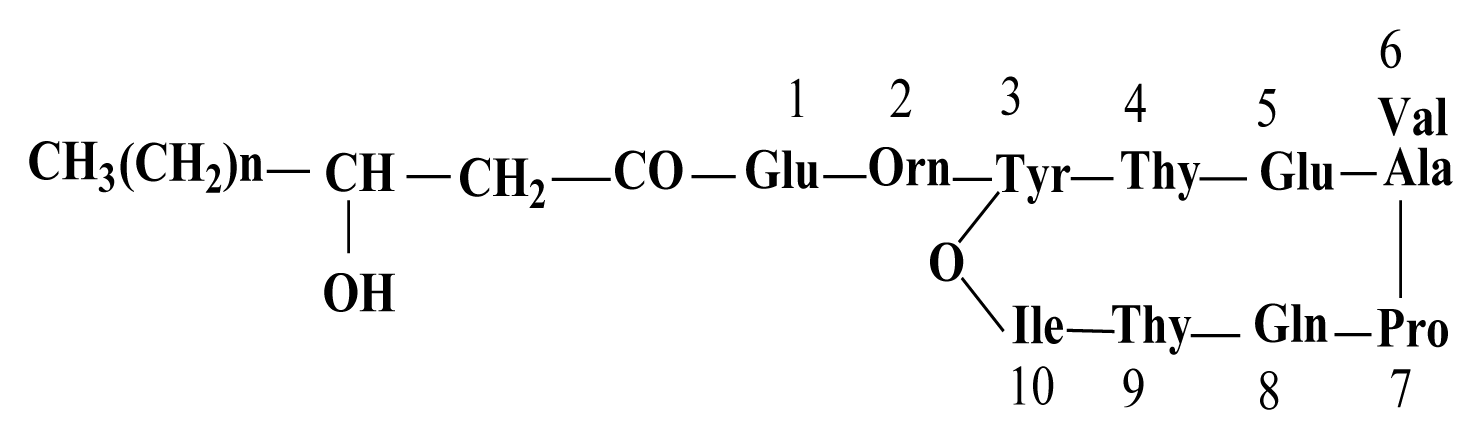Inhibition of the Aspergillus flavus Growth and Aflatoxin B1 Contamination on Pistachio Nut by Fengycin and Surfactin-Producing Bacillus subtilis UTBSP1
Article information
Abstract
In this study, the treatment of pistachio nuts by Bacillus subtilis UTBSP1, a promising isolate to degrade aflatoxin B1 (AFB1), caused to reduce the growth of Aspergillus flavus R5 and AFB1 content on pistachio nuts. Fluorescence probes revealed that the cell free supernatant fluid from UTBSP1 affects spore viability considerably. Using high-performance liquid chromatographic (HPLC) method, 10 fractions were separated and collected from methanol extract of cell free supernatant fluid. Two fractions showed inhibition zones against A. flavus. Mass spectrometric analysis of the both antifungal fractions revealed a high similarity between these anti-A. flavus compounds and cyclic-lipopeptides of surfactin, and fengycin families. Coproduction of surfactin and fengycin acted in a synergistic manner and consequently caused a strong antifungal activity against A. flavus R5. There was a positive significant correlation between the reduction of A. flavus growth and the reduction of AFB1 contamination on pistachio nut by UTBSP1. The results indicated that fengycin and surfactin-producing B. subtilis UTBSP1 can potentially reduce A. flavus growth and AFB1 content in pistachio nut.
Introduction
Aspergillus flavus is a cosmopolitan organism, able to contaminate a wide variety of natural substrates such as grains, nuts and food products (Cotty et al., 1994; González et al., 2005). A. flavus produces aflatoxin which is carcinogenic and toxic to both human and livestock populations (Bennett and Klich, 2003; Shephard, 2005). Furthermore, A. flavus is an opportunistic pathogen causing invasive and non-invasive aspergillosis in humans (Perfect, 2001). In almost all cases, fungal contamination, colonization and infection are usually initiated by airborne conidia that invade the host tissue and subsequently by germination (Samson et al., 1995).
Of the many research approaches being used to reduce and eliminate A. flavus contamination, antifungal agents produced by microorganisms consider as environmentally friendly method to control of A. flavus (Dorner, 2004; Shephard, 2005). Several bacteria particularly Bacillus subtilis strains have been reported as biological agents to control A. flavus (Reddy et al., 2009; Zhang et al., 2007). It is demonstrated that some B. subtilis strains produce antifungal compounds including lipopeptides (LPs) such as surfactin (Hosono and Suzuki, 1983; Vater et al., 2002), fengycin (Vanittanakom et al., 1986), and iturin compounds (Chitarra et al., 2003; Peypoux et al., 1978; Zhang et al., 2007). These antifungal peptides inhibit the growth of large number of fungi, such as Aspergillus, Rhizopus, Botrytis and Penicillium (Chitarra et al., 2003; Munimbazi and Bullerman, 1998; Sun et al., 2006). In some studies, combination of antifungal agents synergistically acted against different plant pathogens (Koumoutsi et al., 2004; Kuroda et al., 2000; Maget-Dana and Ptak, 1990).
In previous study, B. subtilis UTBSP1 considerably remedied aflatoxin B1 in both liquid culture and pistachio nut (Farzaneh et al., 2012). In addition, this strain showed an antifungal activity against A. flavus in dual culture assay (unpublished data). Therefore, the purposes of this work were as follows: (1) Determine the potential of B. subtilis UTBSP1 to inhibit A. flavus and AFB1on pistachio. (2) Evaluate the destructive effect of cell-free supernatant fluid (SF) of B. subtilis UTBSP1 against the A. flavus spore. (3) Separate, purify and identify the anti-A. flavus compounds produced by B. subtilis UTBSP1.
Materials and Methods
Microorganisms
A. flavus R5, with strong ability to produce the high amount of AFB1, and B. subtilis UTBSP1, with destructive effect on AFB1 (Farzaneh et al., 2012), were obtained from University of Tehran.
Inhibition of A. flavus and Aflatoxin B1 in pistachio
Mature pistachios with undamaged and non-adhering hulls were collected from Rafsanjan orchard. The hulls were aseptically removed from the pistachios and a total of 100 pistachios with opened shells were selected and injured by using a scribe to make a puncture wound approximately 1 mm wide by 2 mm deep in each kernel. Ten wounded nuts were placed in 100-ml flasks containing 20 ml of different concentration of bacterial suspension in saline solution, sterile 0.85% NaCl (105, 106, and 107 cfu/ml) for 10 minutes and then were placed on sterile paper. After 15 minutes, approximately 200 spores of A. flavus R5 in 0.05% Tween 80 were inoculated on the wound of kernel and then were placed on 100-ml flasks and incubated for 7 days at 28°C in darkness. The following controls were (1), treated nuts with saline solution and A. flavus spore, and (2), treated nuts with saline solution. The growth of A. flavus (population of spores) on gram nut was evaluated by adding 50 ml saline solution, shaking vigorously at 250 rpm for 30 minutes and counting the spores with hemocytometer and light microscope. In addition, the AFB1 content was extracted and purified by using immune-affinity column cleanup (Stroka et al., 2001) and then determined by high-performance thin-layer chromatography (HPTLC) as well as high-performance liquid chromatographic (HPLC) method (Farzaneh et al., 2012).
Isolation of antifungal compounds produced by B. subtilis UTBSP1
Forty milliliter of 18 hours pre-incubation of UTBSP1 in nutrient broth (NB; 1% peptone, 0.3% beef extract, 0.5% NaCl, pH 7.0) was transferred to 700 ml fresh NB and incubated at 30°C for 48 hours. Bacteria cells were removed by centrifugation at 4,024g for 15 minutes. The pH of cell free SF was adjusted to 2.0 and the precipitate was allowed to settle at 4°C. Subsequently, the acid precipitate was collected by centrifugation at 1,369g for 20 minutes at 4°C. The antifungal compounds from the precipitate were extracted twice using methanol and then methanol evaporated by rotary-evaporator at 45°C under a vacuum and then stored at 4°C as a HCl precipitate.
Inhibitory effects on spore
Inhibitory effects of SF (at the three concentrations; 10%, 25%, and 50% in potato dextrose broth medium) and its HCl precipitate (100, 250, and 500 μg·ml−1) on spore germination were determined after 8 hours at 30°C by using light microscope according to Chitarra et al. (2003). In addition, the effect of HCl precipitate on spore viability was carried out by using fluorescent probes (Chitarra et al., 2003). Briefly, spores were incubated at 30°C for 3 hours in the absence and presence of HCl precipitate (500 μg·ml−1) and were subsequently labeled with the fluorescent probes carboxy-fluorescein (cF) diacetate (cFDA) and propidium iodide (PI; Molecular Probes, Eugene, OR, USA) at a final concentration of 10 μmol·l−1 for 15 minutes at 30°C. The incubated spores in water:methanol solution (50:50 v/v) for 3 hours were considered as control. The staining of the spores was determined by the Inverted Epi-fluorescence microscope (ECLIPSE TE2000-S; Nikon Instech Co., Ltd., Tokyo, Japan) equipped with a 100 W mercury lamp, a laser beam (488 nm), a fluorescent filter set and a CFI Plan Apo TIRF 60X oil objective lens.
Purification of antifungal compounds
The methanol fraction of SF acid precipitation was purified by reverse phase-HPLC (RP-HPLC) with a C18 column (COSMOSIL 5 μm C18-AR-300, 4.6 × 250 mm; Nacalai Tesque, Kyoto, Japan) and injection volume of 100 μl on Agilent 1,100 HPLC and monitored by absorbance at 214 and 280 nm. The solvents were (A) methanol and (B) 0.1% trifluroacetic acid (TFA) in water. Elution (0.9 ml/min) starts with 45% A for 20 minutes, which was continued for 40 minutes with 100% A, holding for 5 minutes and finally continued for 10 minutes with 45% A. This step was repeated several times, and fractions with the same retention time were collected and dried under N2 at 35ºC and finally their antifungal activities were determined against A. flavus (Chitarra et al., 2003).
HPLC/mass spectrometric (MS) analysis of antifungal substances
A coupled liquid chromatography-tandem MS system consisting of an Agilent HP1200 liquid chromatography and a triple-quadrupole mass spectrometer (Agilent 6410 LC/MS; Agilent Technologies, Palo Alto, CA, USA) were employed to separate and determine the molecular weight of compounds in unpurified methanol fraction of acid precipitation and purified antifungal compounds collected from RP-HPLC step. The symmetry ZORBAX SB-C18 column (rapid resolution, 3.5 μm, 30 × 2.1 mm; Agilent Technologies) oven was at 23ºC, the flow rate was 0.4 ml/min, and the injection volume was 5 μl. Solvents (A) 10% formic acid in water and (B) methanol were used as mobile phases. The electrospray source was operated at a capillary voltage of 30 V, a spray voltage of 5 kV, a capillary temperature of 350°C and full scan spectra were recorded over the range m/z 50 to 2,000.
Statistical analysis
The experiment was repeated two times in a randomized complete design with four replicates. Data analysis was performed using the SAS ver. 9.1 software (SAS Institute Inc., Cary, NC, USA). The analysis of variance was done by the GLM module.
Results
Inhibit the A. flavus growth and Aflatoxin B1 contamination in pistachio
There was significant difference among different populations (105, 106, and 107 cfu·ml−1) of B. subtilis UTBSP1 to inhibit A. flavus growth as well as reduce AFB1 accumulation on pistachio nuts (Fig. 1). In control treatment (without UTBSP1 inoculation), the infected pistachio nut was extensively colonized by A. flavus R5 (1.3 × 107 spores/g nut) and its AFB1 content was 12,465.1 ng·g−1 nut. Although the dipping of pistachio nuts in 105 cfu·ml−1 of UTBSP1 couldn’t significantly decrease the A. flavus growth but it could significantly reduce AFB1 accumulation in nut. By using of 106 cfu·ml−1 of UTBSP1, spore population and AFB1 content in pistachio nut decreased to 54.2% and 82.1%, respectively. However, by increasing the cfu of UTBSP1 to 107 per ml, spore population decrease to 94.1% while no AFB1 content was detected.

Reduction of Aspergillus flavus growth (spore population) and aflatoxin B1 (AFB1) content in pistachio nuts by using different concentrations of Bacillus subtilis UTBSP1, including 105, 106, and 107 cfu/ml saline solution. In the infected control (inoculated nut with saline solution and A. flavus R5), 1.3 × 107 spores and 12,465.1 ng AFB1 per g nut were detected. The treatments with same letter were not significantly different (P ≤ 0.05). Error bars indicate standard error of four replications.
Antifungal activity against spore
The germination efficiency of A. flavus spores in the absence of SF or its HCl precipitate was 95%. After 8 hours of incubation, the percentage of spore germination efficiency decreased to 25, 5% and 0%, in the presence of 10%, 25%, and 50% of SF, respectively. In addition, none spore was germinated in the presence of HCl precipitate (at 500 μg·ml−1).
The membrane permeabilization and viability of the spore were assessed using fluorescent probes. The control spores could considerably be stained with cFDA (99% spores), but not significantly with PI (4% spores). Incubation of the spores with HCl precipitate for 3 hours caused to stain 98% spores with PI whereas they could not significantly be stained with cFDA (results are shown in the Fig. 2).
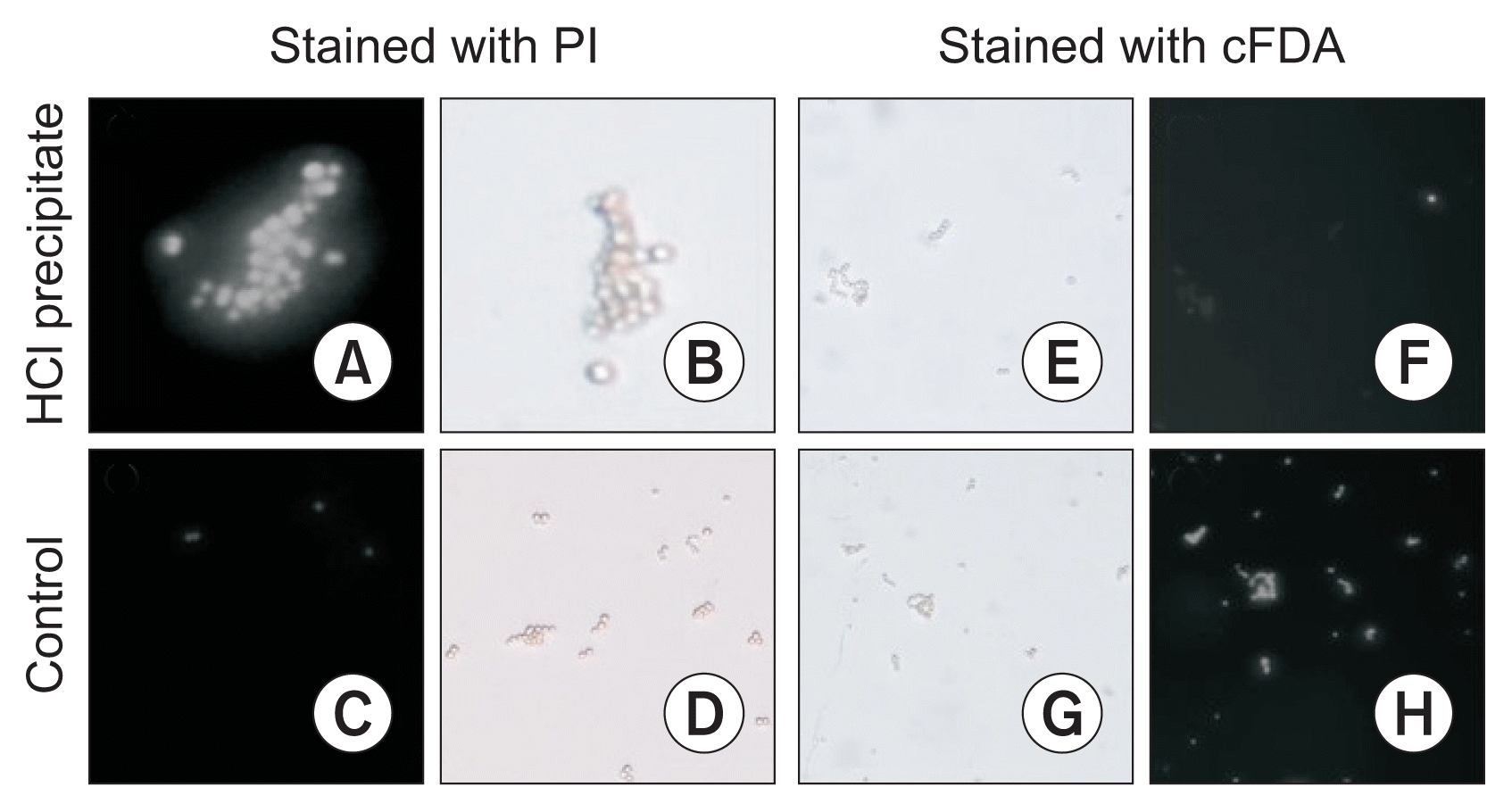
Fluorescence microscopy pictures of Aspergillus flavus spores that were incubated for 3 hours in the HCl precipitate of Bacillus subtilis UTBSP1 (500 μg·ml−1) and then stained with PI (A) or cFDA (F). The fluorescence microscopy pictures of PI-stained (C) or cFDA-stained (H) spores in control (water:methanol [50:50 v/v]) are presented. In addition, the light microscopy pictures of HCl precipitate-treated spores (B, E) and untreated (control) spores (D, G) are shown. PI, propidium iodide; cFDA, carboxyfluorescein diacetate.
Isolation and purification of antifungal compounds
The methanol fraction of HCl precipitate produced several obvious peaks in HPLC (results are shown in the Fig. 3). All the fractions (10 peaks) were collected manually as separated ones. At the concentration of 200 μg·ml−1, two major fractions (A) and (B) showed anti-fungal activities against A. flavus after 4 days. The inhibition zones caused by the fractions (A) and (B) were 17 and 5 mm, respectively. Mixture of the both fractions showed stronger antifungal activity with 19 mm inhibition zone (Fig. 4). The inhibition zone of the fraction (A) and the combination of both fractions were stable after 10 days whereas the inhibitory zone arising from fraction (B) disappeared after 3 days. Therefore, LC/MS was applied to identify both anti-Aspergillus fractions.
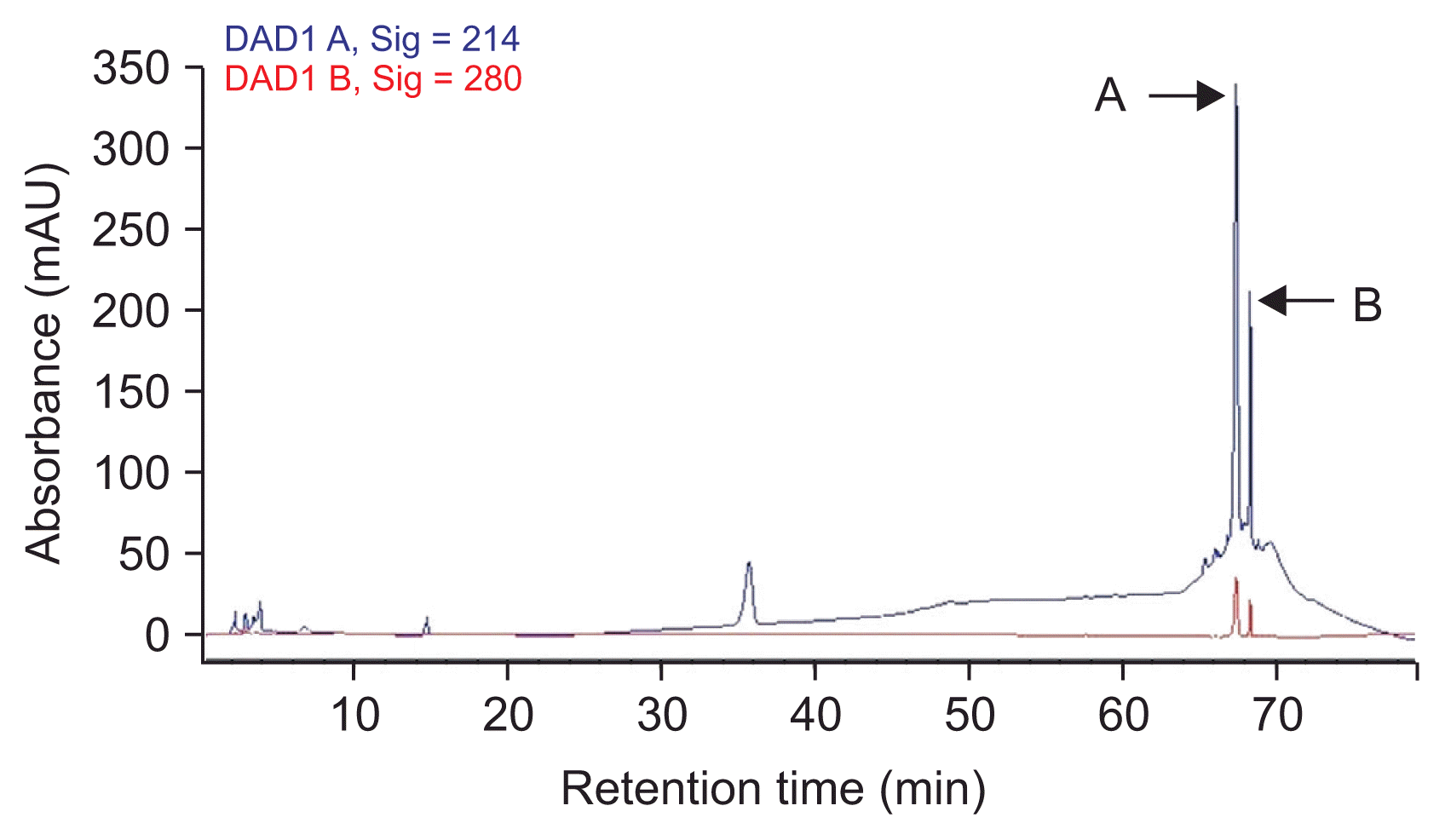
Purification chromatogram of the methanol extraction of the HCl precipitate of Bacillus subtilis UTBSP1 by using reverse phase high-performance liquid chromatography (HPLC) with C18 column. Mobile phase: water (0.1% trifluroacetic acid) and methanol; detector: 214 and 280 nm. All the distinct fractions (10 peaks) were collected manually. Peaks ‘A’ and ‘B’ showed inhibition zones against Aspergillus flavus R5.
Identification using MS analysis
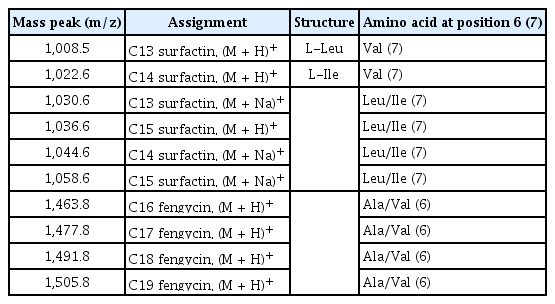
HPLC analysis of methanol fraction of SF acid precipitate and antifungal activity tests, revealed that B. subtilis UTBSP1 produces two peaks with antifungal activities against A. flavus R5 (results are shown in the Fig. 3, 4). Full MS analysis of fraction A showed a series of molecular ions with 1,022.6; 1,036.6 m/z for (M + H)+, and 1,044.6; 1,058.6 m/z for (M + Na)+. Also, the mass spectrum of fraction B showed a series of molecular ions with 1,463.8; 1,477.8; 1,491.8; 1,505.8 m/z, for (M + H)+ (results are shown in the Fig. 5).
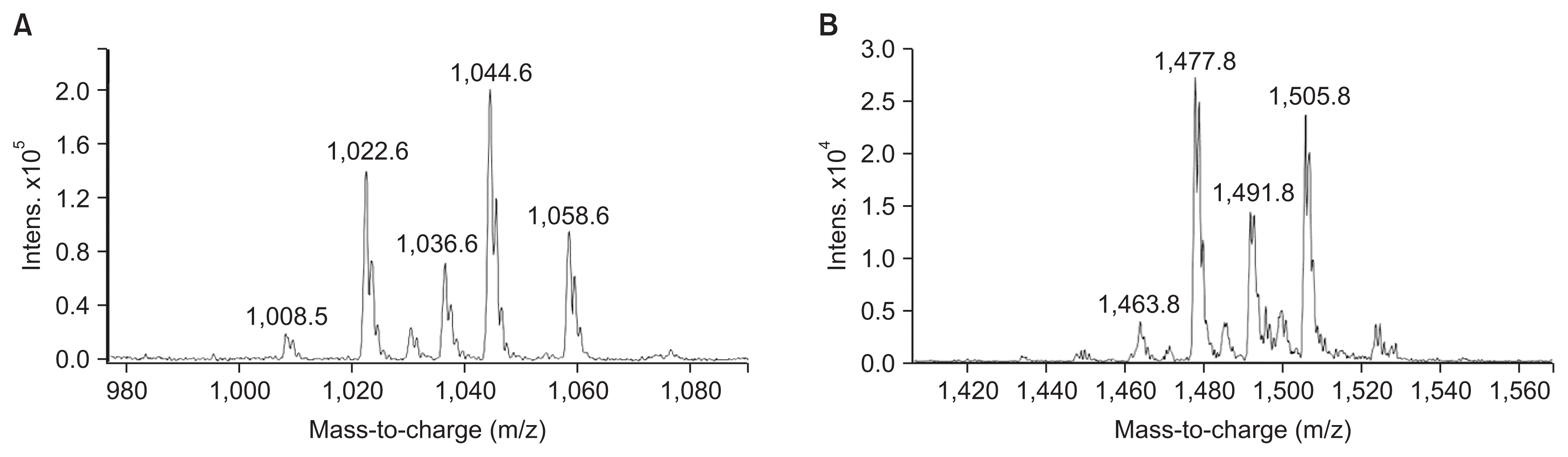
Full mass spectrometric spectra of surfactin (A), and fengycin (B), produced by Bacillus subtilis UTBSP1, and obtained from electrospray ionization triple-quadrupole mass spectrometer. The peaks belong to the HCl precipitate of B. subtilis UTBSP1.
With regards to the comparing of mass data with standard and those reported in previous studies, the antifungal fractions, (A) and (B), represented surfactin homologues and fengycin homologues, respectively (Kowall et al., 1998; Stein, 2005; Vanittanakom et al., 1986; Vater et al., 2002).
HPLC/MS spectra of unpurified methanol fraction of SF acid precipitation exhibited several peaks at different m/z values. Two fractions showed m/z values between 1,000–1,060 as well as 1,460–1,510, symbolized surfactin homologues and fengycin homologues, respectively (Table 1).
Discussion
In this study, B. subtilis UTBSP1 could reduce A. flavus growth and AFB1 contamination in pistachio nuts and there was a positive correlation between the reduction of A. flavus colinization and the reduction of AFB1 accumulation in pistachio nut. Similarly, the antifungal activities of B. subtilis strains and other biological agents against A. flavus and AFB1-production have been reported in several studies (Dorner et al., 1998; Dorner, 2004; Mohammadipour et al., 2009; Reddy et al., 2009; Zhang et al., 2007). However, contrary to our results, several studies indicated that although Bacillus spp., exhibit considerable antifungal activities against some phytopathogenic fungi but their inhibitory activities against A. flavus are no significant (Hu et al., 2009; Sun et al., 2006).
In our study, cell free SF from B. subtilis UTBSP1 showed destructive effect against spore of A. flavus. Therefore, the fluorescent probes including PI and cFDA were applied to perceive the influence of UTBSP1 on fungal spore precisely. The fluorescent probe cFDA is a hydrophobic enzyme activity probe that can pass through the hydrophobic cell membrane and is immediately converted to hydrophilic cF by non-specific esterases and then release the fluorescent light. Therefore, cF slowly accumulates in the cell because of the low permeability of the membrane to cF. In dead cells, there is no cF-accumulation, since the membrane is perforated by many holes (Brul, 1997; Chitarra et al., 2003). In contrast, the fluorescent probe PI is a nucleic acid probe which is supposed to cross only through damaged cell membranes. Therefore, the spore mortality can be correlated with the accumulation of PI. However, fluorescence methods using probes such as cFDA and PI have been used to evaluate conidial viability (Chitarra et al., 2003; Schading et al., 1995). Furthermore, fluorescent labelling in combination with flow-cytometry is a rapid, reliable and sensitive method to assess viability (Brul, 1997; Bunthof et al., 2001). In our study, the incubation of the spores with HCl precipitate for 3 hours, caused to spore staining with PI which indicates that the spores membranes were damaged and permeabilized, similarly to the results reported in the previous studies (Brul, 1997; Chitarra et al., 2003; Schading et al., 1995). It is obvious that in the presence of SF and its HCl precipitate, the fungal spores lose their abilities to initiate biochemical activities and morphological changes as well.
By HPLC analysis and antifungal activity tests, two fractions arising from HCl precipitation with anti-A. flavus activity were isolated in this study. In addition, MS analysis of the two anti-A. flavus fractions unveils an exponential similarity between their compounds and cyclic LPs of surfactin and fengycin families. The mass peak at (M + H)+ m/z = 1,477.8 (Fig. 5B) is attributed to a fengycin isoform containing hydrogen adduct of a β-hydroxy fatty acid with a chain length of 17 carbon atoms and an alanine at position 6 of the octapeptide ring (Vater et al., 2002). A set of mass peaks with an intervals of 14 Da ([M + H]+ ions at m/z 1,463.8, 1,491.8 and 1,505.8) are fengycins with different number of methylene groups (–CH2–). The mass peak at (M + H)+ m/z = 1,022.6 and (M + Na)+ m/z = 1,044.6 (Fig. 5A) is attributed to a surfactin isoform containing proton and sodium adducts respectively, with 14 carbon atoms in carbon chain of β-hydroxy fatty acids and a valine at position 7 of the peptide ring (Vater et al., 2002). Molecular ion with 1,008.5 and 1,036.6 m/z for (M + H)+, and 1,030.6 and 1,058.6 m/z for (M + Na)+ are surfactin isoforms with different length of the carbon chain of β-hydroxy fatty acid (C13 and C15) and a valine or leucine/isoleucine at position 7 of the heptapeptide ring (Morikawa et al., 2000), showed that a set of mass peaks with an intervals of 14 Da are often observed for LPs with different number of methylene groups (–CH2–) in fatty acyl chains.
This study demonstrates that antifungal activity of B. subtilis UTBSP1, producer of both fengycin and surfactin, is attributed to both antibiotics. However, some Bacillus strains which produce surfactin and/or fengycin couldn’t inhibit the growth of A. flavus (Hu et al., 2009; Sun et al., 2006), whereas (Mohammadipour et al., 2009) reported that B. subtilis BS119m, as a surfactin-producer in a high level, completely inhibit the growth of A. flavus. Furthermore, this study indicates that surfactin and fengycin act in a synergistic manner and provide stronger antifungal activity against A. flavus R5, similar to the synergistic antifungal manners of surfactin and iturin (Maget-Dana and Ptak, 1990), surfactin and fengycin (Ongena et al., 2007; Sun et al., 2006) and iturin and fengycin (Romero et al., 2007). Iturin-producer Bacillus strains also showed antifungal activities against A. flavus (Moyne et al., 2001; Zhang et al., 2007).
The surfactin antibiotic has amphiphilic nature and consequently readily associates and tightly anchors into lipid layers. At low concentration it induces only limited perturbation, but at high concentration causes the formation of irreversible pore (Deleu et al., 2005; Heerklotz and Seelig, 2007). Fengycins have strong fungitoxic activity, especially against filamentous fungi (Hu et al., 2007; Sun et al., 2006). Mechanistically, their actions are less well known in comparison with the other LPs, but they readily interact with lipid layers and alter cell membrane structure and permeability in a dose-dependent way (Deleu et al., 2005).
In conclusion, to sum up, B. subtilis UTBSP1, co-producer of surfactin and fengycin, is potentially used to decrease A. flavus growth and aflatoxin contamination in pistachio nut. In addition, B. subtilis UTBSP1 is an AFB1-degrading agent (Farzaneh et al., 2012) and can use for reducing AFB1-content in foods, feeds and agricultural crops. Therefore, the inoculation of agricultural crops by B. subtilis UTBSP1 in cultivation, harvest and post-harvest stages might noticeably reduce aflatoxin in nuts, grains and other susceptible crops. However, the significance of Bacillus species and, on the other hand, their ability to produce toxic compounds call for careful monitoring of their environmental occurrence (Outtrup and Jorgensen, 2002; Schallmey et al., 2004).
Acknowledgments
We gratefully acknowledge University of Tehran and Institute of Food Safety and Quality, Jiangsu Academy of Agricultural Sciences in China for providing financial supports in this study.
Notes
Articles can be freely viewed online at www.ppjonline.org.