|
 |
| Plant Pathol J > Volume 33(1); 2017 > Article |
Abstract
The causal fungus of pear scab, Venturia nashicola, grows slowly and rarely produces conidia on artificial media in the laboratory, but it produced conidia on the Cheongah medium containing Cheongah powder. V. nashicola grew too slow to produce conidia until 15 days after cultivation but produced conidia with 4 × 104 conidia/plate 30 days after cultivation on the Cheongah medium containing 1% Cheongah powder. V. nashicola showed a peak production of conidia with 4.5 × 105 conidia/plate 60 days after cultivation on the carrot medium containing 2% carrot powder, one of the constituents of Cheongah powder. The carrot medium is considered to be the best medium to obtain conidia of V. nashicola in the laboratory until now. This is the first report on the development of a suitable medium for conidia production of V. nashicola, as far as we know.
Pears (Pyrus spp.) have been cultivated for more than 2,000 years and are among the most important fruits in all the temperate regions in about 50 countries of the world (Bell, 1990). The Pyrus species can be traditionally divided into occidental pear and oriental pear groups according to their geographic distribution (Bailey, 1917). Now, European pear (Pyrus communis L.) is considered to be occidental in origin and distinct from oriental pears such as Japanese pear (Pyrus pyrifolia Nakai) and Chineses pear (Pyrus bretschneideri Rehd. and Pyrus ussuriensis Maxim.) in morphological traits as well as molecular divergence (Iketani et al., 1998).
Pear scab, caused by two fungal species, Venturia nashicola and V. pirina, is the most serious disease affecting pears worldwide. V. nashicola is only pathogenic on Japanese and Chinese pears and V. pirina is only pathogenic on European pear (Tanaka and Yamamoto, 1964). Sivanesan (1977) included V. nashicola as a synonym on V. pirina. In recent years, however, Ishii and Yanase (2000) concluded that V. nashicola was distinct from V. pirina on the basis of the pathogenicity tests and morphological characterization of perfect and imperfect states that ascospores of V. pirina were longer and wider than those of V. nashicola and the conidia of V. nashicola were significantly shorter than those of V. pirina (Fig. 1).
Isolation of V. nashicola on selective media is subject to limitations since it grows slowly and could be masked by overgrowth of other faster growing fungi (Koh et al., 2013). Especially, the artificial cultivation of V. nashicola is difficult using common fungal growth medium in the laboratory, since it formed a colony with about 11.02 mm diameter until 60 days after cultivation on potato dextrose agar (PDA) medium but rarely produced conidia (Fig. 1). Although Ryu et al. (2014) found a new culture medium with pear extracts or Ca(NO3)2 for better growth of V. nashicola, 60-day-old colony of V. nashicola cultivated on the new medium was 24.35 mm in diameter and conidia were not produced on the culture medium. Therefore, we screened various media with different commercial crop powders to develop a suitable medium for conidia production of V. nashicola, since Maruyama et al. (2014) reported that the medium containing lotus and burdock roots was the most efficient for conidia mass production of Sphaceloma species through screening of various media with different commercial crop powders.
Five isolates of V. nashicola were cultivated on PDA for 60 days at 20°C in the dark for further experiments (Table 1). Various crop or plant extract powder media (1% crop or plant extract powder and 1.5% agar) containing 20 kinds of commercial crop or plant extract powders were prepared (Table 2) and PDA was used as a control. A mycelial disk of each isolate of V. nashicola cut from 60-day-old colony on PDA using cork-borer with 8 mm diameter was placed on the center of the surface of the prepared media and incubated for 30 days at 20°C in the dark. Each treatment contained 5 replications. The number of conidia harvested from the surface of each medium was measured by hemocytometer. Mycelial growths of V. nashicola measured as diameters of colonies with 8 mm agar plug on the media containing 20 kinds of commercial crop or plant extract powders were somewhat slower than those on PDA (Fig. 2). Of the tested 20 kinds of the media, conidia were produced only on the medium containing Cheongah powder (Fig. 3). V. nashicola grew too slow to produce conidia until 15 days after cultivation on the Cheongah medium but produced gradually conidia thereafter. The number of conidia reached about 4 × 104 conidia/plate 30 days after cultivation on the Cheongah medium containing 1% Cheongah powder.
Since commercial Cheongah powder (300 g/package; Jangmyeong Food Co., Seoul, Korea) consists of major constituents such as 50% carrot powder, 22% apple powder, and 14% mandarin powder and minor constituents such as onion powder, ginger powder, dextrin and glucose, we investigated conidia production of V. nashicola on the medium containing separate constituents of Cheongah powder. Each media containing 1% separate constituents of Cheongah powder (carrot powder, apple powder, mandarin powder, onion powder, ginger powder, and sugar) and 1.5% agar were prepared and a mycelial disk of each isolate of V. nashicola cut from 60-day-old colony on PDA using cork-borer with 8 mm diameter was placed on the center of the surface of the prepared media and incubated at 20°C for 30 days in the dark. V. nashicola grew relatively fast on the medium containing carrot powder (300 g/package; Jangmyeong Food Co.) and followed by apple powder, onion powder, sugar, but it scarcely grew on the media containing mandarin powder and ginger, respectively (Fig. 4). V. nashicola produced about 1 × 104 conidia/plate on the medium containing carrot powder, which were nearly equivalent to the conidia production on the Cheongah medium. On the other hand, V. nashicola produced less than 104 conidia/plate on the media containing apple powder, onion powder, and sugar. However, no conidia were produced on the media containing mandarin powder and ginger powder, respectively. This indicates that the carrot medium is more efficient than the Cheongah medium for conidia production of V. nashicola, since carrot powder is cheaper than Cheongah powder.
We also screened various media with different concentrations of carrot powder to find the optimal concentration of carrot powder in the carrot medium inducing conidia production of V. nashicola in the laboratory. When we cultivated isolates of V. nashicola on the carrot media containing 0.5%, 1%, 2%, and 5% carrot powder, V. nashicola showed a peak production of conidia with 4.5 × 105 conidia/plate 60 days after cultivation on the carrot medium containing 2% carrot powder (Table 3). From the above results, the carrot medium (2% carrot powder and 1.5% agar) is considered to be the best medium to obtain conidia of V. nashicola in the laboratory until now.
V. nashicola was first identified as the causal fungus of pear scab in Korea in 1985 (Cho et al., 1985). Although they described the length of conidia and conidiophores by detaching fungal mass from the diseased leaves of pear using a light microscope, they could not isolate V. nashicola on artificial medium. In recent years, Ryu et al. (2014) observed increased mycelial growth of V. nashicola on PDA media amended with pear extracts or Ca(NO3)2, but there has been no report on the techniques of conidia production on artificial medium in the laboratory until now. Conidia of V. nashicola produced on the carrot medium containing 2% carrot powder was pathogenic on pear leaves and mycological characteristics of V. nashicola produced on the carrot medium containing 2% carrot powder were in accordance with those found on pear trees or fallen leaves (Fig. 1).
Although pear scab has been the most serious disease on pear orchards during the past three decades, there has been no progress on the mycological researches on V. nashicola, since it grows slowly and rarely produces conidia on artificial media. Since use of systemic fungicides is the most common method to control pear scab at farmers’ pear orchards but frequent use of some systemic fungicides has been induced emergence of resistant strains of V. nashicola, development of new type of fungicides is necessary to manage the prevalent resistant populations of V. nashicola against routinely sprayed fungicides (Kwak et al., 2015). Use of resistant cultivars is the most promising method to control pear scab, but resistant cultivars have never been bred until now in Korea. Moreover, it is very urgent to develop resistant cultivars to pear scab, since the most common cultivar ‘Niitaka’ accounting for 82% of areas in pear cultivation in Korea is the most scab susceptible cultivar (Won et al., 2013). The development of the carrot medium suitable for efficient conidia production of V. nashicola will contribute to control pear scab through its utilization for screening of new fungicides and breeding of resistant cultivars in the future.
Acknowledgments
This study was carried out with the support of the Cooperative Research Program for Agricultural Science and Technology Development (PJ01019702), Rural Development Administration, Republic of Korea.
Fig. 1
Mycological characteristics of Venturia nashicola. (A, E) 7-day-old and 60-day-old colonies on potato dextrose agar at 20°C in the dark. (B) Conidia. (C) Conidiophores. (D) Pseudothecium. (F) Ascus. (G) Ascospores released from asci. (H) Ascospores.
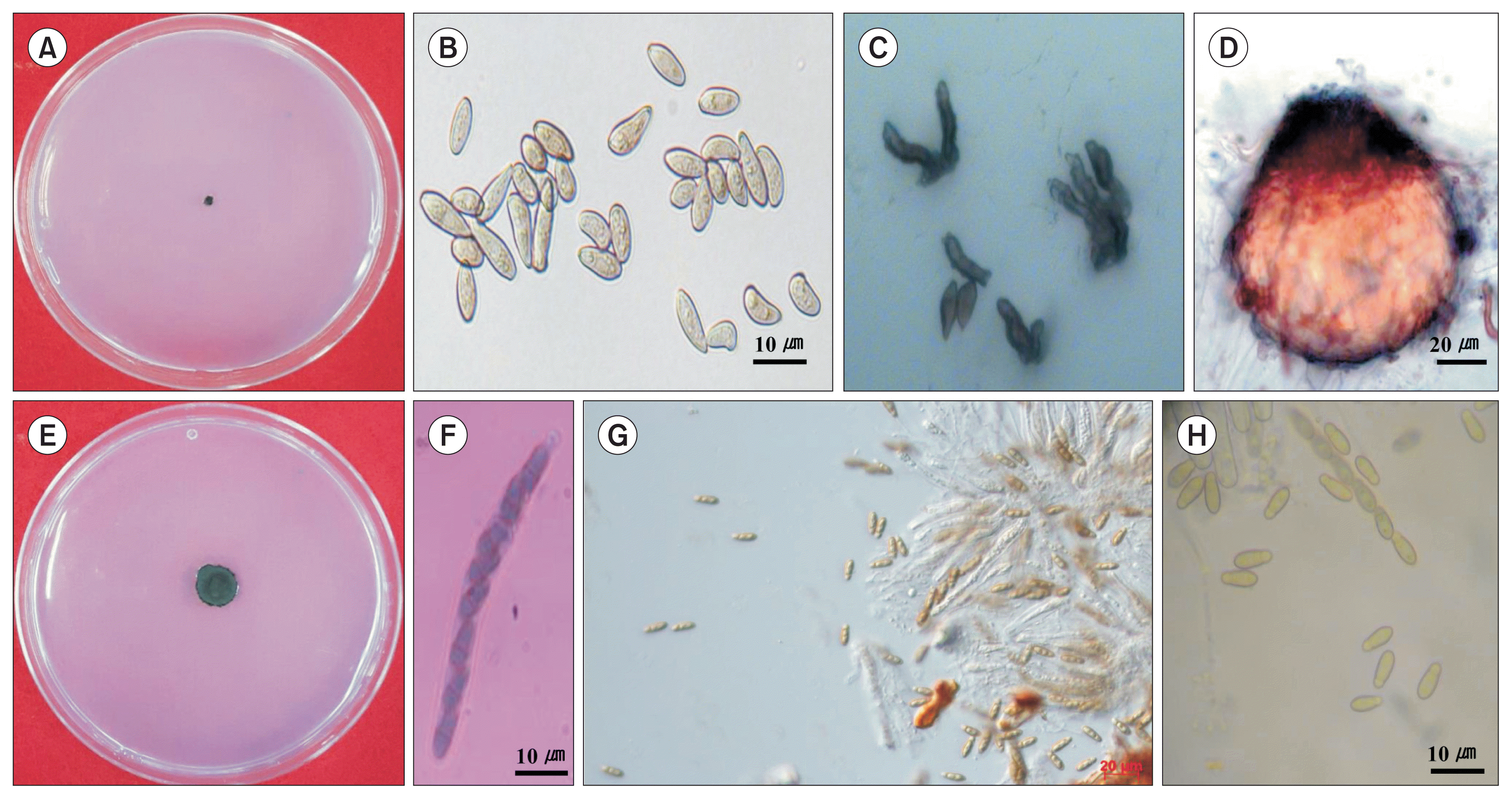
Fig. 2
Mycelial growths of Venturia nashicola 30 days after cultivation on the media containing 20 kinds of commercial crop or plant extract powders and potato dextrose agar (PDA) at 20°C in the dark. Mycelium growths were measured by the colony diameters including 8 mm agar plug.
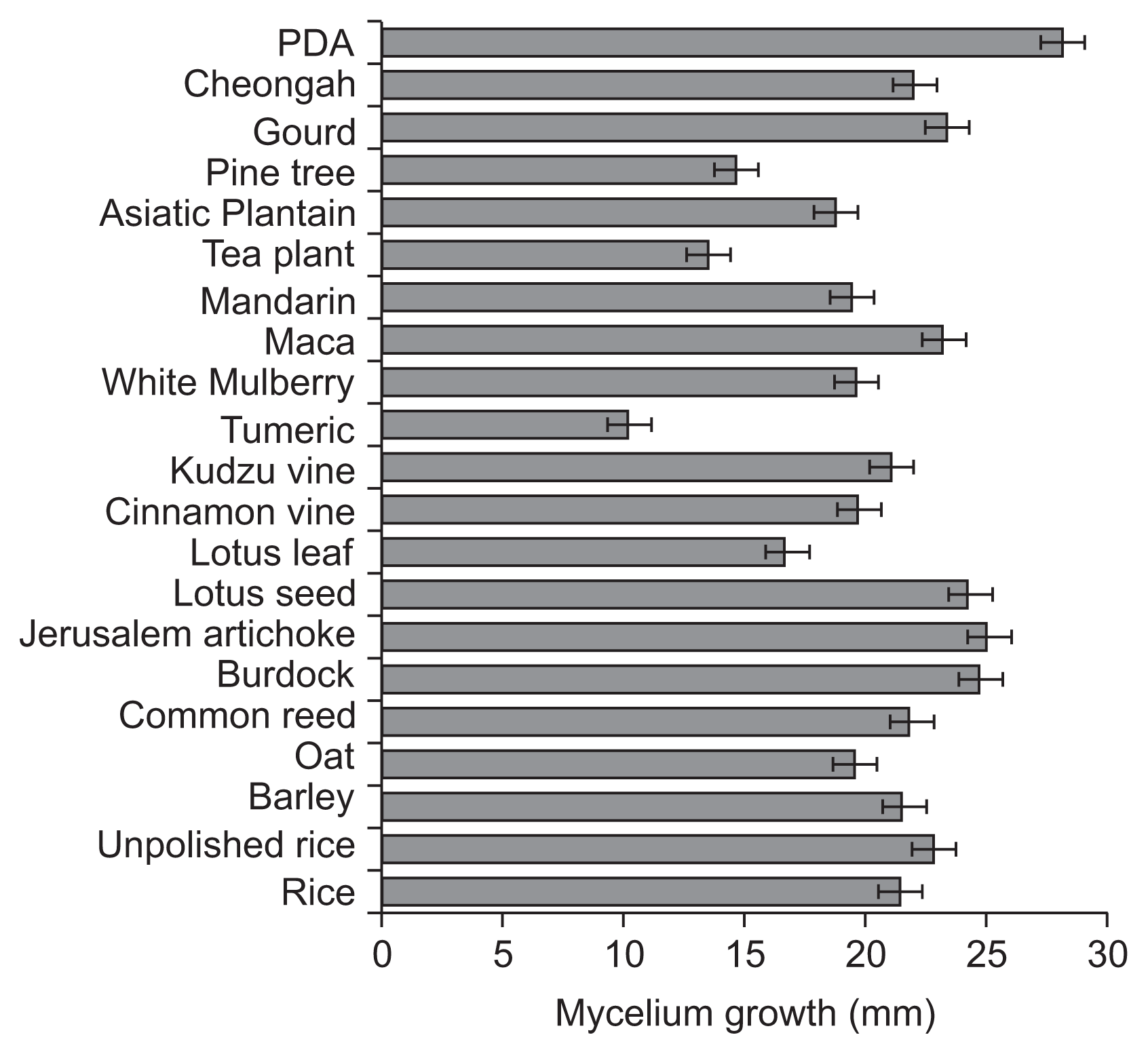
Fig. 3
Colony (A) and conidia (B) with conidiophores of Venturia nashicola 30 days after cultivation on Cheongah medium at 20°C in the dark.
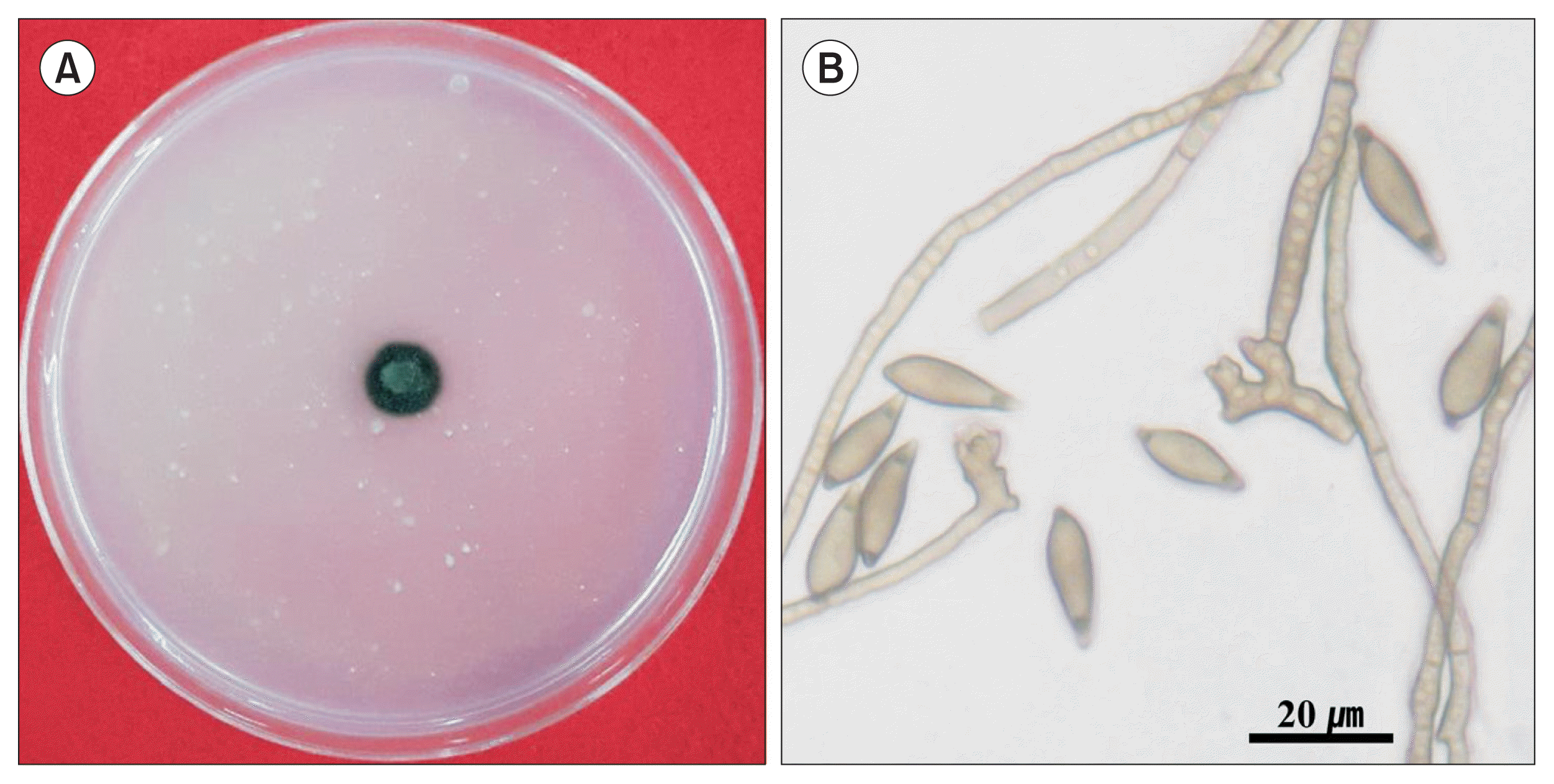
Fig. 4
Mycelial growths of Venturia nashicola 30 days after cultivation on the media containing 1% each of separate constituents, carrot (A), apple (B), mandarin (C), onion (D), ginger (E), and sugar (F) of Cheongah powder at 20°C in the dark.
Table 1
Venturia nashicola isolates tested in this study
Table 2
Various crop or plant extract powders used in this study
Table 3
Mycelial growth and conidia production of Venturia nashicola 60 days after cultivation on the carrot media according to the concentrations of carrot powder
| Concentration (%) | Mycelium growth (mm)* | Conidia/plate (× 105) |
|---|---|---|
| 0.5 | 44.7 ± 0.3 | 1.5 ± 0.6 |
| 1.0 | 45.1 ± 0.2 | 2.0 ± 0.5 |
| 2.0 | 49.2 ± 0.6 | 4.5 ± 1.3 |
| 5.0 | 47.8 ± 1.3 | 2.4 ± 1.0 |
References
Bailey, LH 1917. Pyrus. In: Standard cyclopedia of horticulture, eds. by LH Bailey, 2865-2878. Macmillan, New York, NY, USA.
Bell, RL 1990. Peras (Pyrus). In: Genetic resources of temperate fruit and nut crops I, eds. by JL Moore and JR Ballington, 655-697. International Society for Horticultural Sciences, Wageningen, the Netherlands.
Cho, EK, Cho, WT and Lee, EJ 1985. The causal organism of pear scab in Korea. Korean J Mycol. 13:263-265.
Iketani, H, Manabe, T, Matsuta, N, Akihama, T and Hayashi, T 1998. Incongruence between RFLPs of chloroplast DNA and morphological classification in east Asian pear (Pyrus spp.). Genetic Res Crop Evol. 45:533-539.
Ishii, H and Yanase, H 2000. Venturia nashicola, the scab fungus of Japanese and Chinese pears: a species distinct from V pirina. Mycol Res. 104:755-759.

Koh, HS, Sohn, SH, Lee, YS, Koh, YJ, Song, JH and Jung, JS 2013. Specific and sensitive detection of Venturia nashicola, the scab fungus of Asian pears, by nested PCR. Plant Pathol J. 29:357-363.



Kwak, YS, Min, JY and Kim, HT 2015 Field population monitoring of benzimidazole fungicide resistance using quantitative sequencing in pear scab Venturia nashicola. Res Plant Dis. 21:371.(Abstract).
Maruyama, N, Fukue, N, Kim, OK, Shinohara, H and Negishi, H 2014 Evaluation of media containing crop powder(s) for conidia mass production of Sphaceloma species. Res Plant Dis. 20:332.(Abstract).
Ryu, JP, Min, KH, Yim, SH, Song, JH, Choi, JJ, Lee, SH, Kim, WS, Cho, BH and Yang, KY 2014 Effect of medium compositions on the mycelial growth of Venturia nashicola causing pear scab. Agric Sci Tech Res. 48:9-17 (in Korean).


Sivanesan, A 1977. The taxonomy and pathology of Venturia species. J Cramer, Vaduz, Liechtenstein. 94-99.
Tanaka, S and Yamamoto, S 1964 Studies on pear scab II. Taxonomy of the causal fungus of Japanese pear scab. Ann Phytopathol Soc Jpn. 29:128-136 (in Japanese).
Won, KH, Kang, SS, Kim, YK, Sherzod, R, Lim, KH and Lee, HC 2013. Evaluation of scab resistance and effect of photosynthetic rates on fruit characteristics among elite pear seedings. Korean J Environ Agric. 32:117-122.



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print