Meta-analysis Reveals That the Genus Pseudomonas Can Be a Better Choice of Biological Control Agent against Bacterial Wilt Disease Caused by Ralstonia solanacearum
Article information
Abstract
Biological control agents (BCAs) from different microbial taxa are increasingly used to control bacterial wilt caused by Ralstonia solanacearum. However, a quantitative research synthesis has not been conducted on the role of BCAs in disease suppression. Therefore, the present study aimed to meta-analyze the impacts of BCAs on both Ralstonia wilt disease suppression and plant (host) growth promotion. The analysis showed that the extent of disease suppression by BCAs varied widely among studies, with effect size (log response ratio) ranging from −2.84 to 2.13. The disease incidence and severity were significantly decreased on average by 53.7% and 49.3%, respectively. BCAs inoculation also significantly increased fresh and dry weight by 34.4% and 36.1%, respectively on average. Also, BCAs inoculation significantly increased plant yield by 66%. Mean effect sizes for genus Pseudomonas sp. as BCAs were higher than for genus Bacillus spp. Among antagonists tested, P. fluorescens, P. putida, B. cereus, B. subtilis and B. amyloliquefaciens were found to be more effective in general for disease reduction. Across studies, highest disease control was found for P. fluorescens, annual plants, co-inoculation with more than one BCA, soil drench and greenhouse condition were found to be essential in understanding plant responses to R. solanacearum. Our results suggest that more efforts should be devoted to harnessing the potential beneficial effects of these antagonists, not just for plant growth promoting traits but also in mode of applications, BCAs formulations and their field studies should be considered in the future for R. solanacearum wilt disease suppression.
Introduction
Ralstonia solanacearum, the second most vital soil-borne bacterial phytopathogen, is distributed globally with wide host range (200 plant species over 50 families) (Hayward, 1991; Mansfield et al., 2012). The wilt caused by R. solanacearum is one of the most devastating, systemic wilt diseases of crop plants in the post-genomic era (Huet, 2014; Peeters et al., 2013). Its hosts include economically important crops, such as potato (Solanum tuberosum), tomato (S. lycopersicum), eggplant (S. melongena), tobacco (Nicotiana tabacum), peanut (Arachis hypogaea), pepper (Capsicum annuum), and ginger (Zingiber officinale). R. solanacearum is an aerobic, Gram-negative rod with high degree of phenotypic and genotypic diversity, including five races, five biovars, and is also divided in four phylotypes corresponding roughly to the strains’ geographic origin: Asia (phylotype I), the Americas (II), Africa (III), and Indonesia (IV) (Coll and Valls, 2013; Hayward, 1991; Huet, 2014; Yadeta and Thomma, 2013).
To date, no successful control method exists for this bacterial wilt disease. Field sanitation, crop rotation, resistant varieties, and transgenic resistant plant had been developed with limited success only (Guo et al., 2004; Huet, 2014; Ramesh and Phadke, 2012; Yadeta and Thomma, 2013). Chemical control such as bactericides, in addition to being potentially harmful to the environment, has not proved to be efficient in controlling R. solanacearum. Alternatively, biological control has been proposed to be an effective, safe and eco-friendly approach in plant disease management (Almoneafy et al., 2014; Dey et al., 2014). The public demand for sustainable agriculture has helped to drive research on the biological control of plant diseases and the practical use of antagonistic microorganisms. Recent publications have indicated that biological control of bacterial wilt disease could be achieved by using antagonistic microbes (Guo et al., 2004; Kurabachew and Wydra, 2013; Maji and Chakrabartty, 2014; Ramesh and Phadke, 2012). Several living microbial products have been commercialized as biological control agents (BCAs). A few examples of plant growth promoting bacteria (PGPR) and biological control products are: Bacillus subtilis GBO3 (Kodiak®), Pseudomonas fluorescens A506 (BlightBan®), P. aureofaciens Tx-1 (Spot-Less®), P. syringae ESC-10, and ESC-11 (Bio-save®), P. chlororaphis MA 342 (Cedomon®), Streptomyces griseoviridis K61 (Mycostop®), and S. lydicus WYEC 108 (Actinovate®) (Dey et al., 2014; Figueiredo et al., 2010).
Many of these branded products, including newly emerging products, are evaluated annually in bio-control efficacy tests. When compared with treatments using a BCA alone, mixtures are expected to provide greater efficacy. However, a number of factors, including type of bacterial and/or fungal strains, and the existing pathogen pathovar, influence the effectiveness of single or co-inoculation of BCAs. For example, some studies showed that co-inoculation with bacteria or fungi were less effective in reducing bacterial wilt on plants compared with single inoculation (Almoneafy et al., 2014; Kurabachew and Wydra, 2013; Sarkar and Chaudhuri, 2013; Seleim et al., 2011). Contradictorily, some other earlier studies demonstrated that a co-inoculation was more effective than single inoculation of either bacteria or fungi (Abo-Elyousr et al., 2012; Liu et al., 2009; Zhou et al., 2014). Studies reported that the magnitude of disease reduction is variable among and between studies evaluating the similar bacterial or fungal strains. Furthermore, due to the large number of BCAs and their combinations, no single study can make all the appropriate comparisons. This variability in biocontrol efficacy results leads to different interpretations, as a result different recommendations on treatment methods. Thus, there is a timely need to summarize efficacy of BCAs from available studies in order to draw the most robust conclusions for future field success.
As described in detail previously (Nelson et al., 2015; Rosenberg et al., 2004), meta-analysis is quantitative review of data collected from different studies, which is typically performed in order to estimate the influence of various experimental factors on effect sizes and assessing the publication bias. Now-a-days, meta-analysis has been widely used in plant growth and health management studies to draw a holistic conclusion from many of the primary experimental studies (Kiær et al., 2009; Nelson et al., 2015). In 2005, Stiling and Cornelissen, performed a meta-analysis to evaluate the effect of different types of biocontrol agents (parasitoids, predators, and pathogens) attributes of several weed and pest populations. Ojiambo and Scherm (2006) conducted a meta-analysis to determine the overall effectiveness of biocontrol agents in relation to biological and application oriented factors. Ojiambo et al. (2010) published a quantitative review on fungicide efficacy trials for controlling cucurbit downy mildew in the eastern United States from 2000 to 2008. Moreover, meta-analysis based case study was performed in Western Australia to examine the impact of fungicides on wheat yield loss from yellow spot-septoria nodorum blotch disease (Salam et al., 2013). It is also used to study various questions relevant to plant pathology (Dalla Lana et al., 2015; Huang et al., 2012; Ngugi et al., 2011; Paul et al., 2011). In the present study, we use a meta-analytical approach to quantitatively review the results of studies on biological control efficacy for controlling Ralstonia wilt disease. Hence, the objectives of the present meta-analysis study were to (i) quantify the overall efficacy of BCAs in reducing Ralstonia wilt; (ii) study the effect of BCAs on plant growth promotion and crop yield; (iii) determine what extent biological control efficacy is influenced by plant type, method of BCA applications and experimental conditions (greenhouse vs. field condition); (iv) evaluate differences in efficacy among microbial species used to control Ralstonia wilt.
Materials and Methods
Literature search and data collection
To build a database, searches were conducted in Web of KnowledgeTM and the references cited in publications were retrieved. Articles were also collected from electronic databases (Science, Nature, Elsevier-Science Direct, Springer, and Wiley & Blackwell), and on the Web of Science® and Google ScholarTM. We conducted an extensive literature search in the databases during 15th of October, 2014 to 3rd of February, 2015 by using key words such as biocontrol/biological control of Ralstonia wilt or R. solanacearum, control of R. solanacearum, biocontrol efficacy against R. solanacearum, biological antagonist against R. solanacearum, PGPR against R. solanacearum, and PGPF (plant growth promoting fungi) against R. so-lanacearum.
The titles and abstracts of the collected articles were checked for information about application of BCAs treating R. solanacearum, in addition for increasing shoot, root and total biomass and/or yield. These searches resulted in 650 research publications, which were further screened for meeting our inclusion criteria. A set of criteria was applied to determine whether or not a particular report could be included in the analysis. First, studies need to report at least one measure of disease intensity such as disease incidence, disease severity, and area under the disease progress curve. Second, studies needed to have a treatment with one or more bacterial/fungal species present. Third, we included studies that report data on biological control, but we did not include data from chemical control agents (for e.g., Chitosan, Silicon, etc.). Considering these inclusion criteria, finally, 51 publications were selected. Subsequently, we extracted data for the moderator variables and effect sizes that are described below (see the Appendix 1–3).
Data acquisition
For each study, meta-analysis required the mean, standard deviation (SD) and replicate number/sample size (n) for the control as well as the microbial inoculation. If standard error values (SE) were reported, these were transformed according to the equation: SE = SD (n−1/2) using Meta-Win 2.1 Statistical calculator. When means and errors were presented in the graph, the image was digitized, and Dexter (GAVO data center) software was used to estimate the values (http://dc.zah.uni-heidelberg.de/sdexter/).
Categorical analysis of moderator variables
Categorical analyses were made using the retrieved data in order to determine responses by BCAs against R. solancearum by considering different moderator variables mentioned below:
Microbes/antagonist: Bacillus spp. (352 trials from 29 studies), Pseudomonas spp. (257 trials from 25 studies) and other species (287 trials from 26 studies).
Microbial/Antagonist richness: (i) single inoculation: a single species applied alone (796 trials from 50 studies), (ii) co-inoculation: two different species applied as a co-inoculation or as a consortium (100 trials from 11 studies).
Plant species and plant family: Plant species were represented in the analysis, with data for family members of the Moraceae, Solanaceae, and Zingiberaceae included. The plants were classified by using the PLANTS database of the USDA, Natural Resources Conservation Service (http://plants.usda.gov/java/).
Plant life cycle: Studies were divided into annual (673 trials from 40 studies) and perennial (223 trials from 11 studies) plants. The plants were classified by using the PLANTS database of the USDA, Natural Resources Conservation Service (http://plants.usda.gov/java/).
Method of application: Foliar spray (35 trials from 3 studies), soil drench (451 trials from 32 studies), seed treatment (282 trials from 17 studies), root treatment (26 trials from 5 studies), stem injection method (32 trials from 2 studies), and multiple treatments (70 trials from 3 studies).
Experimental condition: greenhouse (741 trials from 47 studies), containing all studies performed in indoor experiments under controlled environmental conditions, and field (155 trials from 12 studies), containing all outdoor studies.
Effect size calculation
The effect sizes of inoculated microbial effects were calculated as the natural log of the response ratio (LRR) as metric used to evaluate the efficacy of BCAs treatments in each study. According to Rosenberg et al. (2000), the response ratio is the ratio of some measure of outcome in an experimental group to that of the control group. LRR calculations and statistical analysis were conducted by the MetaWin v2.1 software (Rosenberg et al., 2000). For each study, the effect size was calculated as
Where, R is the response ratio, XC is the control mean (without BCAs), XE is the treatment mean (with BCAs), SC is the control SD, SE is the treatment SD, NC is the control replication number and NE is the treatment replication number (Rosenberg et al., 2000). We utilized the fail-safe number calculated by Rosenthal method to determine the number of non-significant, unpublished or missing studies, which would have to be added to our meta-analysis to nullify its overall effect size (Rosenberg, 2005). If this number is larger than 5n + 10 (n, number of studies), then publication bias can be safely ignored as the results are robust even if publication bias exists. We also checked existence of publication bias via scatter-plots and/or funnel plots of effect size vs. sample size or variance, respectively (see the Fig. S1 in Appendix 3).
Estimation of between-studies variance
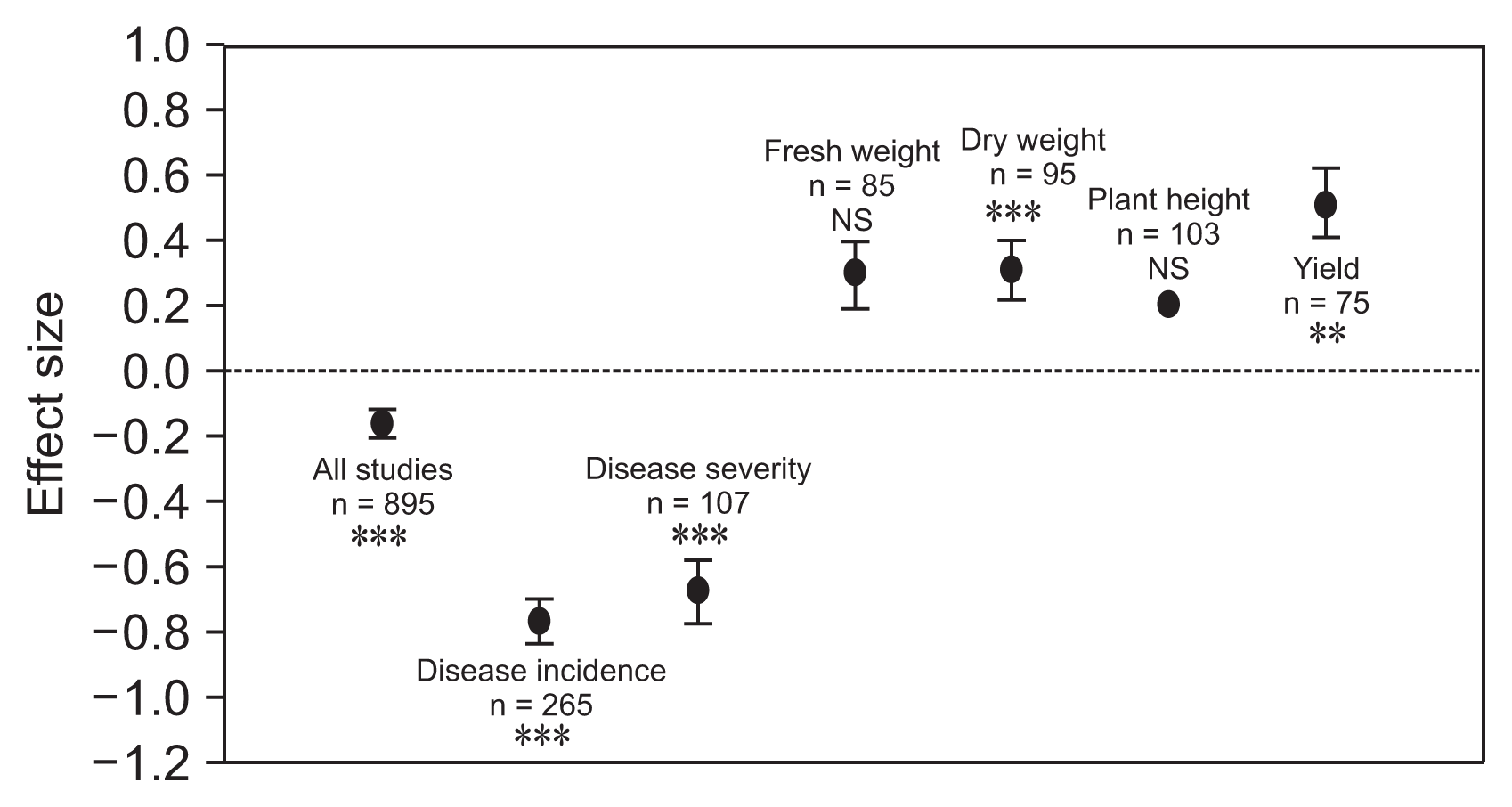
When conducting moderator variable analysis, three Q statistics were generated per factor: one for the variation within categories (QW), one for the variation between categories (QB), and lastly for the total Q (QT), which is the sum of the previous two (QT = QW + QB). A significant QT value means that the variance among studies is greater than expected due to sampling error. Moderator variable had a significant impact on the response ratio when QB was larger than a critical value. In the present meta-analysis, factors were considered as significant when QB was significant and described at least 10% of the total variation (QB / QT ≥ 0.1). A significant QB value indicates that a significant portion of the total heterogeneity can be explained by subdividing the studies into the group of interest (Rosenberg et al., 2000).
The majority of the studies in our data table violated the assumption of study independence. We applied two types of corrections to reduce the non-independence issue (Chandrasekaran et al., 2014; Lehmann et al., 2014, Lehmann and Rillig, 2015): (i) For studies presenting trials with multiple treatments and one common control, we combined and corrected the data to calculate a single effect size value following the approach of Lajeuness (2011); (ii) For studies presenting multiple trials, we combined trials to one effect size value following fixed-effects meta-analytical approach unless trials differed in factors assigned to moderator effects. We performed a sensitivity analysis for all moderator variables that had yielded significant results to test for single studies having disproportional impact (Copas and Shi, 2000; see the Fig. S2–S6 in Appendix 3).
Statistics
We used the statistics software Meta Win v2.1 for the analyses (Rosenberg et al., 2000). We conducted random effects meta-analyses to evaluate the overall biocontrol efficacy and to assess heterogeneity in our effect sizes (Lehmann and Rillig, 2015). Q was compared against a chi-squared distribution with n-1 degrees of freedom (Lehmann and Rillig, 2015). A significant Q indicated that the studies included in our dataset were more heterogeneous than expected by sampling error (Cooper, 1998). Consequently, we analyzed our moderator variables, if they could explain this detected variability in our dataset. Therefore, we investigated the significance of the moderators; P < 0.05 was considered statistically significant.
Results
The overall efficacy of BCAs
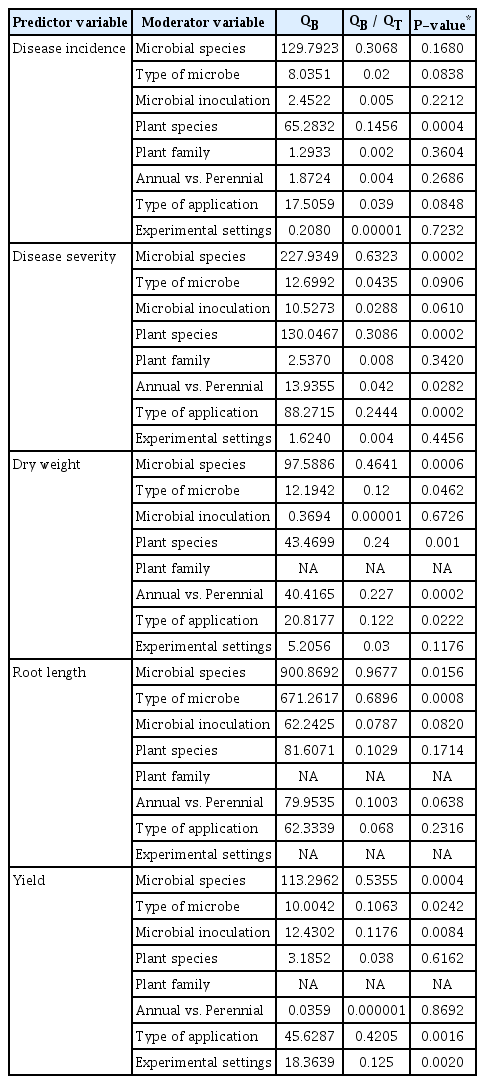
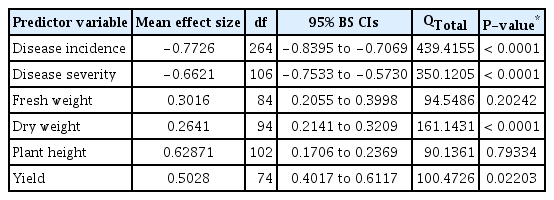
The overall effect size of BCAs against bacterial wilt was negative and significantly different from zero according to 95% boot-strap confidence intervals (BS CIs) (LRR = −0.1630, 95% BS CIs = −0.2094 to −0.1163; Fig. 1). The null hypothesis of homogeneity across studies was rejected (QT = 1,460.1, degree of freedom [df] = 895, P < 0.05), indicating that variability in LRR was greater than would be expected due to random sampling error. Thus, a random-effects meta-analysis was performed to determine the effect of moderator variables on disease suppression.
Effect of biological control agents on disease suppression and plant growth promotion. Error bars are means ± 95% boot-strap confidence intervals (BS CIs). Where the BS CIs do not overlap the horizontal dashed lines, the effect size for a parameter is significant at P < 0.05. All effect sizes differed significantly from zero (chi-square tests, ***P < 0.001, **P < 0.01, NS = P > 0.05). n, number of studies included in the meta-analysis.
Effect of BCAs on disease incidence
BCAs significantly decreased disease incidence (df = 264, LRR = −0.7726, 95% BS CIs = −0.8395 to −0.7069; Fig. 1, Table 1), compared to plants not inoculated with BCAs. Fail safe numbers of these effects tended to be extremely large (78948) compared to the number of independent comparisons included in the meta-analysis, indicating the robustness of the results. Besides, the null hypothesis of homogeneity across studies was rejected (P < 0.05) and thus, we performed moderator analysis for disease incidence.
Statistical results of comparison among variables for biocontrol efficacy of biological control agents against Ralstonia wilt disease
There were no significant effect of moderator variables on the effect sizes of disease incidence, except plant species (QB = 65.2832, QB / QT = 0.1456, P = 0.0004; Fig. 2A, Table 2). Among plant species, S. melongena and S. lycopersicon had a less negative effect than S. tuberosum and C. annuum. BCAs were less negative for annual plants (n = 188, LRR = −0.7961, BS CIs = −0.8790 to −0.7164) than that of perennial plants (n = 77, LRR = −0.7157, BS CIs = −0.8305 to −0.6032). In addition, there were no significant (P > 0.05) effect of microbial species, type of microbes and microbial richness (Fig. 2B). Even though, among microbial species, B. cereus, followed by P. putida, P. fluorescens had highest and B. subtilis and B. amyloliquefaciens showed lowest effect size values, the BS CIs did not overlap with zero. Among genus used as BCAs, Pseudomonas spp. (n = 98, LRR = −0.7298, BS CIs = −0.8355 to −0.6316) were found to be more effective than those of Bacillus spp. (n = 98, LRR = −0.8796, BS CIs = −1.0061 to −0.7541) inoculated plants for disease suppression. For the moderator experimental condition, there was no significant detectable effect. However, greenhouse studies had greater effect size than those of field studies (Fig. 2C).
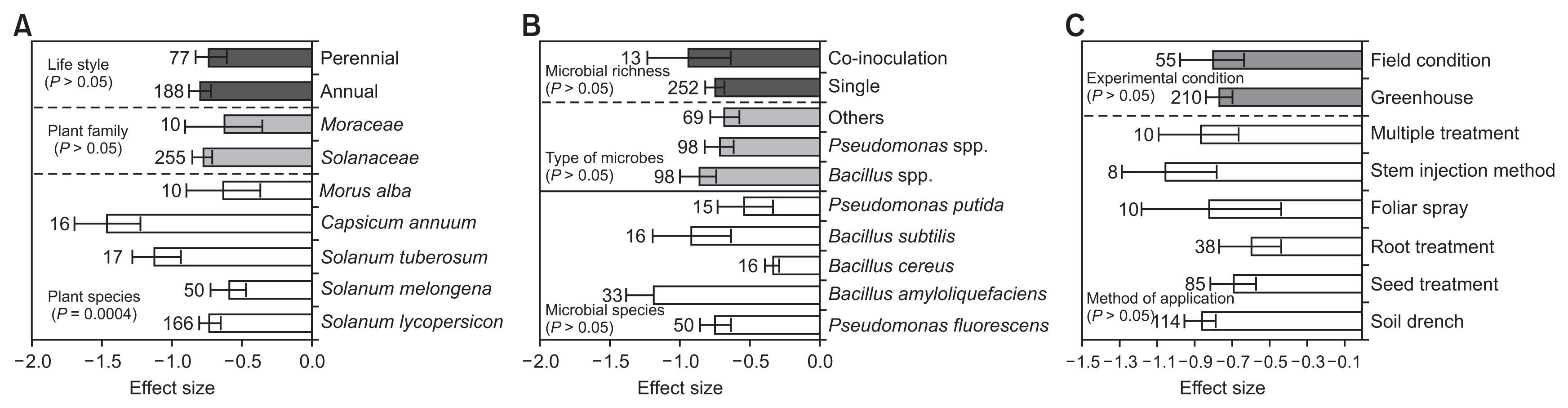
Effect of biological control agents on disease incidence. (A) Effect sizes categorized by plant species, plant family and life style. (B) Effect sizes categorized by microbial species, type of microbes and microbial richness. (C) Effect sizes categorized by method of application and experimental condition. Where the boot-strap confidence intervals do not overlap with zero, the effect size for a parameter is significant at P < 0.05. Numbers of studies are shown above the bars.
Effect of BCAs on disease severity
Disease severity was significantly lower in BCAs inoculated plants compared to control (df = 106, LRR = −0.6621, 95% BS CIs: −0.7533 to −0.5730; Fig. 1, Table 1). There was significant variation among studies BCAs showed decreased disease severity in S. tuberosum and S. lycopersicon than Z. officinale and C. annuum (Fig. 3A). In addition, plant life style had a significant effect on disease severity (QB = 13.9355, QB / QT = 0.042, P = 0.02; Table 2). BCAs mediated decrease in disease severity by perennial plants was significantly higher than that of annual plants (Fig. 3A). Moderator variable analysis showed significant differences between microbial species (QB = 227.9349, QB / QT = 0.6323, P = 0.0002; Table 2). B. cereus, showed greater decrease in disease severity than P. fluorescens and B. subtilis (Fig. 3B). Among type of microbes, Pseudomonas spp. (n = 20, LRR= −0.4789, BS CIs: −0.6571 to −0.3292) had greater effect on disease severity compared to those of Bacillus spp. (n = 47, LRR = −0.6578, BS CIs: −0.8149 to −0.5155). We also found significant effect on method of application for disease severity in BCAs inoculated plants (QB = 88.2715, QB / QT = 0.2444, P = 0.0002; Table 2). Among methods applied, seed treatment had significantly less negative than those of soil drench method in BCAs inoculated plants. We additionally tested for a possible confounding effect of disease severity on experimental condition and found no significant statistical evidence (Fig. 3C).

Effect of biological control agents on disease severity. (A) Effect sizes categorized by plant species, plant family and life style. (B) Effect sizes categorized by microbial species, type of microbes and microbial richness. (C) Effect sizes categorized by method of application and experimental condition. Where the boot-strap confidence intervals do not overlap with zero, the effect size for a parameter is significant at P < 0.05. Numbers of studies are shown above the bars.
Effect of BCAs on plant biomass
We further explored the BCAs mediated impact on plant biomass by investigating different tissues (shoot and root) as response variable. It was found that microbial species varied in their impact on fresh weight (df = 84, LRR = 0.3016, BS CIs: 0.2055 to 0.3998, P > 0.05) and dry weight (df = 94, LRR = 0.2641, BS CIs: 0.2141 to 0.3209, P > 0.001) (Fig. 1). For dry weight, both shoot and root dry weight yielded a positive microbial mediated effect (shoot: 78.5% root: 92.5%). However, the differences between tissue types were not significant for any effect size except overall dry weight.
Moderator variable analysis of dry weight indicated that plant species, life style (annual vs. perennial plants), microbial species, type of microbes, and method of application contributed significantly to dry weight in BCAs inoculated plants, whereas microbial richness and experimental settings were not statistically different between trials (Fig. 4, Table 2). Categorical analysis of the host plant species (QB = 43.4699, QB / QT = 0.24, P = 0.001; Table 2) showed that S. tuberosum followed by S. lycopersicon had highest and C. annuum lowest effect size values (Fig. 4A). When compared within plant life style (QB = 40.4165, QB / QT = 0.227, P = 0.0002), the microbial responses of perennial plants were significantly greater than those of annual plants. Microbial species (QB = 97.5886, QB / QT = 0.4641, P = 0.0006; Table 2) categorical analysis showed the effects of most used species of B. amyloliquefaciens, B. cereus, B. subtilis, and P. fluorescens were found to be effective in plant growth promotion in all studies (Fig. 4B). Among type of microbes (P = 0.04), Bacillus spp. had more positive effect than Pseudomonas spp. In addition, method of application had a significant effect on dry weight (QB = 20.8177, QB / QT = 0.122, P = 0.02). Stem injection method had highest effect size, but sample size was too low (n = 6) to draw a common conclusion. However, seed treatment highest effect size values and soil drenching method had lowest. For the predictor experimental settings, there was no significant effect found. However, greenhouse condition had more positive effect than that of field condition (Fig. 4C).
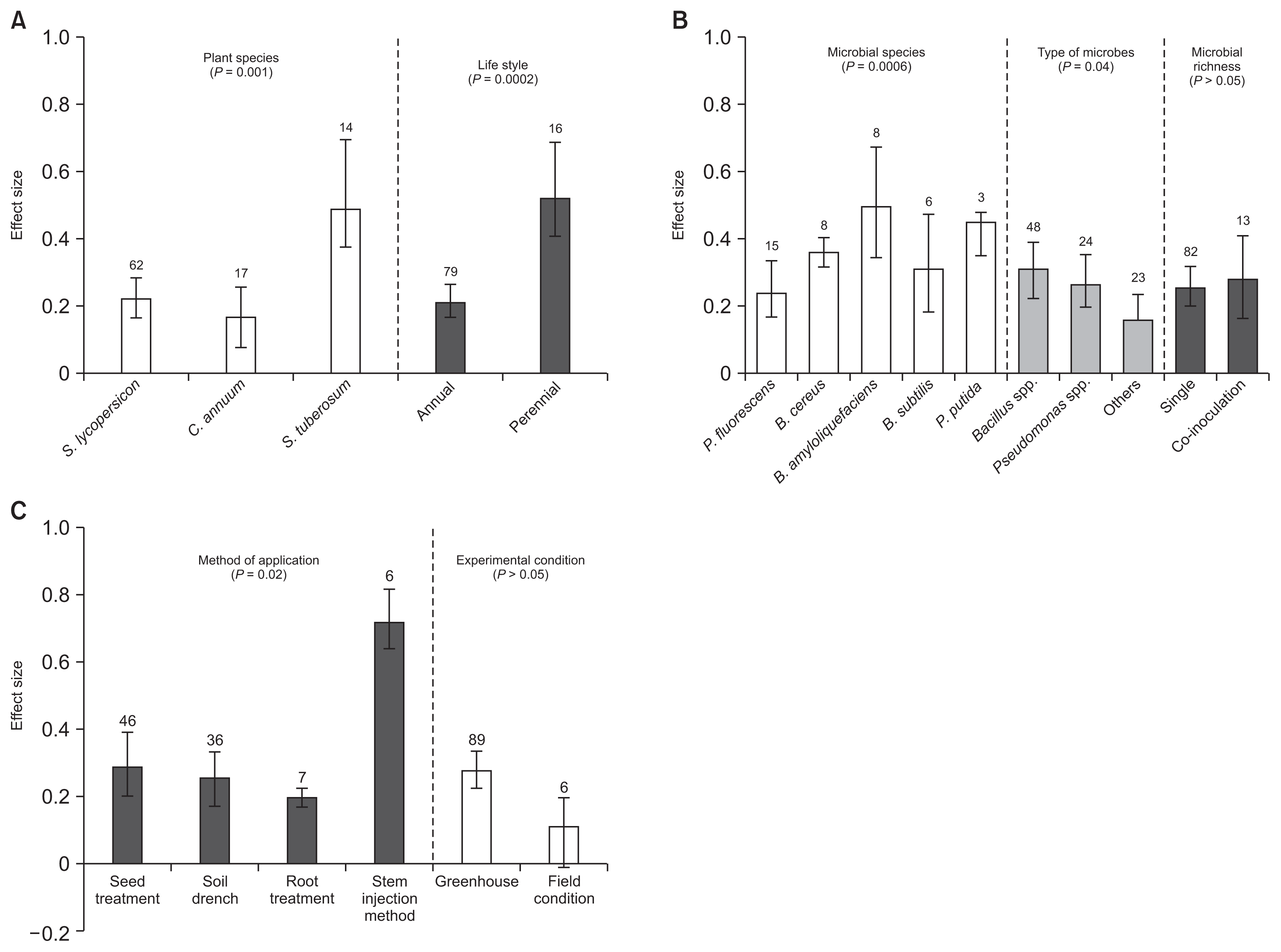
Effect of biological control agents on dry weight. (A) Effect sizes categorized by plant species, and life style. (B) Effect sizes categorized by microbial species, type of microbes and microbial richness. (C) Effect sizes categorized by method of application and experimental condition. Where the boot-strap confidence intervals do not overlap with zero, the effect size for a parameter is significant at P < 0.05. Numbers of studies are shown above the bars. S. lycopersicon, Solanum lycopersicon; C. annuum, Capsicum annuum; S. tuberosum, Solanum tuberosum; P. fluorescens, Pseudomonas fluorescens; B. cereus, Bacillus cereus; B. amyloliquefaciens, Bacillus amyloliquefaciens; B. subtilis, Bacillus subtilis; P. putida, Pseudomonas putida.
Effect of BCAs on yield
We detected a significant positive effect of BCAs on crop yield; BCAs increased crop yield (n = 75, LRR = 0.5028, BS CIs: 0.4017 to 0.6117; Fig. 1, Table 1). The null hypothesis of homogeneity across studies was rejected (QT = 100.4726, df = 74, P < 0.05; Table 2), indicating that variability in LRR was greater than would be expected due to random sampling error. Thus, a random-effects meta-analysis was performed to determine the effect of moderator variables.
Plant species and life style (annual vs. perennial plants) were not significantly affected by BCAs (P > 0.05; Table 2, Fig. 5A). However, significant differences in efficacy were found between microbial species (QB = 113.2962, QB/ QT = 0.5355, P = 0.0004; Table 2) on crop yield. Treatment with Pseudomonas sp. has significantly increased plant yield than that of Bacillus spp. These variations are, in order of the most efficient microbial species, P. fluorescens, followed by P. putida, Serratia spp., B. subtilis and B. amyloliquefaciens. Moreover, our data showed that microbial richness (QB / QT = 0.12, P = 0.008) had significant effect on crop yield (Fig. 5B). Also, the responses to BCAs inoculation for method of application varied significantly under diseased condition (QB / QT = 0.421, P = 0.001; Table 2, Fig. 5C). Crop yield was more pronounced when BCAs were applied as a seed treatment than as a soil drench and root treatment. We found significant variation between studies for crop yield (QB / QT = 0.125, P = 0.002). Greenhouse studies had a significantly greater effect size values than field studies (Table 2, Fig. 5C).
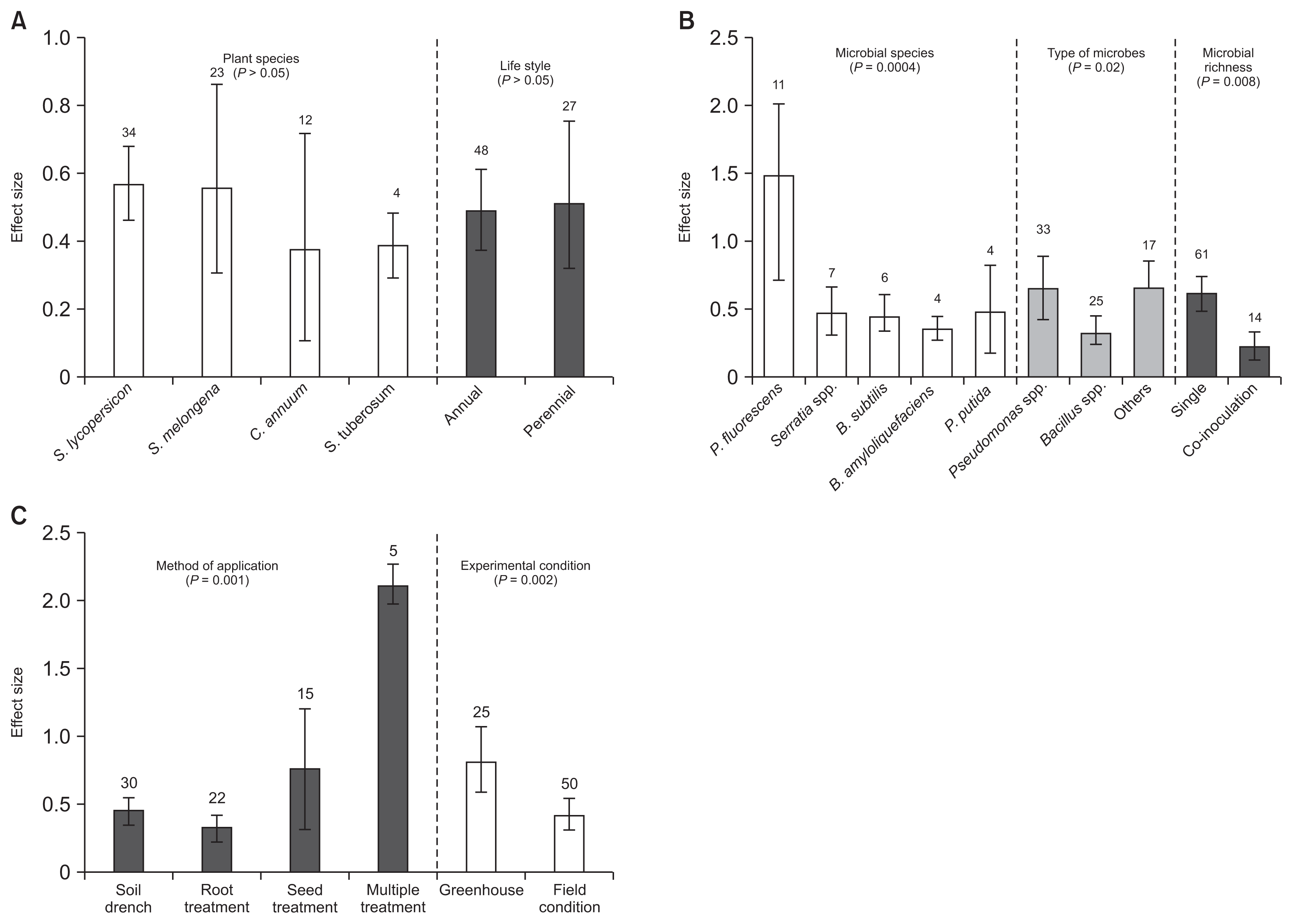
Effect of biological control agents on crop yield. (A) Effect sizes categorized by plant species, and life style. (B) Effect sizes categorized by microbial species, type of microbes and microbial richness. (C) Effect sizes categorized by method of application and experimental condition. S. lycopersicon, Solanum lycopersicon; S. melongena, Solanum melongena; C. annuum, Capsicum annuum; S. tuberosum, Solanum tuberosum; P. fluorescens, Pseudomonas fluorescens; B. subtilis, Bacillus subtilis; B. amyloliquefaciens, Bacillus amyloliquefaciens; P. putida, Pseudomonas putida.
Discussion
Biological control using plant growth promoting microorganisms is an efficient approach to wilt management and is also considered environment-friendly. Recently, several studies focused on biological control of Ralstonia wilt (Ji et al., 2008; Singh and Siddiqui, 2015; Takenaka et al., 2008; Vanitha et al., 2009). Several beneficial microorganisms have been implemented as biocontrol agent for suppressing R. solanacearum. Therefore, we used meta-analysis to interpret results and drawing conclusions from earlier experimental studies (Gurevitch and Hedges, 1999; Koricheva and Gurevitch, 2014). This analytic approach allows for the evaluation of study-specific characteristics that are likely to influence treatment effects (Chandrasekaran et al., 2014; Gurevitch and Hedges, 1999). Several plant pathologists have made use of meta-analysis study to unveil the effects of biocontrol agents (Ojiambo et al., 2010; Ojiambo and Scherm, 2006; Rosenberg et al., 2004; Salam et al., 2013; Stiling and Cornelissen, 2005). In the present meta-analysis study, resolute evidence is provided that BCAs inoculation in controlled or replicated studies causes significant impact on the ecological predictor variables such as disease incidence and disease severity, plant biomass, and yield in R. solancearum infected plants (Fig. 1, Table 1).
The present investigation confirmed that the extent of disease suppression by BCAs varied widely among trials, with LRR ranging from −2.84 (i.e., strong disease suppression by the antagonist) to 2.13 (disease strongly enhanced by application of the bio-control agent). Our data analyses also showed that the applications of BCAs significantly reduced bacterial wilt severity as compared with the untreated plants by more than 49%. Among antagonist, Pseudomonas spp. was found to be more effective BCAs for disease suppression than those of Bacillus spp. Among Pseudomonas spp., P. fluorescens significantly reduced the bacterial wilt incidence and may have increased host resistance to bacterial wilt, hence it has the potential to be used for management of bacterial wilt. There are several bacterial determinants like lipopolysaccharides, siderophore and salicyclic acid production by P. fluorescens stains involved in induce systemic resistance in plants against Ralstonia wilt (Abo-Elyousr et al., 2012; Dey et al., 2014; Maji and Chakrabartty, 2014; Raaijmakers et al., 2006; Vanitha et al., 2009; Zhou et al., 2014).
Nevertheless, members of the genus Bacillus are also often considered to be important biocontrol agents. A total of 29 studies (352 trials) in the dataset used Bacillus spp. as BCAs. Our analysis showed that the mean effect sizes for these trails are significantly lower compared with trials that applied for other BCAs. Previous meta-analysis also reported that there may be inconsistencies in the efficacy of Bacillus spp. as a BCA against soilborne diseases (Ojiambo and Scherm, 2006). According to Cook (1993), the inconsistencies in the efficacy of BCAs may be due to use of single strain as BCAs and a related arbitrary application of few widely used Bacillus strains may be revealed in our dataset. Among Bacillus species, B. cereus, B. subtilis and B. amyloliquefaciens significantly reduced both bacterial wilt incidence and severity under both greenhouse and field conditions than other Bacillus species (Chen et al., 2014; Dey et al., 2014; Kurabachew and Wydra, 2013; Shan et al., 2013).
A general question raised over the technique employed in conventional biocontrol programs has concerned co-inoculation introductions against a single antagonist. It is perceived that co-inoculation of microbial species can result in synergistic, additive, or inhibitory effects on target performance compared to the effect of each antagonist alone. In our analysis, we also found that co-inoculation had greater disease suppression than those of single inoculation. Our results differ from other studies that supported the use of single inoculation compared to co-inoculation against R. solancearum. Previous meta-analysis studies also supported that multi-species application of BCAs were consistently more effective than single species application (Stiling and Cornelissen, 2005).
A suitable field application method is needed once a good potential BCA is identified. The results indicated that type of an application method had a significant impact on the colonization of a BCA on plants. We found that best disease control is obtained when BCAs are applied as seed treatment and soil drench compared with other method of application. Among method of application seed treatment was found to be the best method of application of BCAs. This result agrees with previous literature (Abo-Elyousr et al., 2012).
In our investigation, most of the isolates showed significantly less control efficacy in the field trial than greenhouse. According to previous literature, the BCAs have shown good response against R. solanacearum in vitro and in the greenhouse but have unfortunately failed under field conditions (Liu et al., 2009; Lugtenberg et al., 2001; Wei et al., 2011). This inconsistent performance is probably due to a change in the colonization ability of the antagonistic strains or inadequate delivery of the agent in the rhizosphere environment or prevailing competition in rhizosphere region (Lugtenberg et al., 2001; Yuan et al., 2014). According to Liu et al. (2009) and Yuan et al. (2014), maintaining the population of BCAs above certain level in the soil is an important factor to determine biocontrol efficacy in both greenhouse and field condition. This is also due to greenhouse environment providing a place where manipulation of environmental conditions is feasible to optimize the effectiveness of BCAs (Bélanger and Benyagoub, 1997; Paulitz and Bélanger, 2001).
Plant type significantly affected BCA efficacy. Meta-analyses showed that BCAs were more effective on annual plants than perennial ones. According to Ojiambo and Scherm (2006), the lower degree of disease suppression in perennial plants may be due to the larger number of pathogen inoculum source and absence of crop rotation, which may give rise to polyetic epidemics that generally more difficult to control. Our results showed that greenhouse studies had a greater effect size values than field studies. Paulitz and Bélanger (2001), suggest that the greenhouse environment presents a unique situation that enables disease managers to optimize the effectiveness of BCAs through manipulation of environmental conditions. Thus, it is reasonable to expect greater disease suppression in greenhouse than in the field applications of BCAs. However, our analysis showed no significant difference in mean effect sizes between entries in which biological agents were applied in these two environments.
Our meta-analyses confirmed that fresh weight, dry weight, plant height and root length were higher in with BCA treatment than with no BCA treatment and control, demonstrating that BCAs are not only reduced bacterial wilt and also promoted plant growth. Moderator variable only significantly affected dry weights, but neither fresh weight nor plant height thus; we carried out further categorical analysis only for yield (dry weight).
Our meta-analyses also showed that the BCAs inoculation resulted in higher yields. Yield response varied significantly among microbial species. The relative yield gain was greater for Pseudomonas spp. inoculated plants than for Bacillus spp. inoculated plants. Among microbial species, P. fluorescens was found to be efficient in increasing crop yield compared to other microbial species. Guo et al. (2004) reported comparable R. solanacearum wilt disease reduction and yield increase of tomato plants after treatment by Pseudomonas spp. and Bacillus spp.
Our results confirm the variability in yield response to BCAs inoculated plants, and indicated that there is a relationship between yield response and method of application of the BCA. Seed treatment had shown greatest yield response followed by soil drench method and root treatment. In addition, experimental condition significantly affected yield response; that is, greenhouse studies had a greater positive effect on yield than for field condition. Experiments performed under controlled conditions (greenhouse) allow for sorting out complex interactions but can lead to overestimated effect sizes compared to field trials due to exclusion of potential confounding factors (Lehmann and Rillig, 2015). Also, for our analysis, the number of trials for the experimental condition was unbalanced. Thus, more studies need to be carried out in the field conditions for evaluating the comparability of greenhouse and field studies for BCAs inoculated plants on disease suppression and yield response.
Two Pseudomonas species P. fluorescence and P. putida, and three Bacillus species, B. cereus, B. subtilis and B. amyloliquefaciens, effectively controlled the Ralstonia wilt. Among them P. fluorescence was the best. Co-inoculation method was found in general, to be more effective for the control of Ralstonia wilt than single. However, if there is a possibility to find ecologically significant and better surviving partner (to known best Pseudomonas and/or Bacillus strains), such combinations would be suitable to test in open field applications in future. The inconsistent biocontrol efficacy in the greenhouse and field trials observed in this study was, at least partially, due to the differential colonization abilities of antagonistic strains and prevailing competition in rhizosphere. The seed treatment, soil drench and multiple treatments of antagonistic strains have not only controlled the wilt disease, but also significantly increased the plant yields. Since some of the tested bacterial strains have both growth promoting and disease suppression capabilities together, these strains have great potential to be used for commercial production of bioorganic fertilizer for the control of Ralstonia wilt and can also be extended to mitigate the effect of other phytopathogens as well.
Supplementary Information
Acknowledgments
This work was supported by the KU Brain Pool (2015–2016) of Konkuk University, Seoul, Republic of Korea.
Notes
Articles can be freely viewed online at www.ppjonline.org.