 |
 |
| Plant Pathol J > Volume 32(3); 2016 > Article |
Abstract
Most biocontrol agents for plant diseases have been isolated from sources such as soils and plants. As an alternative source, we examined the feces of tertiary larvae of the herbivorous rhino beetle, Allomyrina dichotoma for presence of biocontrol-active microbes. The initial screen was performed to detect antifungal activity against two common fungal plant pathogens. The strain with strongest antifungal activity was identified as Bacillus amyloliquefaciens KB3. The inhibitory activity of this strain correlated with lipopeptide productions, including iturin A and surfactin. Production of these surfactants in the KB3 isolate varied with the culture phase and growth medium used. In planta biocontrol activities of cell-free culture filtrates of KB3 were similar to those of the commercial biocontrol agent, B. subtilis QST-713. These results support the presence of microbes with the potential to inhibit fungal growth, such as plant pathogens, in diverse ecological niches.
Biological control, in which antagonistic microorganisms such as bacteria, yeasts, and filamentous fungi, fight plant pathogens, is recognized as a safe and sustainable alternative to chemical-based pesticides (Ongena and Jacques, 2008). Many biocontrol agents have been isolated from suppressive soils (Hornby, 1983; Schroth and Hancock, 1982). A microbiome analysis of the rhizosphere in disease-conducive and disease-suppressive soils indicates the importance of certain groups of root-colonizing Pseudomonas spp. (Mendes et al., 2011). Bacillus isolates from various sources have also demonstrated biocontrol capabilities (Choudhary and Johri, 2009; Siripornvisal, 2010), and Bacillus derived-products constitute about half of the commercially available biopesticides (Cawoy et al., 2011; Ongena and Jacques, 2008). Surfactant cyclic lipopeptides (CLP) produced by many Bacillus spp. are one class of metabolites implicated in biocontrol. The surfactants include surfactin, iturin A, and fengycin. These lipopeptides antagonize pathogen growth and induce systemic resistance against plant diseases (Ongena and Jacques, 2008). Additionally microbes have the potential to control insects. The entomopathogenic microbe, B. thuringiensis, is used globally to control various insects (Martin and Travers, 1989). Pseudomonas isolates also display insecticidal activities, in addition to properties that improve plant health (Kupferschmied et al., 2013; Olcott et al., 2010; Ruffner et al., 2013). Thus, insects act as hosts for biocontrol-active microbes (Nadarasah and Stavrinides, 2011; Shanchez-Contreras and Vlisidou, 2008).
In the present study, we screened for the feces of the larva of the herbivorous beetle, the Asian rhino beetle, Allomyrina dichotoma, for active strains of biocontrol agents. In nature, although the adults feed on fruit, nectar, and sap, the larvae consume decaying plant matter, thus, serving as primary decomposers in ecosystems. The enzymes produced by microorganisms in the guts of insects facilitate the breakdown of lignified woody plant materials (Breznak and Brune, 1994). Consequently, in the present study, the larvae were grown on decomposing sawdust as a mimic of natural soil substrates. Additionally, gut microbes aid in insect nutrition, development, and reproduction, and contribute to pathogen resistance (Brune, 2003; Moran et al., 2005). For instance, Bacillus strains can activate immunity against plant diseases, as observed in foliar aphids and whiteflies feeding on pepper (Lee et al., 2012; Song et al., 2015).
Feces of A. dichotoma tertiary larvae were suspended in sterile phosphate buffer (pH 7.5) and serial dilutions were spread evenly on one-third strength KingŌĆÖs B (KB) agar (King et al., 1954). Over 2,000 colonies were isolated from plates incubated at 28┬░C for 14 hours. Stocks of these isolates were maintained in full-strength KB broth (KBB) at ŌłÆ80┬░C. Cultures were revived by streaking suspension onto KB agar and incubating at 28┬░C ┬▒ 2┬░C for 48 hours. Other media used in this study were Luria-Bertani medium (LB; Difco, Sparks, MD, USA), M9 minimal agar (M9; Sambrook and Russell, 2001), Taylor and Francis medium (TF, containing 20 g peptone, 25 g sucrose, and 4.5 g/l yeast extract; Mezghanni et al., 2012), Ok Chun medium (OC, containing 6 g glucose, 8 g yeast extract, 2.5 g K2HPO4, 1.5 g NaCl, 0.5 g Na2CO3, and 1 g/l MgSO4; Ha, 2013), nutrient broth medium (Difco), and potato dextrose broth (PDB; Difco). For growth on plates, liquid PDB was supplemented with agar for potato dextrose agar (PDA; Difco). Phytopathogenic fungal isolates were obtained from the Korean Agriculture Culture Center (Suwon, Korea) included Phytophthora capsici (KACC No. 40111), Botrytis cinerea (KACC No. 40573), Colletotrichum coccodes (KACC No.40010), Rhizoctonia solani (KACC No. 40101), and Fusarium oxysporum (KACC No. 40031). All fungi were cultured on PDA plates at room temperature.
Isolates were first screened for the inhibition of B. cinerea and R. solani growth using a radial diffusion assay (Bajpai and Kang, 2012). Isolates that inhibited these fungi were subsequently screened for biosurfactant production by an assay of their culture filtrates by observing the collapse of oil droplets (Bodour and Miller-Maier, 1998). One hundred and ninety isolates that were positive for both traits were screened further, and two isolates, KB3 and LM11, were selected for further study because they showed the strongest antifungal activity.
The potassium hydroxide test (Gregersen, 1978) and scanning electron microscopy indicated that KB3 and LM11 were both rod-shaped gram-positive bacteria possessing lophotrichous flagella (Fig. 1). Biochemical analysis with the Vitek2 Compact BCL card system (bioM├®rieux, Hazelwood, MO, USA) showed a 90% probability match with Bacillus amyloliquefaciens. Both strains produced beta-xylosidase, Ala-Phe-Pro arylamidase, L-pyrrolydonyl-arylamidase, alpha-galactosidase, phenylalanine arylamidase, and beta-glucosidase, and utilized D-mannose, D-trehalose, D-mannitol, pyruvate, palatinose, and myo-inositol. In addition, both strains exhibited esculin hydrolysis, methyl-a-D-glucopyranoside acidification, tetrazolium red production, and polymyxin B and kanamycin resistance. The isolates tolerated high salinity, growing at 6.5% NaCl.
The identification of KB3 and LM11 as B. amyloliquefaciens isolates was confirmed by their 16S rRNA sequences. The gene was amplified by PCR using universal primers, forward primer 5ŌĆ▓-AGA GTT TGA TCC TGG CTC AG-3ŌĆ▓ and reverse primer 5ŌĆ▓-ACG GCT ACC TTG TTA CGA CTT-3ŌĆ▓ (Weisburg et al., 1991) from KB3 and LM11 genomic DNAs extracted from log-phase cultures in LB broth using an ExiPrepŌäó bacteria genomic DNA Kit (Bioneer Inc., Daejeon, Korea). The PCR reaction was performed with a Bioneer premix (Bioneer Inc.), and the 1.4 kb 16S rRNA sequence was obtained by Solgent ASSA service (Solgent, Daejeon, Korea). Nucleotide sequences were analyzed using BLASTN and the National Center for Biotechnology Information database. A phylogenetic comparison of the 16S rDNA sequence with related Bacillus spp. was performed. First, the sequence was aligned using CLUSTAL X and edited using BioEdit version 5. Then, a phylogenetic tree was constructed with MEGA6 software (Tamura et al., 2013) using the neighbor-joining method with the Kimura two-parameter model (Kimura, 1980) and bootstrap values based on 1,000 replications. The phylogenetic analysis showed that the 16S rDNA sequences of both isolates were 99.99% identical to the B. amyloliquefaciens subsp. plantarum CAU B946 sequence (Fig. 1). Consequently, the KB3 and LM11 isolates were identified as isolates of B. amyloliquefaciens.
B. amyloliquefaciens strains from other sources are used commercially in agriculture as biopesticides and biofertilizers (Li et al., 2015; P├®rez-Garc├Ła et al., 2011). Interestingly, endophytic B. amyloliquefaciens isolated from the seeds of ornamental hostas induced host resistance against plant diseases and insects (Li et al., 2015). However, there are limited reports on the isolation of Bacillus strains from insects (Sreerag et al., 2014).
Biocontrol-active isolates of B. amyloliquefaciens and B. subtilis produce various antimicrobial CLP, including surfactin, iturin A, and fengycin (Chowdhury et al., 2015; Ongena and Jacques, 2008; P├®rez-Garc├Ła et al., 2011). These metabolites have antifungal and antibacterial activities, affect bacterial motility and biofilm formation, and act as effectors to induce systemic resistance (P├®rez-Garc├Ła et al., 2011).
Isolates KB3 and LM11 produced surfactants that caused oil drops to collapse in the procedures to identify changes in surface tension as described by Bodour and Miller-Maier (1998). The method involved adding 2 ╬╝l of 10W-40 Pennzoil (Pennzoil, Houston, TX, USA) to wells of a 4-well cell culture plate (SPL Lifesciences, Pocheon, Korea) followed by the application of 5 ╬╝l of the cell-free cultures of KB3 and LM11. Cells were removed from 2-day cultures, grown in KBB with shaking at 200 rpm at 30┬░C, by centrifugation for 20 minutes at 10,000g (Vision, Bucheon, Korea). The supernatants were filtered through 0.2-╬╝m filters to remove all cells. Cell-free culture filtrates from biocontrol bacteria, Lysobacter antibioticus HS124 (Gardener et al., 2014) and Pseudomonas sp. NJ134 (Kang, 2012), which do not produce CLPs, were used as negative controls. B. subtilis QST-713, the main microbial ingredient of the biocontrol product, Serenade (AgraQuest, Davis, CA, USA), was used as a positive control. The collapse of an oil droplet in a well indicated surfactant activity; cell-free filtrates of B. amyloliquefaciens KB3 and LM11, and B. subtilis QST-713, caused droplet collapse (Fig. 2A).
The chemistry of the surfactants from isolate KB3 was investigated by thin layer chromatography (TLC) and high performance liquid chromatography (HPLC). Standards for surfactin and iturin A were used (Sigma-Aldrich, St. Louis, MO, USA). B. amyloliquefaciens KB3 was grown for 2 days at 30┬░C in a shaking incubator at 180 rpm in KBB. The culture filtrate was adjusted to pH 2.0 using 6 N HCl centrifuged for 20 minutes at 10,000g. After incubation at 4┬░C for 24 hours, the suspension was centrifuged and the supernatant collected and dried by rotary vacuum concentration. The dry materials were dissolved in chloroform and methanol (65:15, v/v) and spotted onto TLC plates (HX246144; Merck KGaA, Darmstadt, Germany) with chloroform:methanol:water (65:25:1, v/v/v) as the mobile phase solvent. Surfactin at 100 and 10 ppm (Sigma-Aldrich) was applied as a standard. Plates were developed and compounds detected as previously described (Nitschke and Pastore, 2006). Both the surfactin standard and the KB3 sample produced a white spot with a retention factor of 0.55 (Fig. 2B). The concentration of the KB3 culture extract was estimated to be approximately 20 ppm.
Qualitative and quantitative analyses of iturin A and surfactin in the lipopeptide extracts were carried out by comparing HPLC chromatogram peaks of iturin A and surfactin standards with those observed from the extracts (Fig. 2C). The supernatant was mixed 1:1 (v/v) with ethyl acetate, and the upper layer removed and filtered with a filter paper (Cat. No. 1006-110, Whatman; GE Healthcare, Buckinghamshire, UK). The sample was concentrated under nitrogen gas, weighed, and then re-dissolved in methanol. The methanol extract was passed through an Isolute C-18 CE type cartridge (International Sorbent Technology Ltd., Hengoed, UK) and injected onto a reverse-phase C18 column (Shimadzu Shim-pack VP-ODS, 250 l ├Ś 4.6 mm; Shimadzu, Kyoto, Japan) using an HPLC instrument (Shimadzu Prominence LC-20AD; Shimadzu). The column oven temperature was set at 40┬░C, and different ratios of distilled water, tetrahydrofuran, and acetonitrile were used as mobile solvents. The biosurfactants were detected at 214 nm. Iturin A and surfactin (Sigma-Aldrich) were dissolved in methanol to a final concentration of 1 mg/ml and used as standards (Kim et al., 2010). Retention times of the iturin A standard were 4.1, 4.9, and 6.5 minutes. Chromatograms of the culture filtrates from KB3 showed these same three peaks (Fig. 2C). Retention times of the surfactin standard were 25.2, 26.3, 28.0, and 29.1 minutes, and peaks at these times were detected from the KB3 extracts (Fig. 2C). The concentrations of the surfactants, calculated by peak areas, suggest that iturin A and surfactin were present at 1,064 ┬▒ 236 ╬╝g/ml and 3,779 ┬▒ 899 ╬╝g/ml, respectively.
PCR was used to identify genes that might be involved in the biosynthesis of surfactin and iturin A by strain KB3. Biocontrol lipopeptides are non-ribosomally synthesized by modularly organized mega-enzymes called, peptide synthases (NRPS). NRPS genes were clustered in an operon (Arguelles-Arias et al., 2009; Chen et al., 2007; Chowdhury et al., 2015). Primers used to amplify the genes encoding the lipopeptides were based on sequences from B. amyloliquefaciens FZB42 (Arguelles-Arias et al., 2009; Chen et al., 2007). Primers specific for the gene sfp involved in surfactin production were: forward, 5ŌĆ▓-ATG AAG ATT TAC GGA ATT TA -3ŌĆ▓ and reverse 5ŌĆ▓-TTA TAA AAG CTC TTC GTA CG -3ŌĆ▓. The primers for the ituD gene involved in iturin A production were: forward, 5ŌĆ▓-ATG AAC AAT CTT GCC TTT TTA-3ŌĆ▓ and reverse, 5ŌĆ▓-TTA TTT TAA AAT CCG CAA TT -3ŌĆ▓. The expected PCR had sizes of 600 bp for sfp and 1,203 bp for ituD. PCR reactions were performed in a thermocycler (Bioneer) in a 50 ╬╝l reaction volume containing KB3 genomic DNA with HelixAMŌäó Premium-Taq Polymerase Kit (NanoHelix Co., Daejeon, Korea). Negative controls without DNA and with the genomic DNA of Bacillus thuringiensis KACC12072 isolate that does not produce these surfactants were included in the assay. The amplified products were visualized in a 1.5% agarose gel, stained by EcoDye (SolGent). The expected PCR products were efficiently amplified from KB3, but not from B. thuringiensis KACC12072 (data not shown).
Our chemical analyses and the genetic information indicate that Isolate KB3 produces surfactants similar to surfactin and iturin A. These findings suggest that isolate KB3 expresses biocontrol activity in plants through several mechanisms, including inhibition of fungal pathogen growth, induction of systemic resistance in plants, and prevention of adherence of pathogens to plant surfaces (Chen et al., 2013; Chowdhury et al., 2015; Dietel et al., 2013; Kim et al., 2010; Ongena and Jacques, 2008; Wang et al., 2010). Surfactins from B. amyloliquefaciens S499 have been detected in a gnotobiotic rhizosphere (Nihorimbere et al., 2012), showing that nutrition from plants is adequate for their production. Whether lipopeptides increase plant growth requires further investigation (Buensanteai et al., 2008; Ernst et al., 1971). Surfactants are insecticidal in the larval midgut of the leaf miner Tuta absoluta (Ben Khedher et al., 2015) and the aphid Myzus persicae (Yun et al., 2013). The insecticidal activity for isolate KB3 is under investigation, and whether rhino beetle larvae are resistant has been considered. However, these findings illustrate that insects are valuable sources of microbes with the potential for agricultural significance in improving plant health.
For an organism to be commercially viable, methods for growth must be cost-effective. Consequently, antifungal activity was compared after growth of KB3 for 5 days at 30┬░C in a shaking incubator at 180 rpm on different growth media, including; LB, M9 containing glucose as the sole carbon source, TF, OC, and one-third strength KB. Cell-free culture filtrates were prepared by centrifugation at 13,000 rpm for 20 minutes at 4┬░C and filtration through a sterile membrane with a 0.2 ╬╝m pore size (Minisart, 0.2 ╬╝m; Sartorius Stedim Biotech, Melsungen, Germany). The cell-free filtrates were added to PDA at final concentrations between 0% and 10% (v/v). PDA was amended with the same amounts of sterile medium to allow measurement of control growth. Mycelial plugs from 7-day-old cultures of the fungal plant pathogens, R. solani, P. capsici, C. coccodes, and F. oxysporum were placed in the center of the PDA plates and incubated at 28┬░C in the dark. Mycelial growth was measured 1 week post incubation. Growth inhibition was determined using the following formula. Growth inhibition percentage = (R ŌłÆ r) / R ├Ś 100; where R is the radius of the fungal growth in the control plates, and r is the radius of the fungal growth in the plates containing the bacterial culture filtrates. Each experiment was repeated at least two times with three replicates per experiment.
The antifungal activities of KB3 significantly differed with the growth media (Fig. 3). The cell-free bacterial culture of KB3 grown in TF broth showed the highest in vitro antifungal activity for all fungal pathogens. The differences in antifungal activity were not related to bacterial cell number; the number of culturable cells in onethird KB and M9 media was 2.5 ┬▒ 1.4 ├Ś 107 and 3.5 ┬▒ 2.5 ├Ś 105 cfu/ml, respectively, whereas, in other media, the means were 5.8 ┬▒ 4.6 ├Ś 108 cfu/ml. Differential effects of media have been observed with other bacteria. The biocontrol activity of Pseudomonas chlororaphis O6 was influenced by the presence of glucose (Park et al., 2011), and biocontrol-active B. subtilis has produced high yields of biosurfactants with glucose as a carbon source, urea or ammonium chloride as a nitrogen source, and oxygenlimited conditions (Ghribi and Ellouze-Chaabouni, 2011).
The biocontrol activity of the culture filtrates of B. amyloliquefaciens KB3 and B. subtilis QST-713 as a positive control, grown on KB, was tested in planta against six different plant pathogens causing rice blast, rice sheath blight, tomato gray mold, tomato late blight, wheat leaf rust, barley powdery mildew, and pepper anthracnose (Kim et al., 2001). Briefly, plants were grown in vinyl pots in a green house at 25┬░C ┬▒ 5┬░C for 1-4 weeks. The plants were sprayed with 1:3 diluted cell-free 5-day-old culture filtrates grown in one-third KB. Tween 20 was used as a wetting agent. Distilled water with Tween 20 was used as the negative control. The treated plant seedlings were inoculated with spores or mycelial suspensions from one of the six plant pathogens 24 hours after exposure to culture filtrate, as described previously (Kim et al., 2001). Disease symptoms were assessed 3-7 days after inoculation, depending on the pathogen. The pots were arranged in a randomized complete-block design, with three replicates per treatment, and each replicate consisted of nine plants. The disease index was determined by measuring the percentage of the leaf area or plants that was infected. The experiment was conducted three times.
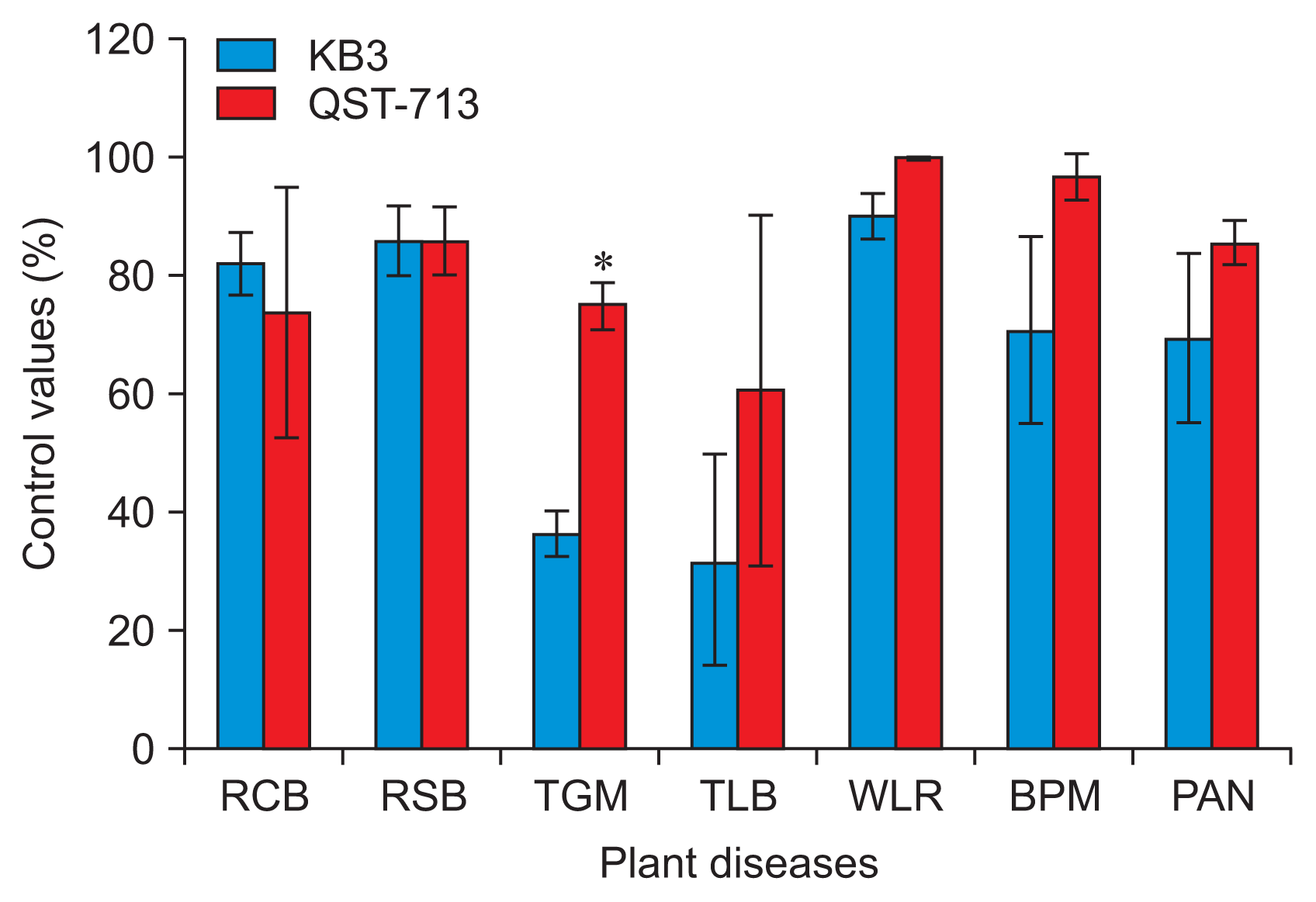
Foliar application of the KB3 bacterial culture filtrates significantly reduced disease incidence in four pathosystems, similar to the control achieved with B. subtilis QST-713 (Fig. 4). The preparations were least effective against tomato gray mold and tomato late blight (Fig. 4).
In conclusion our studies show that the feces of the tertiary larvae of the rhino beetle, A. dichotoma possess microbes with the potential for biocontrol of plant pests. Thus, these studies expand potential sources for such microbes. The KB3 strain isolated in this study showed broad-spectrum antifungal activity and disease control. Surfactin and iturin A, metabolites of importance in biocontrol, were detected. We are currently investigating the roles of CLPs in plant disease and insect control and the molecular regulatory mechanisms involved in the expression of CLP genes in the rhizosphere and insect guts.
Acknowledgments
This work was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through the Agri-Bio Industry Technology Development Program, funded by the Ministry of Agriculture, Food and Rural Affairs (MAFRA) (grant 314084-3).
Fig.┬Ā1
(A) Transmission electron microscopic image of the cell of isolate KB3 harvested at logarithmic growth phase from nutrient broth. (B) The phylogenetic tree of 16S rRNA sequences of isolates KB3 and LM11 showing 100% identity over the 1.4 kb sequence with those reported for reference Bacillus strains. The phylogenetic tree was constructed using the neighbor-joining method and KimuraŌĆÖs two-parameter model. The numbers at nodes indicate consensus bootstrap values based on 1,000 replications. Scale bar = 0.002 substitutions per nucleotide position. Pseudomonas tremae was used as the out group.
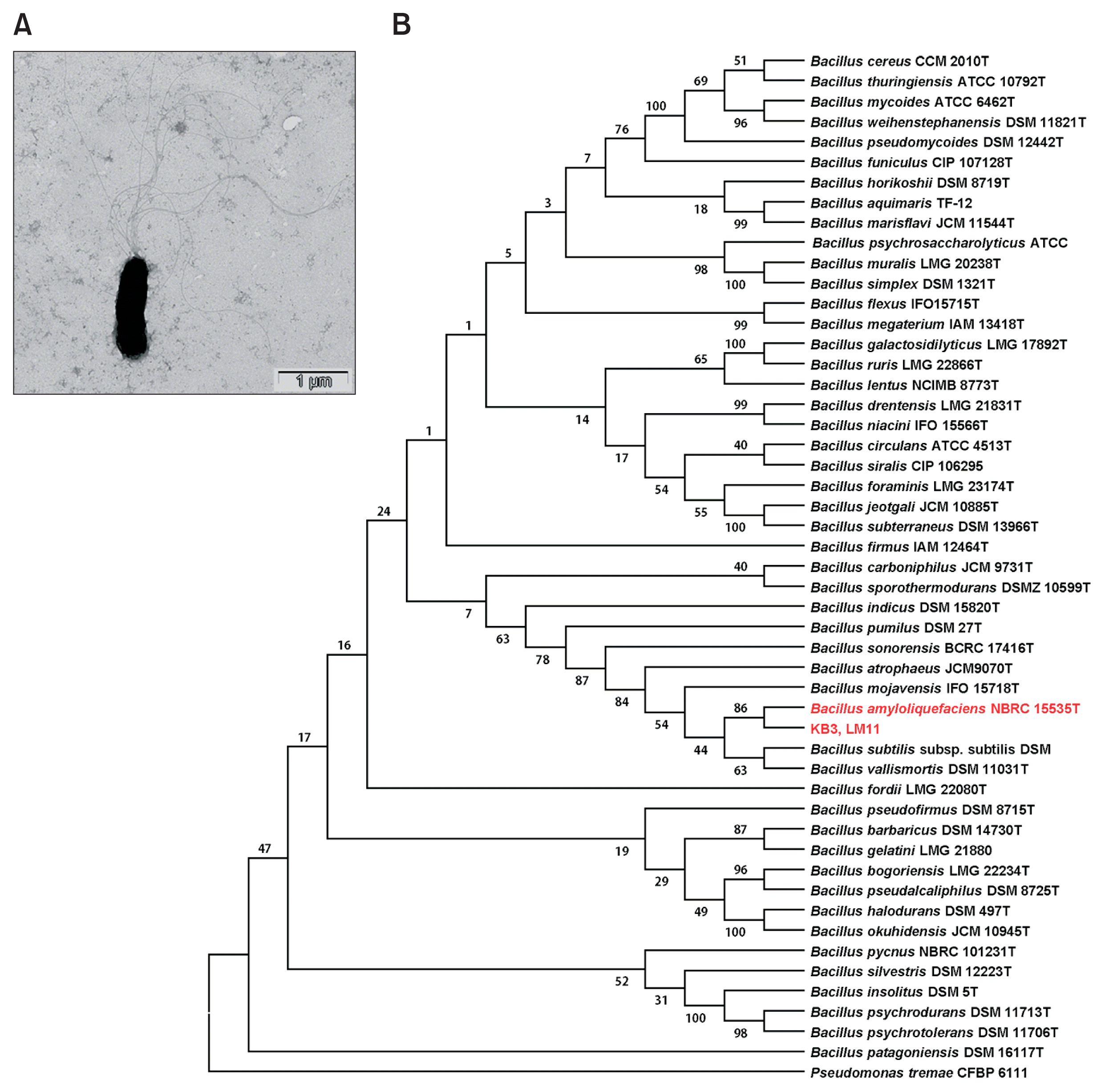
Fig.┬Ā2
Detection of surfactants produced by Bacillus amyloliquefaciens KB3. (A) The wells of six plates containing oil and surfactants in the drop collapse assay. The lanes contained: 1, distilled water; 2, Lysobacter antibioticus HS124; 3, B. thuringiensis KACC12072; 4, B. amyloliquefaciens KB3; 5, B. amyloliquefaciens LM11; 6, B. subtilis QST-713. (B) Detection of surfactant activity by thin layer chromatography. The lanes show results from with standard surfactin (100 ppm), standard surfactin (10 ppm), and B. amyloliquefaciens KB3. (C) High-performance liquid chromatograms for the metabolites eluted from KB3 versus the iturin A and surfactin standards.
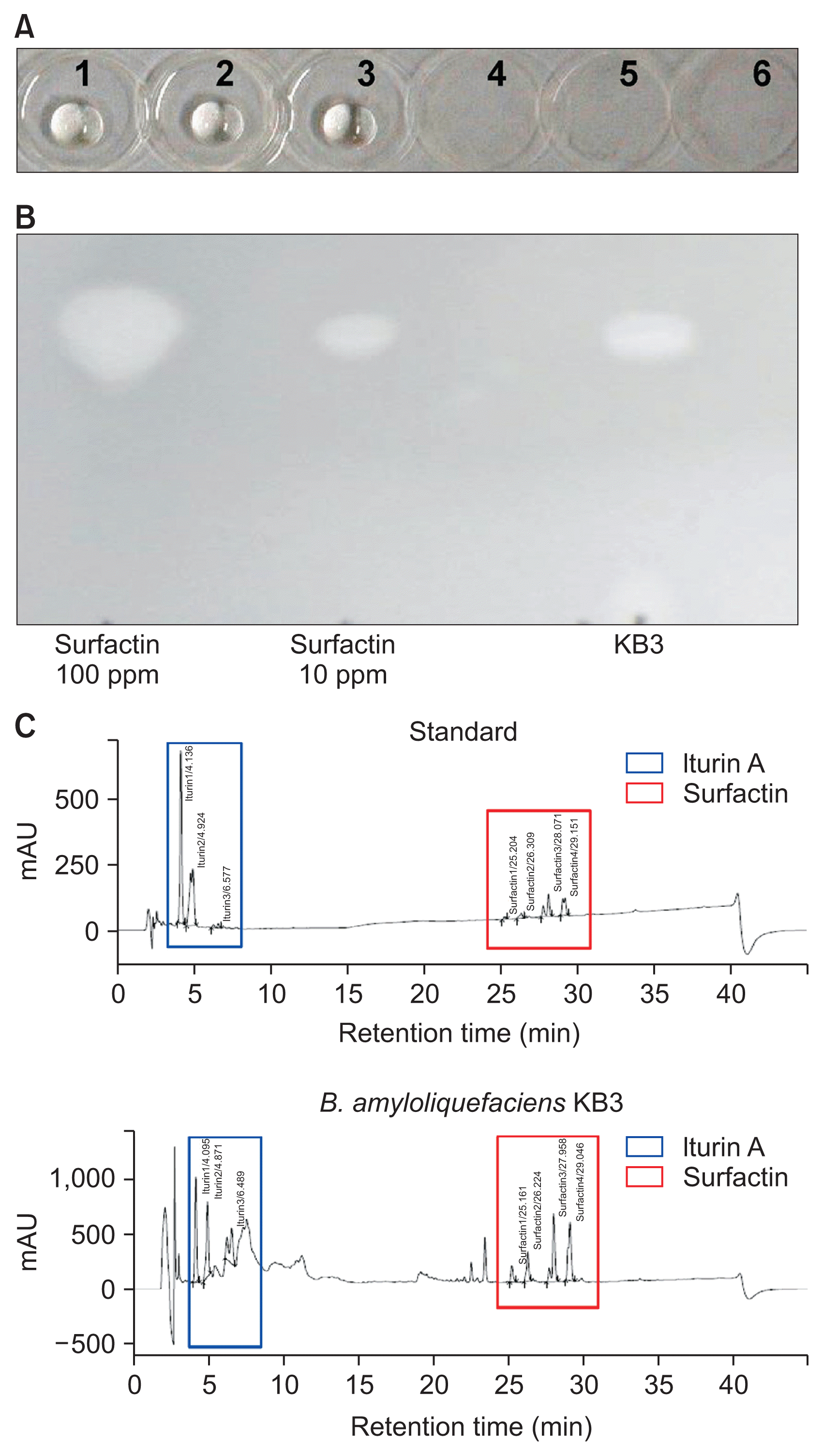
Fig.┬Ā3
Effects of growth media for isolate KB3 on in vitro antifungal activity. Data are shown for growth on potato dextrose agar amended with 10% cell-free filtrates from each of the five media. Different letters indicate significant differences between different growth media according to a one-way ANOVA (P < 0.05). Data are expressed as the means of three replicates in each of three plates, and error bars show the standard deviations. 1/3KB, one-third strength KingŌĆÖs B medium; M9, M9 minimal medium; LB, Luria-Bertani medium; OC, Ok Chun medium; TF, Taylor and Francis medium; R. solani, Rhizoctonia solani; P. capsici, Phytophthora capsici; C. coccodes, Colletotrichum coccodes; F. oxysporum, Fusarium oxysporum.
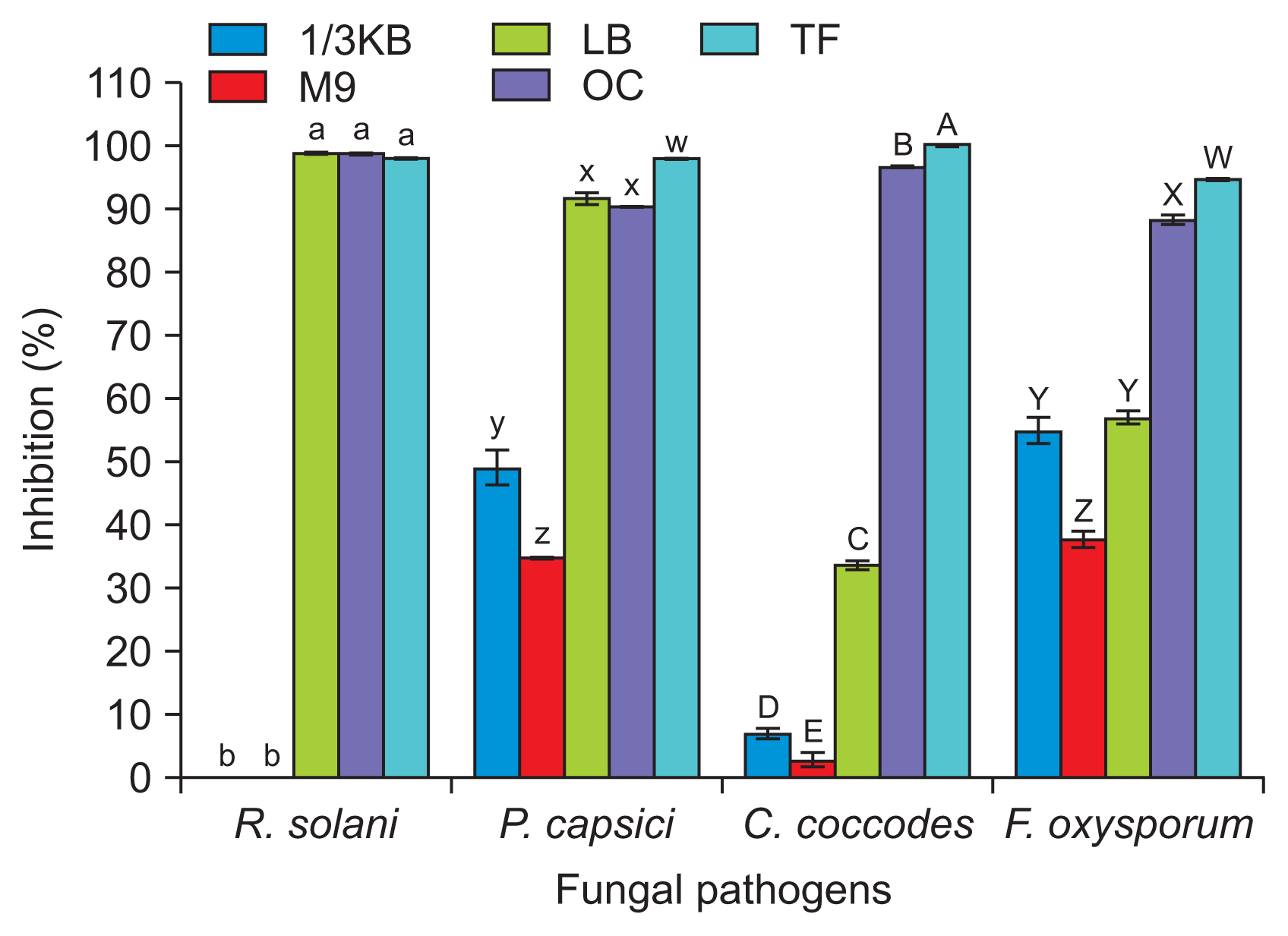
Fig.┬Ā4
In planta biocontrol efficacies of the cell-free filtrates from Bacillus amyloliquefaciens KB3 and B. subtilis QST-713. Intact plants were sprayed with cell-free filtrates from B. amyloliquefaciens KB3 and B. subtilis QST-713 grown in one-third KingŌĆÖs B (KB) at 30┬░C for 2 days (1:3 dilution). Water was used as the negative control. The disease index for each pathogen was based on the areas lesions on infected plants. Data are presented as the means ┬▒ standard deviations of three independent experiments with nine plants per treatment. Bars labeled with * for each pathosystem show significantly difference (P < 0.05) in disease based on StudentŌĆÖs t-test. RCB, rice blast; RSB, rice sheath blight; TGM, tomato gray mold; TLB, tomato late blight; WLR, wheat leaf rust, BPM; barley powdery mildew; PAN, red pepper anthracnose.
References
Arguelles-Arias, A, Ongena, M, Halimi, B, Lara, Y, Brans, A, Joris, B and Fickers, P 2009. Bacillus amyloliquefaciens GA1 as a source of potent antibiotics and other secondary metabolites for biocontrol of plant pathogens. Microb Cell Fact. 8:63




Bajpai, VK and Kang, SC 2012. In vitro and in vivo inhibition of plant pathogenic fungi by essential oil and extracts of Magnolia liliflora Desr. J Agr Sci Tech. 14:845-856.
Ben Khedher, S, Boukedi, H, Kilani-Feki, O, Chaib, I, Laarif, A, Abdelkefi-Mesrati, L and Tounsi, S 2015. Bacillus amyloliquefaciens AG1 biosurfactant: putative receptor diversity and histopathological effects on Tuta absoluta midgut. J Invertebr Pathol. 132:42-47.


Bodour, AA and Miller-Maier, RM 1998. Application for a modified drop-collapse technique for surfactant quantitation and screening of biosurfactant-producing microorganisms. J Microbiol Methods. 32:273-280.
Breznak, JA and Brune, A 1994. Role of microorganisms in the digestion of lignocellulose by termites. Annu Rev Entomol. 36:453-487.

Brune, A 2003. Symbionts aiding digestion. In: Encyclopedia of insects, eds. by VH Resh and RT Card├©, 1102-1107. Academic Press, New York, NY, USA.

Buensanteai, N, Yuen, GY and Prathuangwong, S 2008. The biocontrol bacterium Bacillus amlyliquefaciens KPS46 produces auxin, surfactin and extracellular proteins for enhanced growth of soybean plant. Thai J Agri Sci. 41:101-116.
Cawoy, H, Bettiol, W, Fickers, P and Ongena, M 2011. Bacillus-based biological control of plant diseases. In: Pesticides in the modern world: pesticides use and management, eds. by M Stoytcheva, InTech, Rijeka, Croatia.

Chen, XH, Koumoutsi, A, Scholz, R, Eisenreich, A, Schneider, K, Heinemeyer, I, Morgenstern, B, Voss, B, Hess, WR, Reva, O, Junge, H, Voigt, B, Jungblut, PR, Vater, J, S├╝ssmuth, R, Liesegang, H, Strittmatter, A, Gottschalk, G and Borriss, R 2007. Comparative analysis of the complete genome sequence of the plant growth-promoting bacterium Bacillus amyloliquefaciens FZB42. Nat Biotechnol. 25:1007-1014.



Chen, Y, Yan, F, Chai, Y, Liu, H, Kolter, R, Losick, R and Guo, JH 2013. Biocontrol of tomato wilt disease by Bacillus subtilis isolates from natural environments depends on conserved genes mediating biofilm formation. Environ Microbiol. 15:848-864.


Choudhary, DK and Johri, BN 2009. Interactions of Bacillus spp. and plants--with special reference to induced systemic resistance (ISR). Microbiol Res. 164:493-513.


Chowdhury, SP, Hartmann, A, Gao, X and Borriss, R 2015. Biocontrol mechanism by root-associated Bacillus amyloliquefaciens FZB42: a review. Front Microbiol. 6:780



Dietel, K, Beator, B, Budiharjo, A, Fan, B and Borriss, R 2013. Bacterial traits involved in colonization of Arabidopsis thaliana roots by Bacillus amyloliquefaciens FZB42. Plant Pathol J. 29:59-66.



Ernst, R, Arditti, J and Healey, NP 1971. Biological effects of surfactants. I. Influence on the growth of orchid seedlings. New Phytol. 70:457-475.

Gardener, BB, Kim, IS, Kim, KY and Kim, YC 2014. Draft genome sequence of a chitinase-producing biocontrol bacterium, Lysobacter antibioticus HS124. Res Plant Dis. 20:216-218.

Ghribi, D and Ellouze-Chaabouni, S 2011 Enhancement of Bacillus subtilis lipopeptide biosurfactants production through optimization of medium composition and adequate control of aeration. Biotechnol Res Int. 2011 653654.


Gregersen, T 1978. Rapid method for distinction of gram-negative from gram-positive bacteria. Eur J Appl Microbiol Biotechnol. 5:123-127.


Ha, BD 2013. Isolation of antagonistic microorganism against plant pathogen from feces of Allomyrina dichotoma larva and large scale fermentation of Bacillus amyloliquefaciens KB3. MS thesis. Chonnam National University, Gwangju, Korea.
Kang, BR 2012. Biocontrol of tomato Fusarium wilt by a novel genotype of 2,4-diacetylphloroglucinol-producing Pseudomonas sp. NJ134. Plant Pathol J. 28:93-100.

Kim, JC, Choi, GJ, Park, JH, Kim, HT and Cho, KY 2001. Activity against plant pathogenic fungi of phomalactone isolated from Nigrospora sphaerica. Pest Manag Sci. 57:554-559.


Kim, PI, Ryu, J, Kim, YH and Chi, YT 2010. Production of biosurfactant lipopeptides iturin A, fengycin and surfactin A from Bacillus subtilis CMB32 for control of Colletotrichum gloeosporioides. J Microbiol Biotechnol. 20:138-145.


Kimura, M 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 16:111-120.



King, EO, Ward, MK and Raney, DE 1954. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 44:301-307.

Kupferschmied, P, Maurhofer, M and Keel, C 2013. Promise for plant pest control: root-associated pseudomonads with insecticidal activities. Front Plant Sci. 4:287



Lee, B, Lee, S and Ryu, CM 2012. Foliar aphid feeding recruits rhizosphere bacteria and primes plant immunity against pathogenic and non-pathogenic bacteria in pepper. Ann Bot. 110:281-290.



Li, H, Soares, MA, Torres, MS, Bergen, M and White, JF Jr 2015. Endophytic bacterium, Bacillus amyloliquefaciens, enhances ornamental hosta resistance to diseases and insect pests. J Plant Interact. 10:224-229.

Martin, PA and Travers, RS 1989. Worldwide abundance and distribution of Bacillus thuringiensis isolates. Appl Environ Microbiol. 55:2437-2442.




Mendes, R, Kruijt, M, de Bruijn, I, Dekkers, E, van der Voort, M, Schneider, JH, Piceno, YM, DeSantis, TZ, Andersen, GL, Bakker, PA and Raaijmakers, JM 2011. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science. 332:1097-1100.


Mezghanni, H, Khedher, SB, Tounsi, S and Zouari, N 2012. Medium optimization of antifungal activity production by Bacillus amyloliquefaciens using statistical experimental design. Prep Biochem Biotechnol. 42:267-278.


Moran, NA, Russell, JA, Koga, R and Fukatsu, T 2005. Evolutionary relationships of three new species of Enterobacteriaceae living as symbionts of aphids and other insects. Appl Environ Microbiol. 71:3302-3310.




Nadarasah, G and Stavrinides, J 2011. Insects as alternative hosts for phytopathogenic bacteria. FEMS Microbiol Rev. 35:555-575.


Nihorimbere, V, Cawoy, H, Seyer, A, Brunelle, A, Thonart, P and Ongena, M 2012. Impact of rhizosphere factors on cyclic lipopeptide signature from the plant beneficial strain Bacillus amyloliquefaciens S499. FEMS Microbiol Ecol. 79:176-191.


Nitschke, M and Pastore, GM 2006. Production and properties of a surfactant obtained from Bacillus subtilis grown on cassava wastewater. Bioresour Technol. 97:336-341.


Olcott, MH, Henkels, MD, Rosen, KL, Walker, FL, Sneh, B, Loper, JE and Taylor, BJ 2010. Lethality and developmental delay in Drosophila melanogaster larvae after ingestion of selected Pseudomonas fluorescens strains. PLoS One. 5:e12504



Ongena, M and Jacques, P 2008. Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol. 16:115-125.


Park, JY, Oh, SA, Anderson, AJ, Neiswender, J, Kim, JC and Kim, YC 2011. Production of the antifungal compounds phenazine and pyrrolnitrin from Pseudomonas chlororaphis O6 is differentially regulated by glucose. Lett Appl Microbiol. 52:532-537.


P├®rez-Garc├Ła, A, Romero, D and de Vicente, A 2011. Plant protection and growth stimulation by microorganisms: biotechnological applications of Bacilli in agriculture. Curr Opin Biotechnol. 22:187-193.


Ruffner, B, P├®chy-Tarr, M, Ryffel, F, Hoegger, P, Obrist, C, Rindlisbacher, A, Keel, C and Maurhofer, M 2013. Oral insecticidal activity of plant-associated pseudomonads. Environ Microbiol. 15:751-763.


Sambrook, J and Russell, D 2001. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, USA.
Schroth, MN and Hancock, JG 1982. Disease-suppressive soil and root-colonizing bacteria. Science. 216:1376-1381.


Shanchez-Contreras, M and Vlisidou, I 2008. The diversity of insect-bacteria interactions and its applications for disease control. Biotechnol Genet Eng Rev. 25:203-243.


Siripornvisal, S 2010. Biocontrol efficacy of Bacillus subtilis BCB3-19 against tomato gray mold. KMITL Sci Tech J. 10:37-44.
Song, GC, Lee, S, Hong, J, Choi, HK, Hong, GH, Bae, DW, Mysore, KS, Park, YS and Ryu, CM 2015. Aboveground insect infestation attenuates belowground Agrobacterium-mediated genetic transformation. New Phytol. 207:148-158.



Sreerag, RS, Jayaprakas, CA, Ragesh, L and Kumar, SN 2014 Endosymbiotic bacteria associated with the mealy bug, Rhizoecus amorphophallis (Hemiptera: Pseudococcidae). Int Sch Res Not. 2014 268491.
Tamura, K, Stecher, G, Peterson, D, Filipski, A and Kumar, S 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 30:2725-2729.



Wang, Y, Lu, ZX, Bie, XM and Fengxia, L 2010. Separation and extraction of antimicrobial lipopeptides produced by Bacillus amyloliquefaciens ES-2 with macroporous resin. Eur Food Res Technol. 231:189-196.





 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




