 |
 |
| Plant Pathol J > Volume 32(5); 2016 > Article |
Abstract
Acknowledgments
Fig. 1
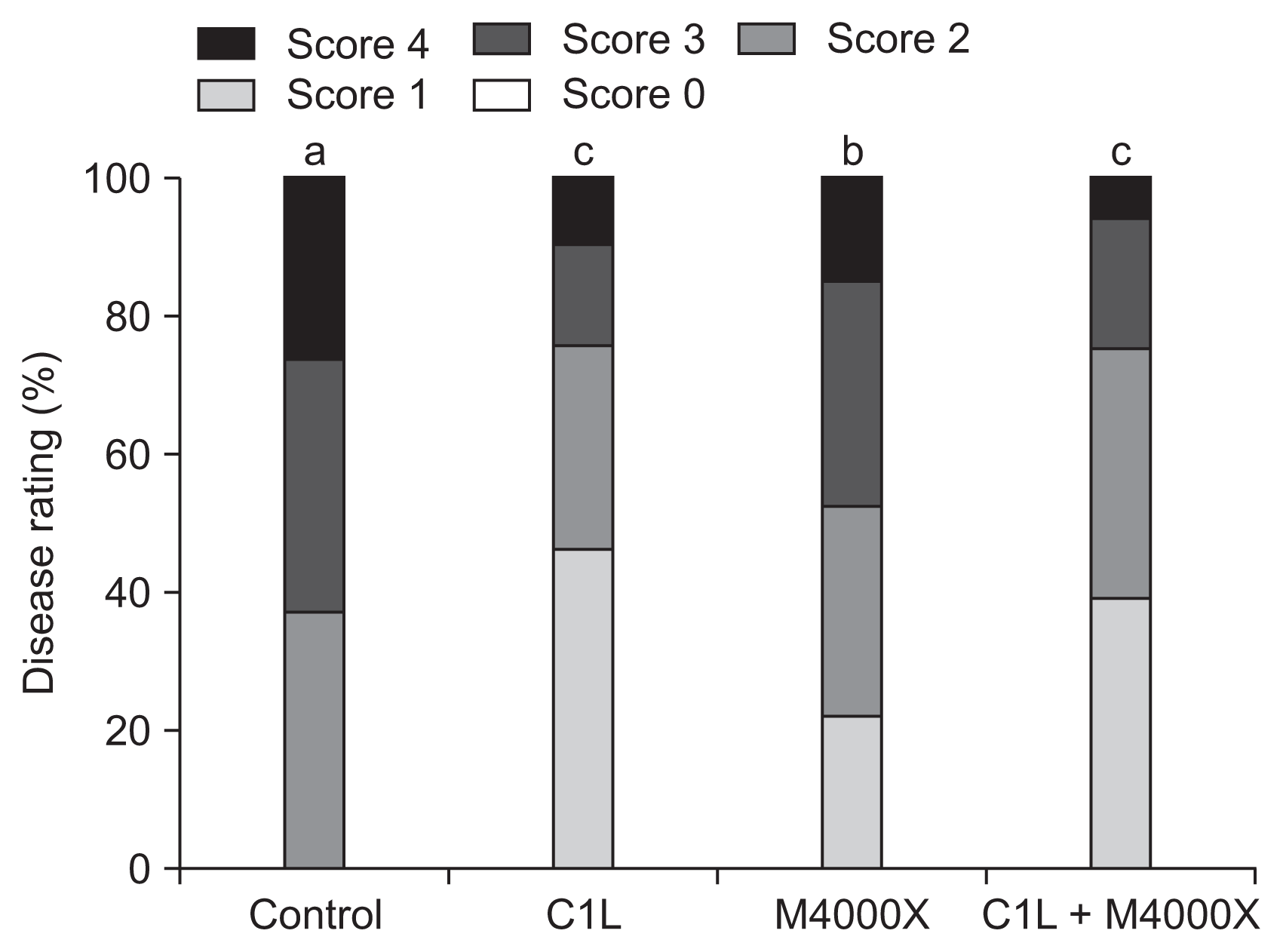
Fig. 2
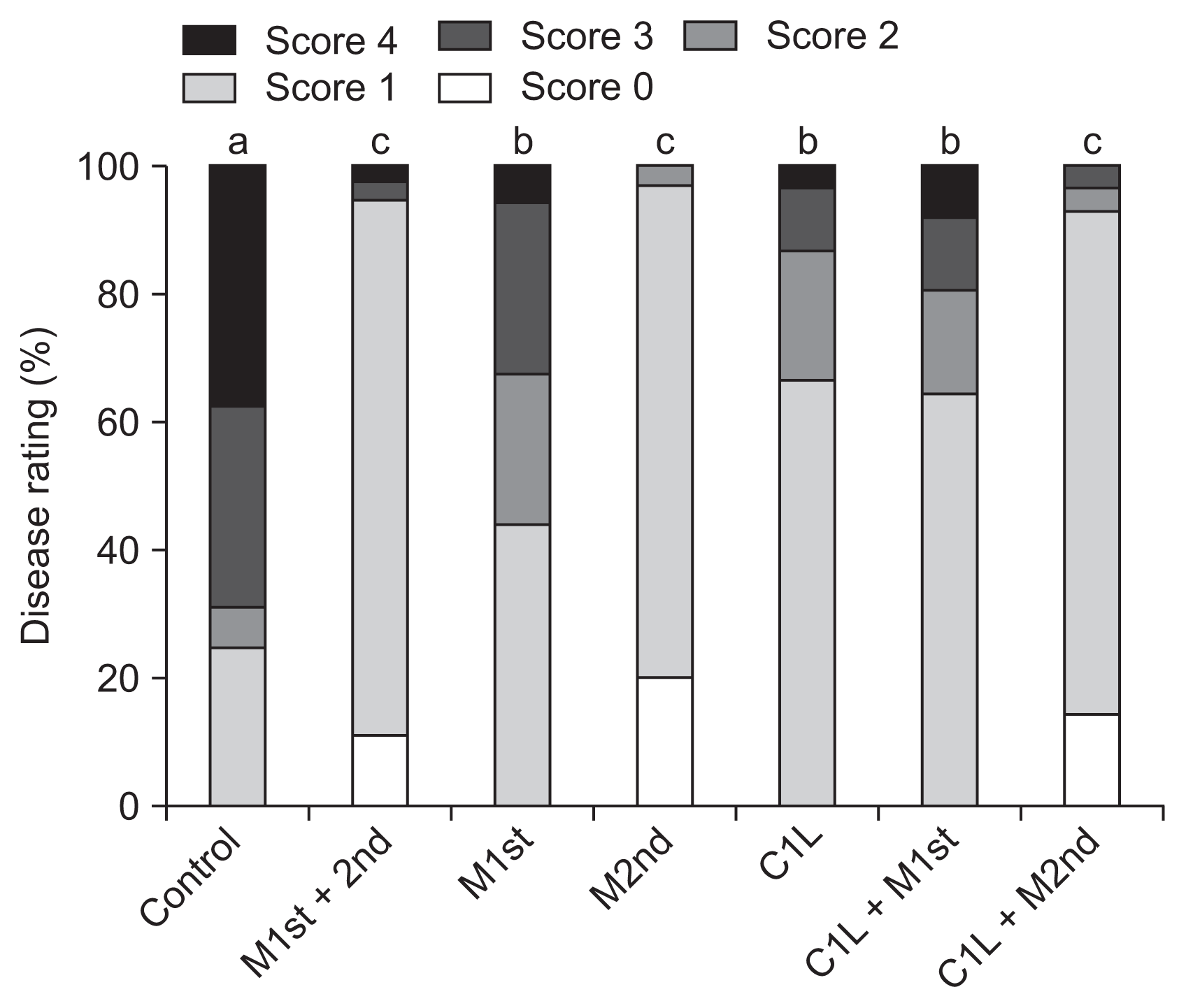
Fig. 3
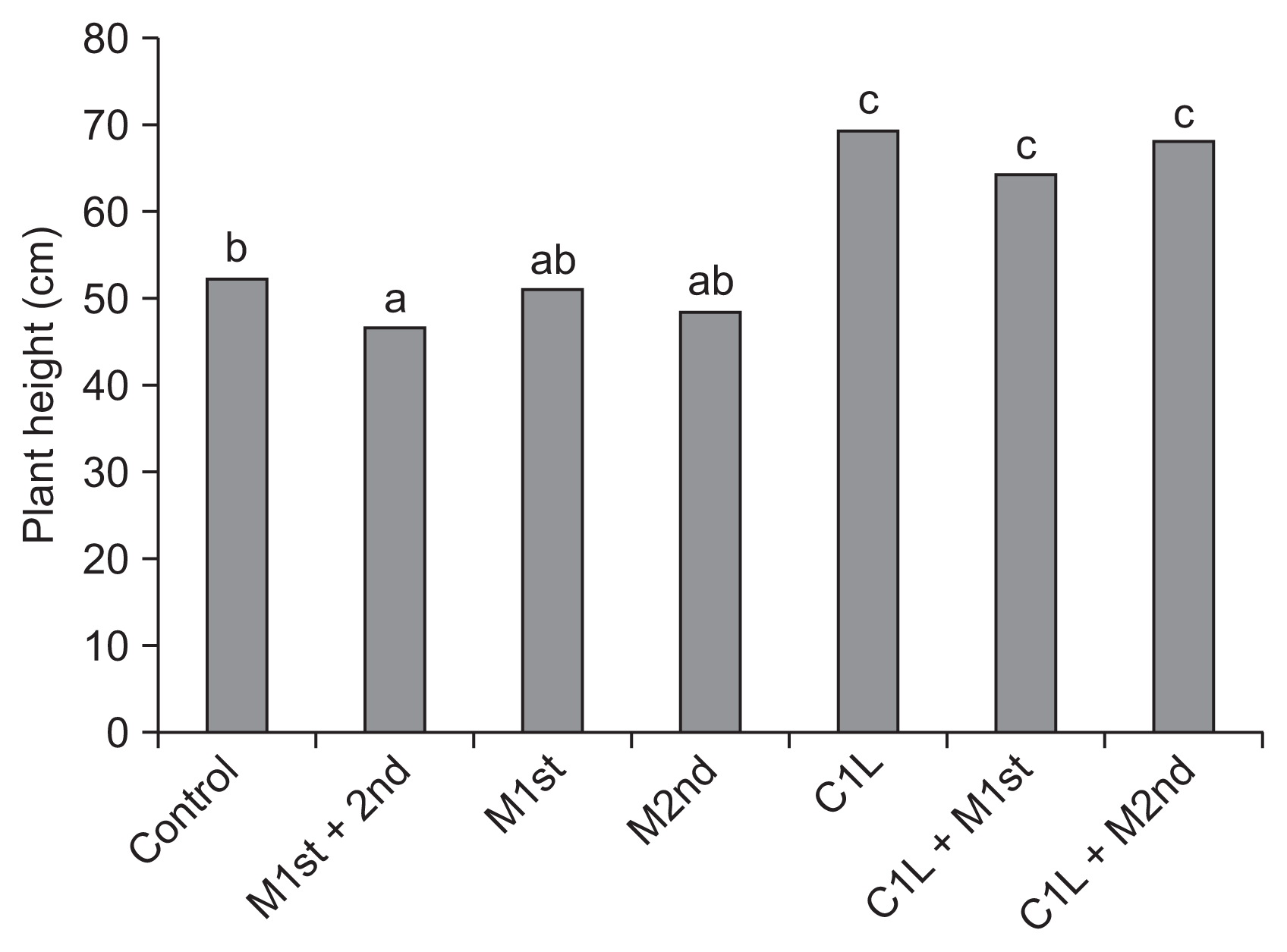
Table 1
| Treatment | Applications* | ||
|---|---|---|---|
|
|
|||
| Before planting | 2 wk after planting | 4 wk after planting | |
| Control | - | - | - |
| M1st + 2nd | - | Foliar sprays of mancozeb | Foliar sprays of mancozeb |
| M1st | - | Foliar sprays of mancozeb | - |
| M2nd | - | - | Foliar sprays of mancozeb |
| C1L | Root dipping | Drench treatment | Drench treatment |
| C1L + M1st | Root dipping | Drench treatment with foliar sprays of mancozeb | Drench treatment |
| C1L + M2nd | Root dipping | Drench treatment | Drench treatment with foliar sprays of mancozeb |
Table 2
| Treatment* | Disease severity† |
|---|---|
| Control | 2.81 ± 0.79 a |
| M4000X | 2.41 ± 0.99 b |
| C1L | 1.87 ± 0.98 c |
| C1L + M4000X | 1.91 ± 0.89 c |
Values followed by the same letter are not statistically different based on non-parametric Kruskal-Wallis and Mann-Whitney comparisons (P < 0.05).
* Detailed applications of maneb and rhizobacterial inoculum are described in the materials and methods. M4000X, 4,000-fold diluted maneb (i.e., 2.5 mg active ingredient (a. i.)/l); C1L, B. cereus C1L; C1L + M4000X, a soil drench with B. cereus C1L together with a foliar spray of 4,000-fold diluted maneb.
Table 3
| Treatment* | Disease severity† |
|---|---|
| Control | 2.81 ± 1.18 a |
| M1st + 2nd | 0.97 ± 0.50 c |
| M1st | 1.94 ± 0.97 b |
| M2nd | 0.83 ± 0.45 c |
| C1L | 1.50 ± 0.81 b |
| C1L + M1st | 1.64 ± 0.98 b |
| C1L + M2nd | 0.96 ± 0.57 c |
Values followed by the same letter are not statistically different based on non-parametric Kruskal-Wallis and Mann-Whitney comparisons (P < 0.05).
* The composition and application program of each treatment is present in Table 1. Detailed applications of mancozeb and rhizobacterial inoculum are described in the materials and methods. Manzozeb was applied at the recommended dose (i.e., 25 mg active ingredient (a. i.)/l).
Table 4
| Treatment* | Disease severity† |
|---|---|
| Control | 1.39 ± 1.29 a |
| M1st + 2nd | 0.34 ± 0.71 bc |
| M1st | 0.50 ± 0.80 b |
| M2nd | 0.30 ± 0.46 b |
| C1L | 0.37 ± 0.78 bc |
| C1L + M1st | 0.67 ± 0.99 b |
| C1L + M2nd | 0.13 ± 0.61 c |
Values followed by the same letter are not statistically different based on non-parametric Kruskal-Wallis and Mann-Whitney comparisons (P < 0.05).
* The composition and application program of each treatment is present in Table 1. Detailed applications of mancozeb and rhizobacterial inoculum are described in the materials and methods. Manzozeb was applied at the recommended dose (i.e., 25 mg active ingredient (a. i.)/l).
Table 5
| Treatment* | Strain C1L population (in log cfu/g of fresh root) | Significance |
|---|---|---|
| Control | - | - |
| C1L | 6.17 ± 0.07 | a |
| C1L + M1st | 5.94 ± 0.24 | b |
| C1L + M2nd | 6.00 ± 0.08 | ab |
M, mancozeb; C1L, B. cereus C1L; -, indicates that no colony of B. cereus C1L was detected in the control treatment.
Values followed by the same letter are not statistically different based on Tukey’s multiple comparisons (P < 0.05).
* The composition and application program of each treatment is present in Table 1. Detailed applications of mancozeb and rhizobacterial inoculum are described in “Materials and Methods”. Manzozeb was applied at the recommended dose (i.e., 25 mg active ingredient (a. i.)/l).
References
- TOOLS



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




