 |
 |
| Plant Pathol J > Volume 33(2); 2017 > Article |
Abstract
Tan spot caused by the fungus Pyrenophora triticirepentis is a serious disease of wheat, which is on increase in recent years in Mediterranean region. In the field this fungus produces a diamond-shaped necrotic lesions with a yellow halo on wheat foliage. The objective of this study was to characterize and compare several monospore isolates of P. tritici-repentis collected from different infected wheat fields in various locations of Algeria, and find the morphological differences between them, if any. The results revealed wide morphologically variation among the isolates based on colony colors and texture, mycelial radial growth and conidial size.
Tan spot caused by the fungus Ascomycetes/Dothideomycetes Pyrenophora tritici-repentis is a serious disease of wheat. The fungus has a wide host range including numerous non-cereal grasses (Krupinsky, 1992; Morrall and Howard, 1975). It is one of the pathogen associated with leaf spot diseases of wheat. The disease is on increase in both incidence and severity on wheat grown in the Mediterranean region (Benslimane et al., 2006, 2011; Nsarellah and Mergoum, 1997). The initial infection occurs through the ascospores that infect and produce lesions on young wheat seedlings (Adee and Pfender, 1989; Howard and Morrall, 1975; Wiese, 1987). The initial symptoms of tan spot are small dark brown spots that expand to become tan diamond-shaped lesions with a yellow halo (Marshall, 2009). Frequently, there is a small black spot in the center of the lesion. The lesions often coalesce as they grow, resulting in large necrotic area and hence reduces the photosynthetic area.
The etiology, biology and epidemiology of the fungus have been studied extensively by several researchers (Adee and Pfender, 1989; da Luz and Bergstrom, 1986; Hosford, 1971; Howard and Morrall, 1975; Morrall and Howard, 1975; Rees et al., 1982; Sutton and Vyn, 1990; Wright and Sutton, 1990). Variability among P. tritici-repentis populations was demonstrated by several workers around the world (Ali and Francl, 1998; Ali et al., 2010; Benslimane et al., 2013; da Luz and Hosford, 1980; dos Santos et al., 2002; Friesen et al., 2005; Lamari and Bernier, 1989, 1991; Leisová et al., 2008; Misra and Singh, 1972; Moreno et al., 2008; Schilder and Bergstrom, 1990; Singh and Hughes, 2006). Studying variability within the population in a geographical region is important because it documents the changes occurring in the population. A number of authors have studied monospore isolates of P. tritici-repentis their biology, morphology and culture peculiarities on different nutrient media and found variation among the isolates (Ali and Francl, 1998; Friesen et al., 2003; Hunger and Brown, 1987; Mielke, 1999; Wolf, 1991; Wolf and Hoffmann, 1993). A little or no information is available on P. tritici-repentis populations present on wheat in various geopgraphical regions of Algeria. This would be a prerequisite for a further population analysis of pathogen virulence and that ultimately would help in evaluation wheat cultivars for tan spot resistance grown in Algeria.
The objective of the present study was to characterize and compare several monospore isolates of P. tritici-repentis, collected from different infected wheat fields from various locations of Algeria, to find the morphological culture differences among them, if any.
In a previous study (Benslimane et al., 2011), eighty-two mono-conidial isolates of P. triticirepentis were recovered from Triticum aestivum and T. durum diseased leaves sample. The samples were collected from different geographical wheat growing regions of in Algeria (Table 1, Fig. 1). Briefly, leaf spotted area were cut into 3 cm pieces, surface sterilized in 5% hypochlorite solution for 3 min, then rinsed thrice in sterile water (5 min each time). The fragments were blotted on tissue paper to remove the excess water and placed in Petri dishes with three layers of dampened Whatman filter paper. The plates were incubated at 22°C for 24 h under light then in dark for 24 h. Single conidia from conidiophores (Fig. 2) developing close to the edge of each lesion were transferred to potato dextrose agar (PDA).
All 82 isolates were grown individually on PDA in plastic Petri dishes at 20°C in the dark. After one week, 5 mm diameter plugs were taken aseptically from the margins of actively growing cultures and placed fungus-side down in the center of fresh PDA dishes. Each isolate was replicated 4 times. Petri dishes were incubated in dark at 20°C, and then macroscopic characters (color, sector, and texture) of each colony were recorded after 7 days. PDA was choosing for this step and the next, because it is commonly used for the isolation and growth of wide range of fungi in laboratories.
All isolate hyphal growths were determined on PDA medium. To assess the effects of temperature on in vitro colony radial growth, small plugs, 5 mm in diameter were transferred singly to 9 cm Petri dishes containing PDA. Cultures were incubated in the dark at three temperatures (20°C, 25°C, and 30°C), mycelium radial growth was recorded for each isolate in mm at 24 h interval until the colonies had reached the plate edge. Four diameters of linear growth of each plate were measured at right angles to each other and the values were averaged. Four replications (one plate/rep) were used for each isolate and for each temperature. Means values were used to perform principal component analysis using STATISTICA software (StatSoft Inc., Tulsa, OK, USA).
To determine whether any differences in conidial width and length exist within or among the isolates, 42 isolates were selected randomly and spores were produced of each isolate following the protocols of Lamari and Bernier (1989). Small plugs, 0.5 cm in diameter, from 8 day culture were transferred singly to 9 cm Petri plates, containing V8-PDA. The cultures were incubated in the dark until the colonies reached 4 cm in diameter. The cultures were then flooded with sterile distilled water and the mycelium flattened with the bottom of flamed test tube. After the water was decanted, the cultures were subjected to a regime of 18 h of light at room temperature followed by 24 h of dark at 15°C. Conidia size (length, width) and septation number of 50 spores/each isolate were counted under 40× magnifications with the aid of ocular and stage micrometer in compound microscope. Spore measurements were compared among the isolates and data were analyzed statistically by ANOVA method using SPSS software (SPSS Inc., Chicago, IL, USA).
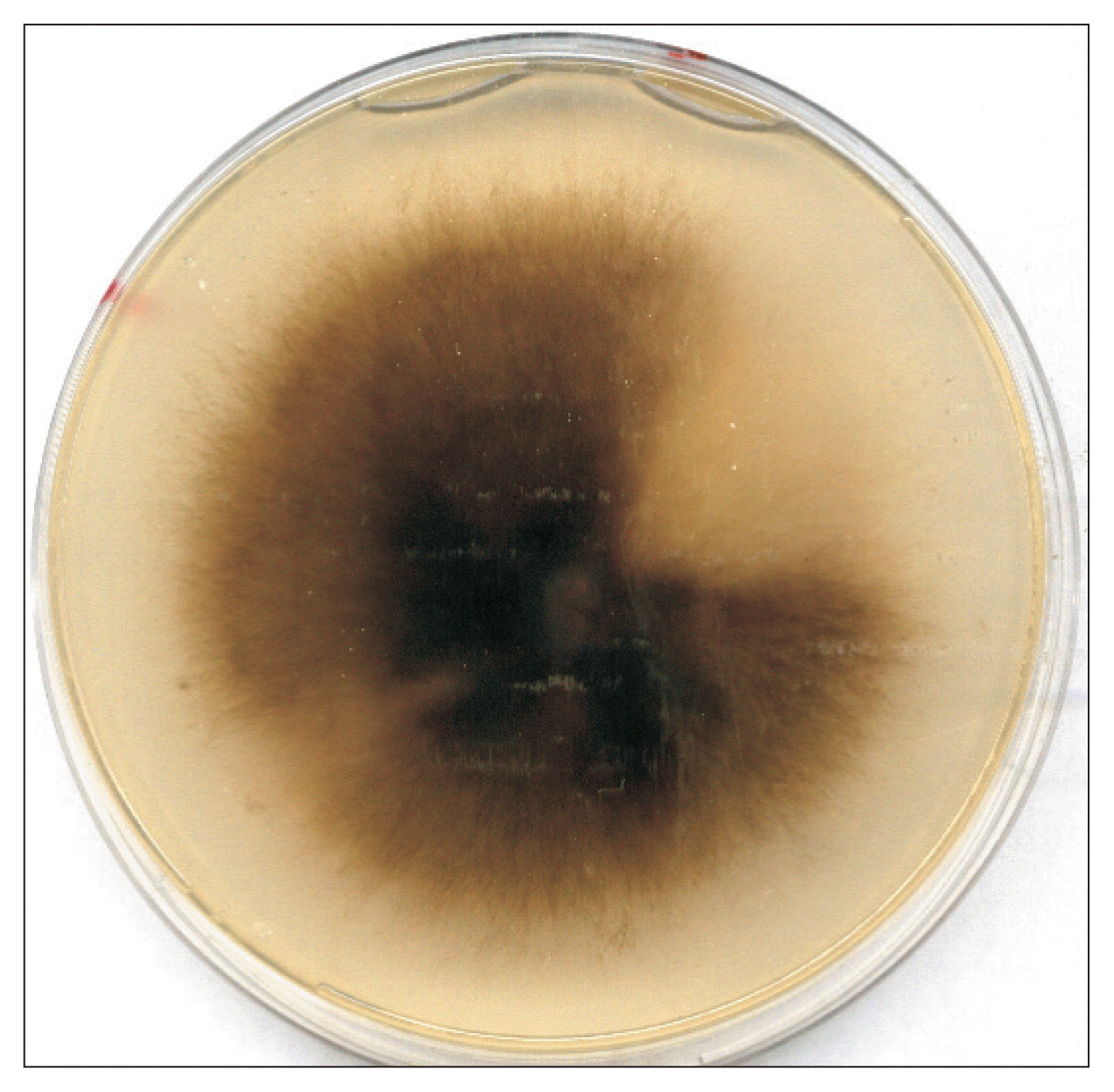
The P. tritici-repentis isolates used in this study varied in colony color and mycelium compactness. Cultures growth on PDA medium showed usually a thick cottony mycelium sometimes fluffy, often gray-green (Fig. 3), and rarely whitish (Fig. 4). Underside the colony color was green (Fig. 5). The old cultures about two weeks showed black spherical mycelia aggregations (Fig. 6). Moreover we found that when the mycelium color became orange, the isolates loosed the ability to produce spores as a result of a repeated sub-culture in PDA medium (Fig. 7).
The results on radial growth of all isolates colonies at different temperatures showed that the mycelium growth is temperature depended (Table 2). It was observed that the temperature range of 25-30°C was optimum for mycelial growth of P. tritici-repentis on PDA. Indeed, most of the isolates (57) showed a better (3.72-6.95 mm) growth at 25°C; however, some (10) of the isolates grew better at 30°C. Only five isolates (Ptr16, Ptr20, Ptr41, Ptr48, and Ptr72) grew (3.45-5.55 mm) better at 20°C. Based on radial growth at the three different temperatures, the principal component analysis categorized the isolates into six groups (Fig. 8); some of these groups stand out more than others; such as the group consisting of a single isolate Ptr6, or the group combining Ptr52 and Ptr64 (Table 1).
Even the different analyses of the results of mycelia growth showed that there was some difference between the studied isolates, the effect of temperatures, expressed trough the radial growth, showed that the difference or the approximation among studied isolates, seems to have no relationship with the geographical origin of the isolates. It was found that pathogen population in closely located fields or in the same, consisted of specimens of large phenotypical variability. Otherwise, it does not show any relationship with the climate of the area from where the sample was collected. In deed in Fig. 8, we can see isolates from different geographical regions grouped together, as well as other isolates from nearby areas classified statistically in groups far away each other.
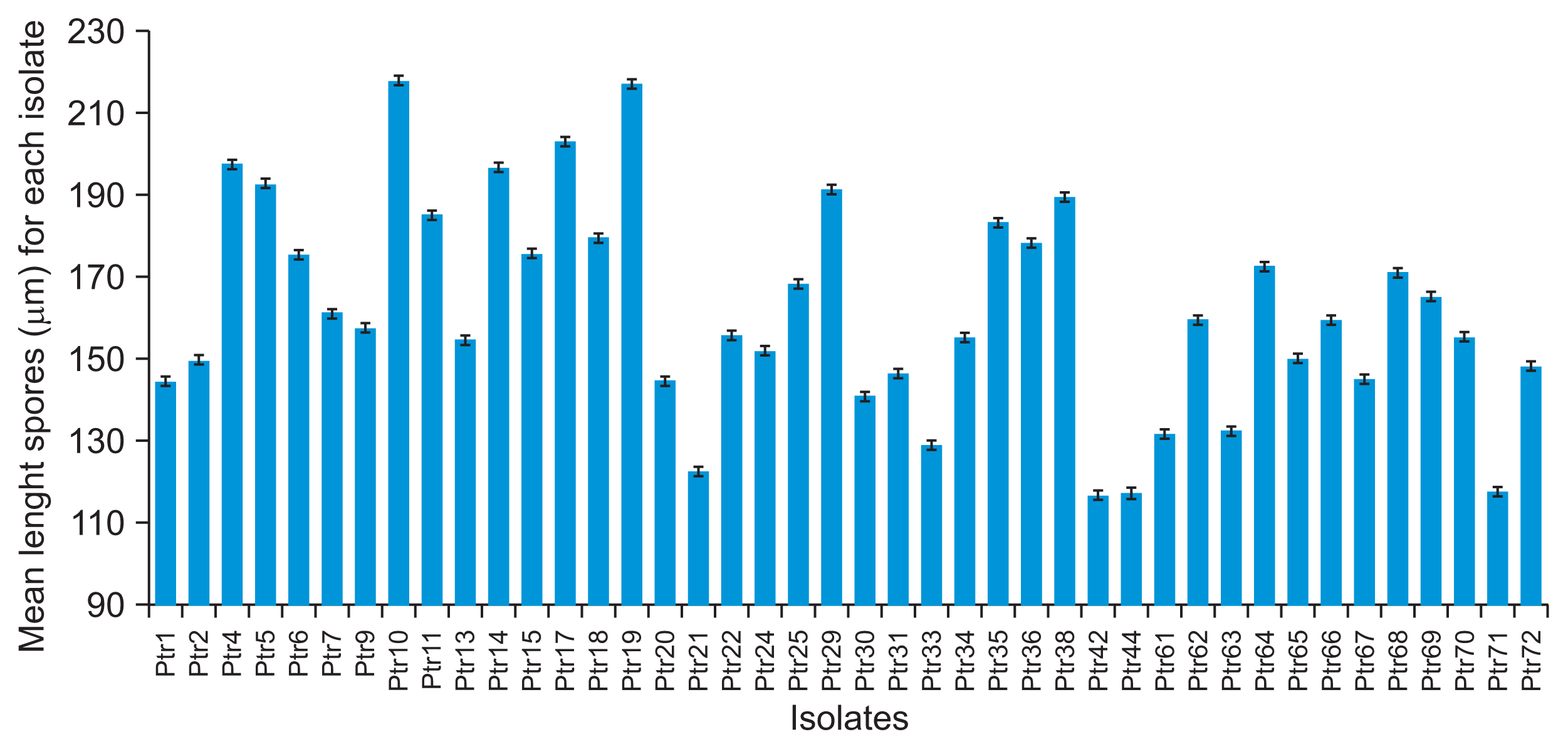
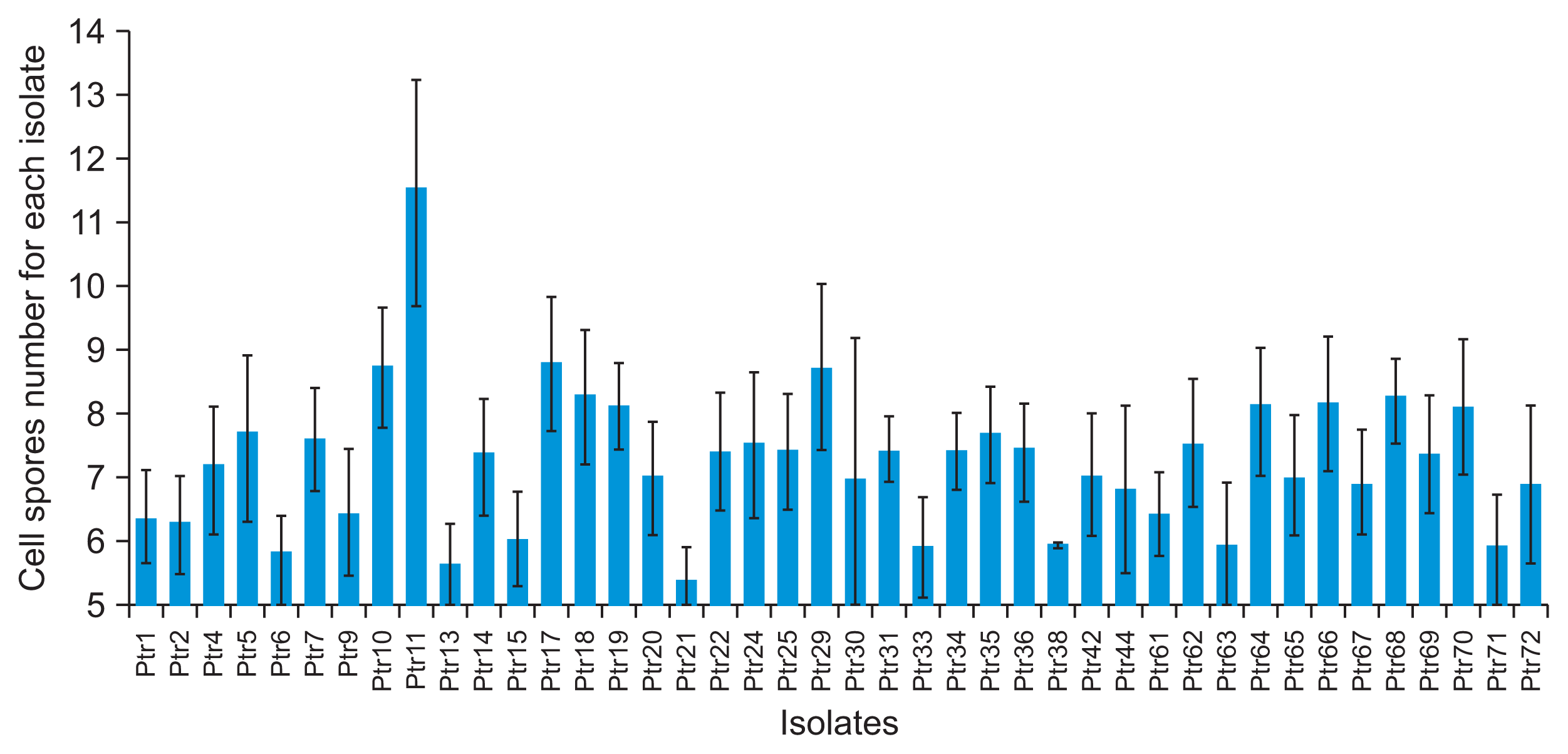
P. tritici-repentis isolates produced abundant conidia on V8-PDA which were consistent in morphology. Significant differences were founded in conidial length and number of cells among the isolates (P = 0.000). Dimensions of the conidia for each isolate are presented in Fig. 9 and Fig. 10. Average conidial length was maximum (217.67 μm) in isolate Ptr10 and minimum (117.15 μm) in Ptr71 whereas the number of septa varied from 11.52 in isolates Ptr11 to 5.42 in isolate Ptr21. However, no significant variation was observed in conidial width; only two values were founded 15.6 μm and 18.2 μm.
The results of the present study revealed wide morphological variation among P. tritici-repentis isolates based on colony color and texture, mycelial radial growth, and conidial size prevalent on wheat in Algeria.
Several researchers observed variation in mycelia color and colony morphology in P. tritici-repentis and its related species isolates collected from different geographical regions. Dos Santos et al. (2002) studied P. tritici-repentis, McDonald (1967) and Frazzon et al. (2002) studied Pyrenophora teres isolates, and observed significant morophological variation based on mycelial colony colors. Similar results were reported by Benslimane (2002) and Christensen and Graham (1934) when they studied Pyrenophora graminea isolates for their morphological variation. Hosford (1971) observed that P. tritici-repentis isolates lose their sporulation when the culture became orange colored due to frequent subcultuing on PDA. This phenomenon also occurred frequently as sectoring in an otherwise typical colony and was not always associated with slower growth.
In Bipolaris sorokiniana isolates, Valim-Labres et al. (1997), Oliveira et al. (1998), and Matsumura (1991) observed and reported variability based on mycelium color and colony morphology grown on PDA. Some isolates exhibited white tufts while others showed fan shaped sectors, although the surface of all isolates was plain.
Conidia of Drechslera tactylidis significantly vary in length, diameter and number of septations (Zeiders, 1980). This also holds true for the closely related specie B. sorokiniana, when 87 isolates representing different agroecological regions of Pakistan studied for morphological variation and observed differences in colony size and conidial color and size (Asad et al., 2009).
Morphological variability is also common in several other plant pathogens population. In Fusarium oxysporum f.sp lentis, 32 isolates collected in western Algeria showed variability in the cultural colony appearance and size of conidia (Belabid, 2002). Similarly, 29 isolates of Sphaeropsis sapinea from Canada revealed several morphotypes based on their appearance of colony, their radial growth, and conidial size (Hausner et al., 1999).
Morphological variation within a taxon is well known in fungi (Harrington and Rizzo, 1999). For example it is known that the morphology of conidiophores and conidia in several asexual fungi is strongly influenced by the culture medium (Booth, 1971). Morphological characters are the main tool in identifying and describing of a species (Harrington and Rizzo, 1999). This is more useful for quantitative characters, because they can be used in defining species phylogeny (Luckow, 1995). Among the morphological quantitative characters in fungal species, spore size is probably the most commonly character used (Parmasto and Parmasto, 1992). However, if these characters have long been used to identify the pathogenic fungi and to compare the isolates of different origins, analysis has several major drawbacks. These characters are highly variable in many fungi, which limit the scope of their significance in determining population structures. Moreover, in general, these characters (with rare exceptions) cannot be a precise genetic analysis; genes involved in expression are being too numerous (Lourd, 1995).
In this study, we found that P. tritici-repentis isolates showed significant differences in many mprophological characters such as spore size, colony color, etc. grown on the same medium and similar growing conditions. Obtaining fungal isolates information characterized for color, growth, and spore size facilitates further research in the fungus in a multitude of discipline. For example, to study the genetics of a fungus or its interaction with a host, mutants of the fungus are produced. However, to determine if mutagenesis altered these characters, the range of variation in the original isolates for each character must be determined. In addition, isolates with defined characteristics would facilitate studies involving the epidemiology of tan spot.
Fig. 1
Map of wheat growing areas in Algeria showing the 14 provinces where Pyrenophora tritici-repentis isolates were collected. 1, Mascara; 2, Ain Defla; 3, Tipaza; 4, Médéa; 5, Blida; 6, Algiers; 7, Bouira; 8, Boumerdès; 9, Tiziouzou; 10, Bejaia; 11, Sétif; 12, Mila; 13, Skikda; 14, Constantine; 15, Guelma.
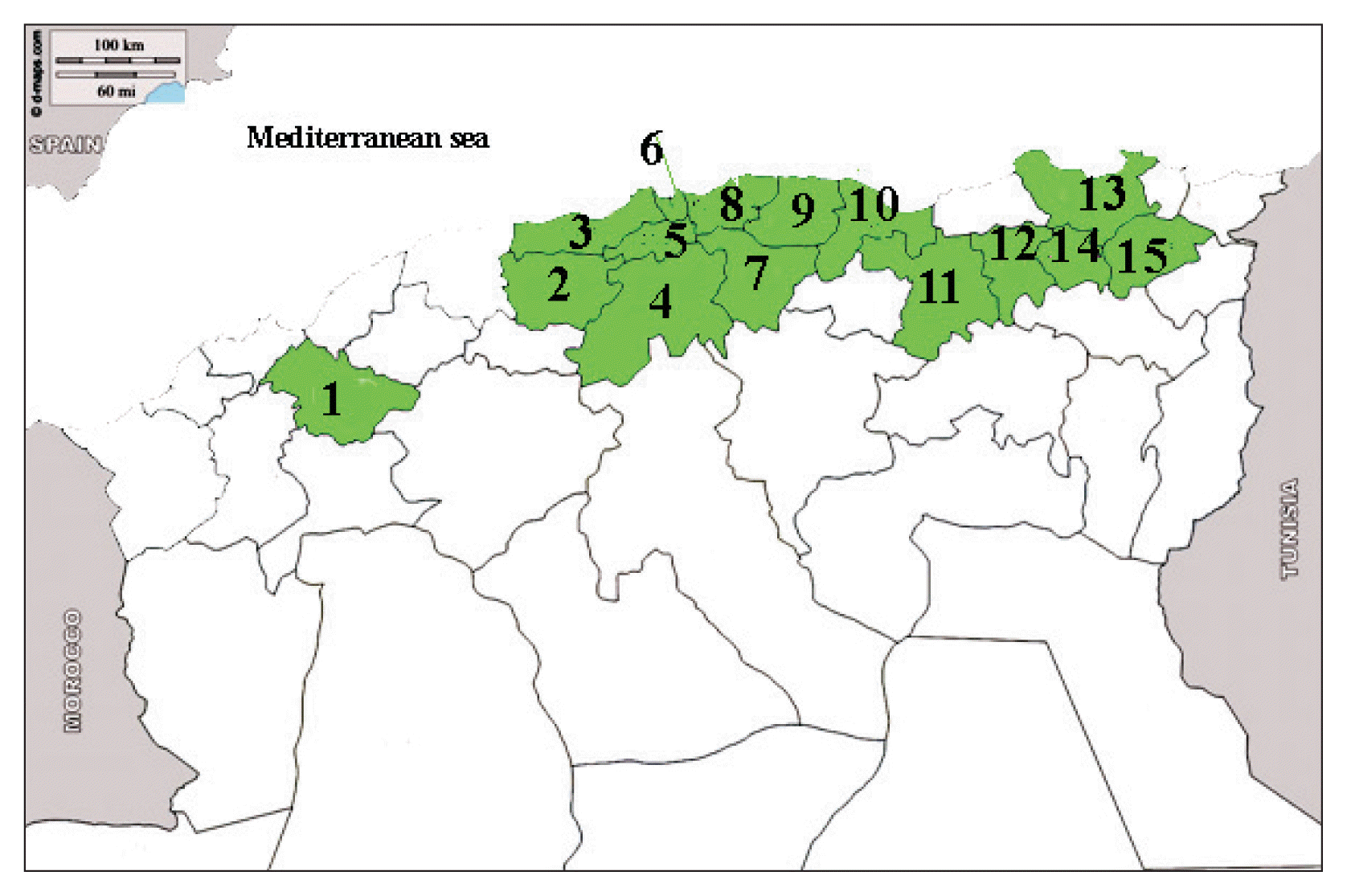
Fig. 3
Culture of Pyrenophora tritici-repentis on potato dextrose agar showing a gray-green thick fluffy mycelium.
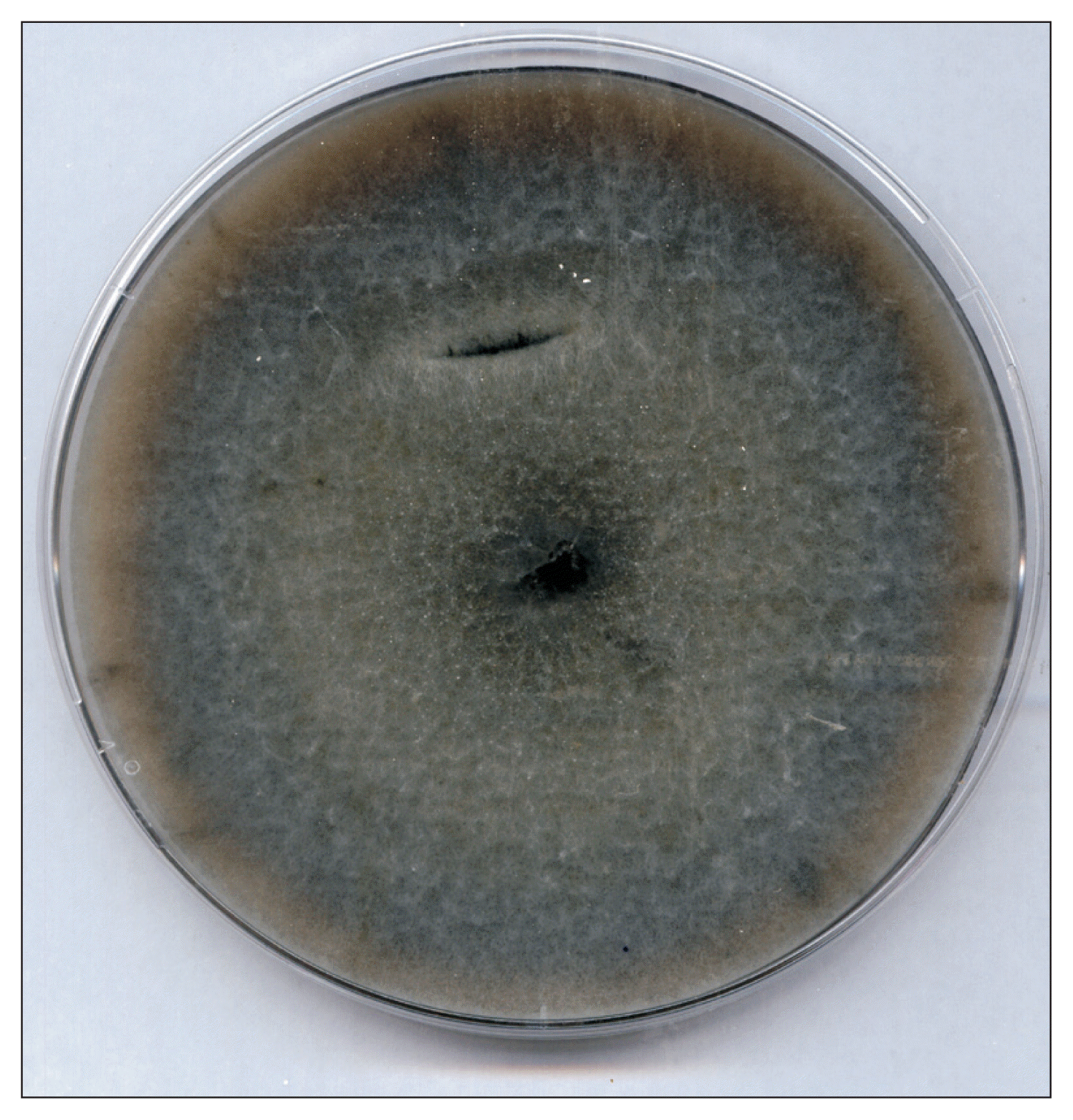
Fig. 4
Culture of Pyrenophora tritici-repentis on potato dextrose agar depicting a cottony whitish mycelial growth.
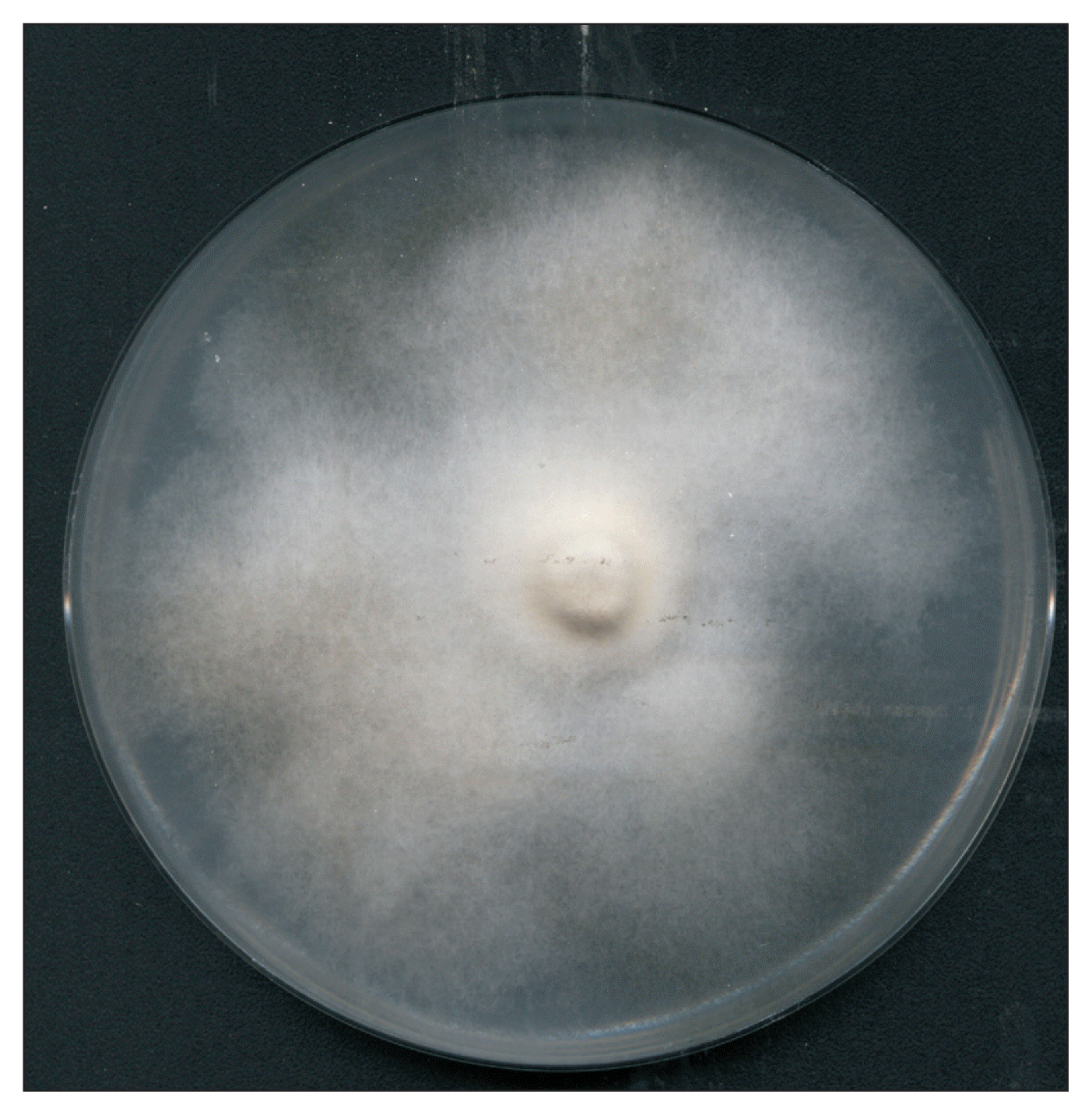
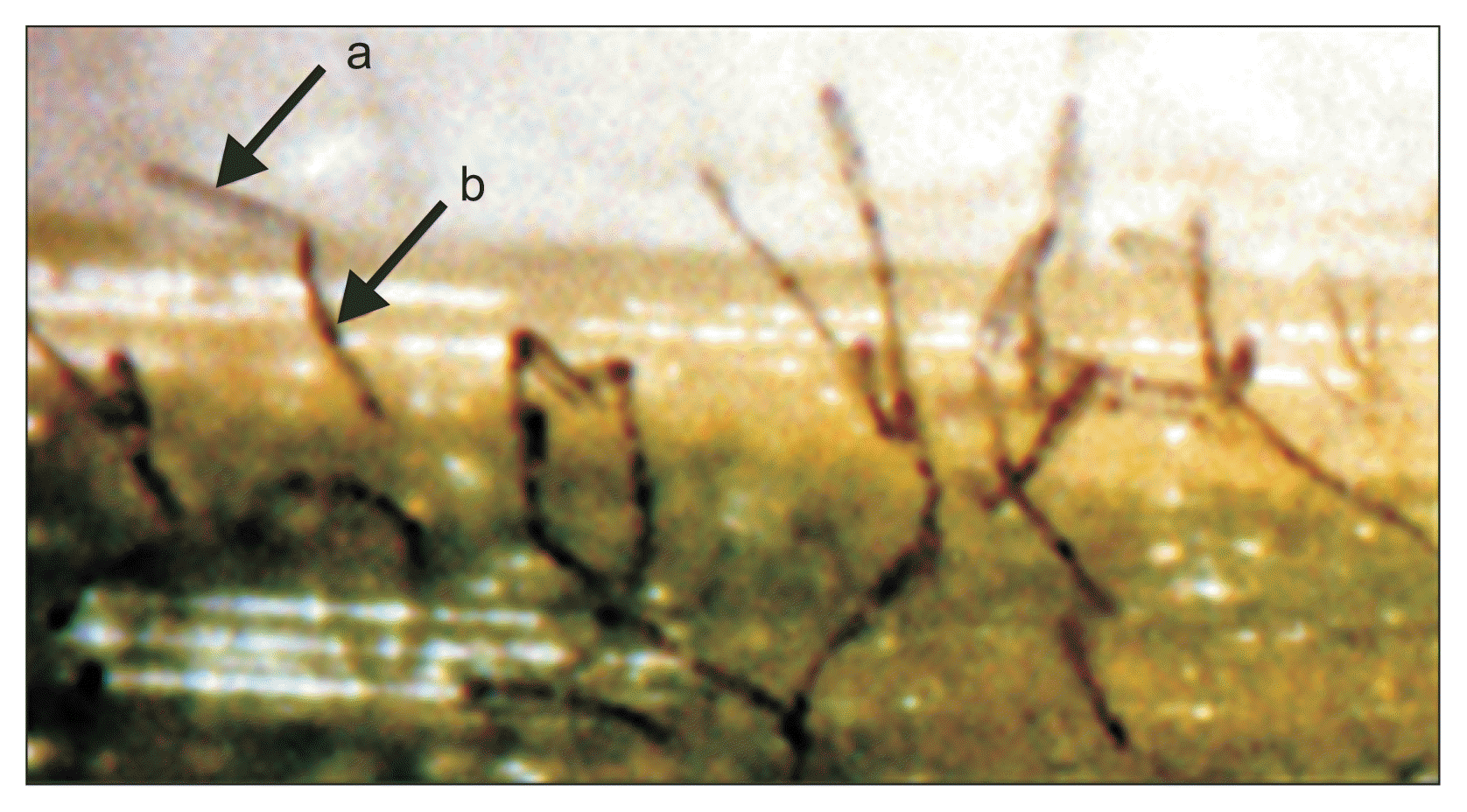
Fig. 6
Fourteen-day-old Pyrenophora tritici-repentis culture showing black spherical mycelia aggregations (arrows).

Fig. 7
Changing into orange color (arrows) observed in Pyrenophora tritici-repentis subcultures maintained on potato dextrose agar medium.
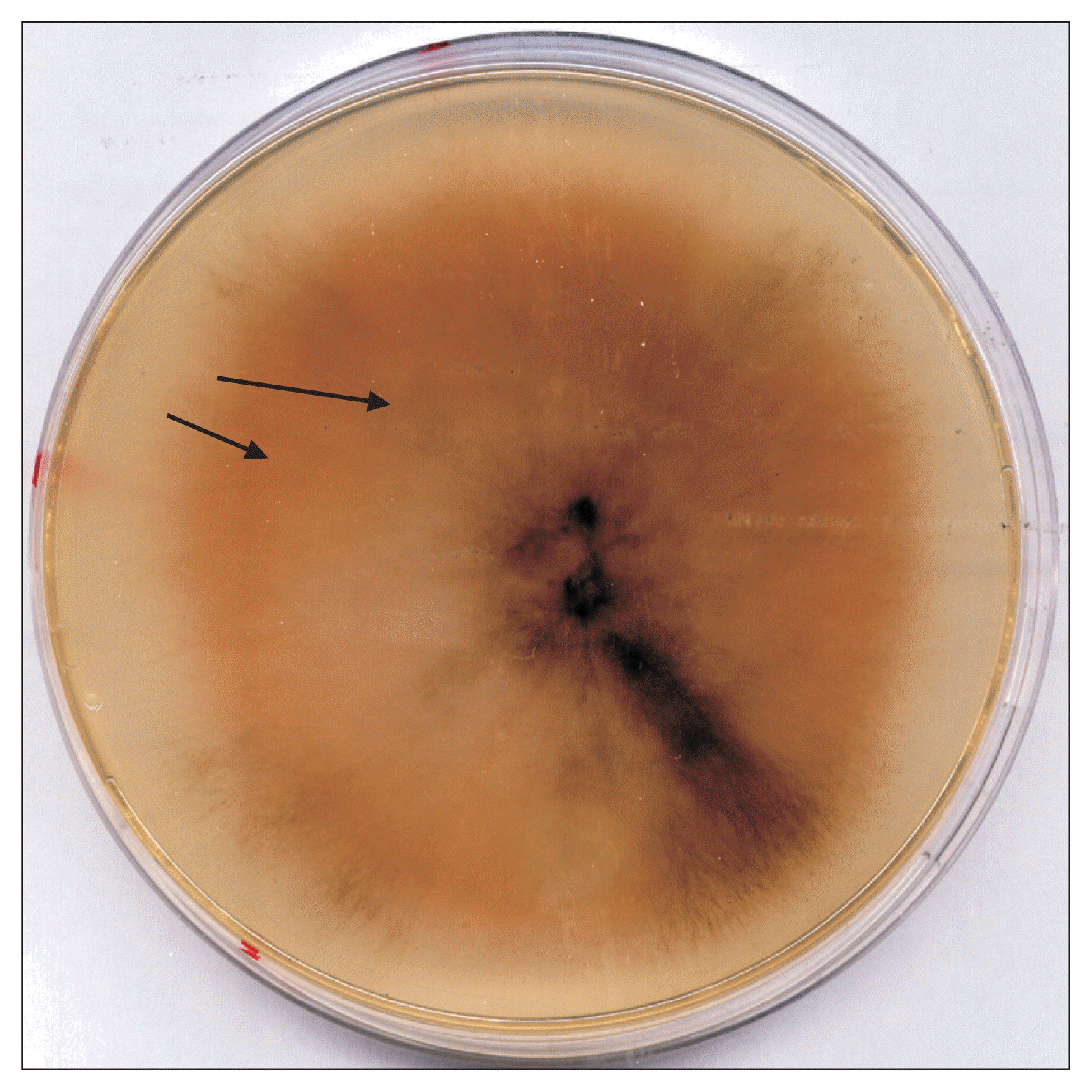
Fig. 8
Groups of isolates as reveled by the principal component analysis based on mycelia radial growth at three different temperatures.
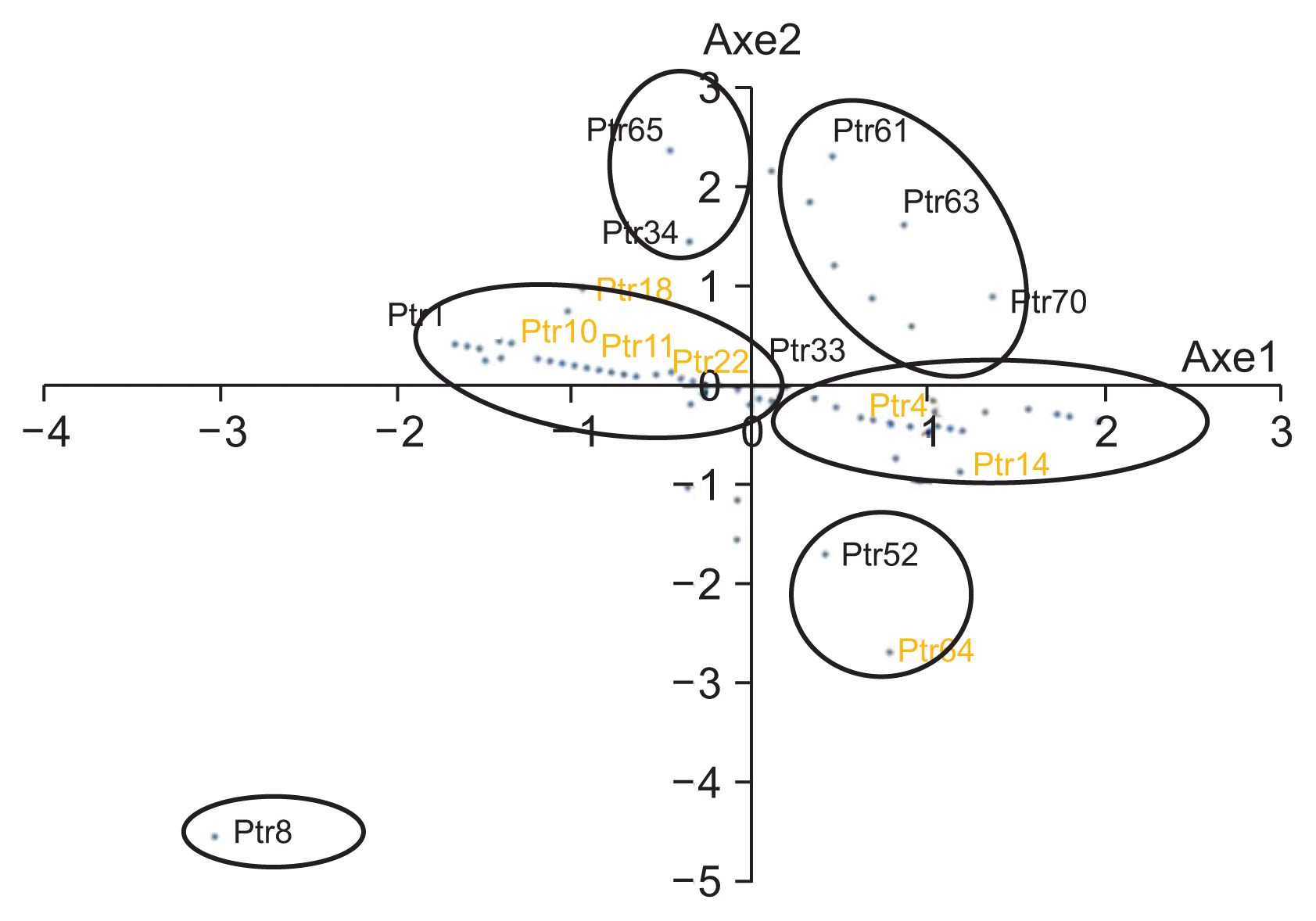
Table 1
Pyrenophora tritici-repentis isolates used in this study
Table 2
Daily means growth (mm) of Pyrenophora tritici-repentis mycelia at different temperatures
References
Adee, EA and Pfender, WF 1989. The effect of primary inoculums level of Pyrenophora tritici-repentis on tan spot epidemic development of wheat. Phytopathology. 79:873-877.

Ali, S and Francl, L 1998. Race structure of Pyrenophora tritici-repentis isolates from wheat and grasses in the US Great Plains. Phytopathology. 88:S114.
Ali, S, Gurung, S and Adhikari, TB 2010. Identification and characterization of novel isolates of Pyrenophora triticirepentis from Arkansas. Plant Dis. 94:229-235.


Asad, S, Iftikhar, S, Munir, A and Ahmad, I 2009. Characterization of Bipolaris sorokoniana isolated from different agro-ecological zones of wheat production in Pakistan. Pak J Bot. 41:301-308.
Belabid, L 2002. La Fusariose vasculaire de la lentille (Lens culinaris Med.) dans le nord-ouest algérien: morphologie et diversité génétique chez Fusarium oxysporum (Schlecht.) Emend. S. & H. f. sp. lentis (Vasud. & Srini.) en relation avec la repartition geographique et le pouvoir pathogène. PhD thesis. Université d’Oran, Oran, Algeria.
Benslimane, H 2002. Caractérisation de quelques isolats de Pyrenophora graminea S. Ito & Kuribay et leur comportement à l’égard de cinq génotypes d’orge. Master thesis. Institut National Agronomique, Alger, Algeria.
Benslimane, H, Bouznad, Z, Aouali, S, Khalfi, A, Benbelkacem, A and Sayoud, R 2006. Prévalence de la tache bronzée du blé causée Pyrenophora tritici-repentis en Algérie. 20-21 Jun, 6; In: éme journées scientifiques et techniques phytosanitaires; Alger, Algeria.
Benslimane, H, Lamari, L, Benbelkacem, A, Sayoud, R and Bouznad, Z 2011. Distribution of races of Pyrenophora tritici-repentis in Algeria and identification of a new virulence type. Phytopathol Mediterr. 50:203-211.
Benslimane, H, Lababidi, S, Yahyaoui, A, Ogbonnaya, F, Bouznad, Z and Baum, M 2013. Genetic diversity of Pyrenophora tritici-repentis in Algeria as revealed by amplified fragement length polymorphism (AFLP) analysis. Afr J Biotechnol. 12:4082-4093.
Booth, C 1971. The genus Fusarium. Commonwealth Mycological Institute, Kew, UK. 137.
Christensen, JJ and Graham, TW 1934. Physiologic specialization and variation in Helminthosporium gramineum Rab. Tech Bull Minn Agric Expt Stn. 95:40.
da Luz, WC and Bergstrom, GC 1986. Effect of temperature on tan spot development in spring wheat cultivars differing in resistance. Can J Plant Pathol. 8:451-454.

da Luz, WC and Hosford, RM Jr 1980. Twelve Pyrenophora tricostoma races for virulence to wheat in Central Plains of North America. Phytopathology. 70:1193-1196.

dos Santos, AMPV, Matsumura, ATS and Van Der Sand, ST 2002. Intraspecific genetic diversity of Drechslera tritici-repentis as detected by random amplified polymorphic DNA analysis. Genet Mol Biol. 25:243-250.

Frazzon, APG, Matsumura, ATS and Van Der Sand, ST 2002. Morphological characterisation and genetic analysis of Drechslera teres isolates. Genet Mol Biol. 25:235-241.

Friesen, TL, Ali, S, Kianian, S, Francl, LJ and Rasmussen, JB 2003. Role of host sensitivity to Ptr ToxA in development of tan spot of wheat. Phytopathology. 93:397-401.


Friesen, TL, Ali, S, Klein, KK and Rasmussen, JB 2005. Population genetic analysis of a global collection of Pyrenophora tritici-repentis, causal agent of tan spot of wheat. Phytopathology. 95:1144-1150.


Harrington, TC and Rizzo, DM 1999. Defining species in the fungi. In: Structure and dynamics of fungal populations, eds. by JJ Worrall, 43-71. Kluwer Press, Dordrecht, the Netherlands.

Hausner, G, Reid, J, Hopkin, AA and Davis, CN 1999. Variation in culture and rDNA isolates of Sphaeropsis sapinea from Ontario and Manitoba. Can J Plant Pathol. 21:256-264.
Hosford, RM Jr 1971. A form of Pyrenophora trichostoma pathogenic to wheat and other grasses. Phytopathology. 61:28-32.

Howard, RJ and Morrall, RAA 1975. The epidemiology of leaf spot disease in a native prairie. I. The progression of disease with time. Can J Bot. 53:1040-1050.

Hunger, RM and Brown, DA 1987. Colony color, growth, fungicide sensitivity, and pathogenicity of Pyrenophora tritici-repentis. Plant Dis. 71:907-910.

Lamari, L and Bernier, CC 1989. Evaluation of wheat lines and cultivars to tan spot [Pyrenophora tritici-repentis] based on lesion type. Can J Plant Pathol. 11:49-56.

Lamari, L and Bernier, CC 1991. Genetics of tan necrosis and extensive chlorosis in tan spot of wheat caused by Pyrenophora tritici-repentis. Phytopathology. 81:1092-1095.

Leisová, L, Hanzalová, A and Kucera, L 2008. Genetic diversity of Pyrenophora tritici-repentis isolates as revealed by AFLP analysis. J Plant Pathol. 90:233-245.
Lourd, M 1995. Diversité génétique des populations de parasites des plantes: structures des populations et analyse de la diversité. In: Modélisation en protection des cultures, eds. by S Savary, 172-186. Académie d’Agriculture de France, Paris, France.
Marshall, D 2009. Disease which challenge global wheat production powdery mildew and leaf and head blight. In: Wheat: science and trade, eds. by BF Carver, 155-168. Wiley-Blackwell, Ames, IA, USA.
Matsumura, ATS 1991. Variabilidade intraespecífica quanto a patogenicidade, características de cultura e padrão isoenzimático em populações naturais de Bipolaris sorokiniana (Helminthosporium sativum). PhD thesis. Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil.
McDonald, WC 1967. Variability and inheritance of morphological mutants in Pyrenophora teres. Phytopathology. 57:747-755.
Mielke, H 1999. Studien zur biologie des erregers drechslera tritici-repentis, zur anfelligkeit des Weizens und verschiedener artverwandten sowie zur Bekampfung der DTR-Weizenblattdurre. Parey Buchverlag, Berlin, Deutschland.
Misra, AP and Singh, RA 1972. Pathogenic differences amongst three isolates of Helminthosporium tritici-repentis and the performance of wheat varieties against them. Indian Phytopathol. 25:350-353.
Moreno, MV, Stenglein, SA, Ballati, PA and Perello, AE 2008. Pathogenic and molecular variability among isolates of Pyrenophora tritici-repentis, causal agent of tan spot of wheat in Argentina. Eur J Plant Pathol. 122:239-252.


Morrall, RAA and Howard, RJ 1975. The epidemiology of leaf spot disease in a native prairie. II. Air borne spore population of Pyrenophora tritici-repentis. Can J Bot. 53:2345-2353.

Nsarellah, N and Mergoum, M 1997. Effect of crop rotation and straw mulch inoculation on tan spot and root rot in bread and durum wheat. In: Heminthosporium blights of wheat: spot blotch and tan spot, eds. by In : E Duveiller, HJ Dubin, J Reeves and A McNab, 157-161. In: Proceedings of an International Workshop Held at CIMMYT; EI Batan, Mexico.
Oliveira, AMR, Matsumura, ATS, Prestes, AM, Matos, GS and Van Der Sand, ST 1998. Variabilidade patogênica e morfológica em isolado de Bipolaris sorokiniana. Fitopatol Bras. 23:349-353.
Parmasto, E and Parmasto, I 1992. Size and shape of basidiospores in the Hymenomycetes. Mycol Helv. 5:47-78.
Rees, RG, Platz, GJ and Mayer, RJ 1982. Yield losses in wheat from yellow spot: comparison of estimates derived from single tillers and plots. Aust J Agric Res. 33:899-908.

Schilder, AMC and Bergstrom, GC 1990. Variation in virulence within the population of Pyrenophora tritici-repentis in New York. Phytopathology. 80:84-90.

Singh, PK and Hughes, GR 2006. Genetic similarity among isolates of Pyrenophora tritici-repentis, causal agent of tan spot of wheat. J Phytopathol. 154:178-184.

Sutton, JC and Vyn, TJ 1990. Crop sequences and tillage practices in relation to diseases of winter in Ontario. Can J Plant Pathol. 12:358-368.
Valim-Labres, ME, Prestes, AM, Van der Sand, S and Matsumura, ATS 1997. Variação no aspecto cultural, morfologia e virulência em isolados de Bipolaris sorokiniana de trigo. Fitopatol Bras. 22:483-487.
Wiese, MV 1987. Tan spot (yellow leaf spot). In: Compendium of wheat diseases, eds. by MV Wiese, 42-43. APS Press, St. Paul, MN, USA.
Wolf, P 1991. Biologie Epidemiologie Schadrelevanz Konzeption fûr ein integrierte Bekämpfung von Drechslera tritici-repentis (Died.) Drechs. dem Erreger einer Blattfleckenkrankheit an Weizen. PhD thesis. TU München, Weihenstephan, Germany.
Wolf, PFJ and Hoffmann, GM 1993. Biological studies on Drechslera tritici-repentis (Died.) Shoem. (teleomorph Pyrenophora tritici-repentis (Died.) Drechsler) the casual agent of a leaf spot disease on wheat. Z Pflanzenkrankh Pflanzenschutz. 100:33-48.
Wright, KH and Sutton, JC 1990. Inoculum of Pyrenophora tritici-repentis in relation to epidemics of tan spot of winter in Ontario. Can J Plant Pathol. 12:149-157.
- TOOLS
-
METRICS

- Related articles
-
Characteristics of a
Lettuce mosaic virus Isolate Infecting Lettuce in Korea2014 June;30(2)



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



