamense (n = 10), C. fructicola (n = 7), C. theobromicola (n = 2), and C. temperatum (n = 1), based on multi-locus analyses of ITS, gapdh, chs-1, tub2 and (his3, act) or (cal and ApMat). The results of this study also revealed four fungal species, C. jiangxiense, C. temperatum, C. theobromicola, and C. yulongense, were first recorded in Korea.
C. siamense (n = 20), C. fructicola (n = 19), and C. gloeosporioides sensu stricto (n = 9) are the most dominant species among KACC isolates in the C. gloeosporioides species complex. According to previous studies and this work, C. gloeosporioides, C. siamense, C. fructicola, C. aenigma, C. jiangxiense, C. theobromicola, C. viniferum, and C. temperatum have a wide range of hosts, whereas C. musae has a narrow host range, on Musa spp. and Mangifera indica, and C. alatae is a host-specific fungus, on the genus Dioscorea (Farr and Rossman, 2021; Jayawardena et al., 2021; Li et al., 2019; Liu et al., 2022; Weir et al., 2012; Zhang et al., 2023). In addition, C. yulongense was originally described by Wang et al. (2019a) on Vaccinium dunalianum (Ericaceae family) and this fungus was found on Camellia japonica (Theaceae family) in this study as the second host plant. Another species, C. camelliae was also reported only on the genus Camellia of the family Theaceae (Liu et al., 2022), but this fungal species was herein from the different family Vitaceae (Cayratia japonica).
C. aenigma was introduced by Weir et al. (2012) and this fungus later became a very popular species, causing anthracnose on the grapevine, Asian pear, and walnut in China (Fu et al., 2019; Wang et al., 2021; Yan et al., 2015), on dragon fruits in Thailand (Meetum et al., 2015), on avocado in Israel (Sharma et al., 2017), on miracle in Japan (Truong et al., 2018), on grape and orange stonecrop in Korea (Choi et al., 2017; Kim et al., 2021). In our study, this fungus was isolated from five new hosts (Ampelopsis glandulosa f. citrulloides, Boehmeria japonica, Ilex rotunda, Laurus nobilis, and Zanthoxylum schinifolium) belonging to the five different families.
Apple (Malus domestica) is one of the most economically important crops in Korea. Kim et al. (2006) and Lee et al. (2007) implicated that C. gloeosporioides and C. acutatum were serious pathogens causing bitter rots of apple fruits in Korea. However, the identified species were unreliable as mentioned above. Later, causal agents of apple bitter rot disease in the country were authentically identified as C. siamense, C. fructicola, C. nymphaeae, C. fioriniae, C. gloeosporioides, and C. aenigma based on multi-locus sequence analyses (Cheon et al., 2016; Kim et al., 2020; Lee et al., 2021; Oo et al., 2018). The Colletotrichum isolates from apple in this work and in our earlier study (Thao et al., 2023) have been collected since 1997 from many different geographic locations in Korea, and the most prevalent species on apple were C. siamense (10 isolates), followed by C. fructicola (seven), C. nymphaeae (five) and C. fioriniae (four), while only one isolate of C. gloeosporioides sensu stricto was from apple and no C. acutatum sensu stricto isolates were found in KACC. Additionally, a fungal species C. temperatum, originally described by Doyle et al. (2013) on Vaccinium macrocarpon, was first detected on apple in this study.
Grape (Vitis vinifera) is an extensively cultivated crop worldwide and in Korea. The crop was seriously damaged by the infection of C. acutatum and C. gloeosporioides (Hong et al., 2008), C. viniferum (Oo and Oh, 2017), and C. aenigma (Kim et al., 2021). In this study, two isolates on grape (Vitis sp.) were identified as C. viniferum, whereas the other two isolates on Vitis vinifera were identified as C. siamense.
Kiwiberry (Actinidia spp.) has been widely cultivated in the country and worldwide (Kim et al., 2017; Latocha, 2017). The anthracnose symptoms caused by C. nymphaeae on kiwiberry fruits were observed in Jeonnam province (Kim et al., 2018b). Many other Colletotrichum species also infected kiwi plants worldwide such as C. aenigma on Actinidia arguta in China (Wang et al., 2019b), C. fioriniae, C. karsti, C. gloeosporioides, C. fructicola, and C. siamense on Actinidia spp. in Japan (Poti et al., 2023). A new combination of kiwiberry and C. theobromicola was found in this study.
The main causal fungi of chili pepper (Capsicum annuum) anthracnose in Korea were known as C. gloeosporioides sensu lato, C. acutatum s. lat., C. dematium s. lat., and C. trucatum s. lat. (Oo and Oh, 2016, 2020; Oo et al., 2017; Park and Kim, 1992). However, only one strain of C. fructicola in the C. gloeosporioides species complex from chili pepper is stored in KACC. While 16 strains of the C. acutatum species complex from chili pepper are preserved in KACC (Thao et al., 2023). It suggests that C. gloeosporioides s.c. is not a main causal fungus on chili pepper anthracnose in Korea.
Machilus thunbergii is a medicinal plant and it was widely distributed in the west-southern islands of Korea and some other Asian countries (Park et al., 1990). Only Glomerella cingulate and C. fructicola were formerly known as pathogens on this plant in Japan (Kobayashi, 2007) and China (Liu et al., 2022), respectively. A new species candidate (KACC47776) from Machilus thunbergii in Jeju, Korea was identified in this study. The second new species candidate (KACC48700) was from Paederia foetida, this host was also infected by many different Colletotrichum species (Liu et al., 2022). The third new species candidate (KACC47035) was the first report of a Colletotrichum fungus on coastal hogfennel (Peucedanum japonicum), an edible wild vegetable and medicinal resource.
A reassessment of members of the C. gloeosporioides species complex in Korea revealed new records of four Colletotrichum species and 27 combinations between plants and fungi in Korea. Of which, new findings of C. fructicola on pepper, C. siamense on grape, C. temperatum on apple, and C. theobromicola on kiwiberry could play an important role in the agricultural sciences and practices of Korea. However, the new combinations found in this study need to be clarified by pathogenicity tests in further studies.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this work was reported.
Acknowledgments
This study was supported by a grant (PJ017286) from the National Institute of Agricultural Sciences and was a part of the “2023 KoRAA Long-term Training Program”, Rural Development Administration, Republic of Korea.
Electronic Supplementary Material
Supplementary materials are available at The Plant Pathology Journal website (http://www.ppjonline.org/).