 |
 |
| Plant Pathol J > Volume 33(2); 2017 > Article |
Abstract
The effect of temperature on the rate of systemic infection of potatoes (Solanum tuberosum L. cv. Chu-Baek) by Potato virus Y (PVY) was studied in growth chambers. Systemic infection of PVY was observed only within the temperature range of 16°C to 32°C. Within this temperature range, the time required for a plant to become infected systemically decreased from 14 days at 20°C to 5.7 days at 28°C. The estimated lower thermal threshold was 15.6°C and the thermal constant was 65.6 degree days. A systemic infection model was constructed based on experimental data, using the infection rate (Lactin-2 model) and the infection distribution (three-parameter Weibull function) models, which accurately described the completion rate curves to systemic infection and the cumulative distributions obtained in the PVY-potato system, respectively. Therefore, this model was useful to predict the progress of systemic infections by PVY in potato plants, and to construct the epidemic models.
Potato virus Y (PVY) is a member of the Potyvirus genus. It is an important aphid-transmitted virus species that causes economically important diseases in potatoes (Solanum tuberosum L.) and many other plant species throughout the world (Hull, 2002). The peach aphid (Myzus persicae) is an important vector in transmitting PVY (Radcliffe, 1982). However, only winged (alatae) aphids have the ability to transmit the virus horizontally, from an infected potato plant to other healthy plants, because of the short time they remain viruliferous and are able to transmit the virus (Broadbent, 1948) in a non-persistent manner (Watson and Roberts, 1939). The timing of the occurrence of these alatae aphids, their numbers, and their flight behavior affect the spread of PVY in potatoes. Forecasting models, based on monitoring aphids, have been developed to predict the movement time of these aphids (Robert et al., 2000).
The spread of PVY infection in a crop is not only dependent on the aphid transmission ability, but also on the susceptibility of the host to infection. Older plants often show a stronger resistance to infection than younger and -tender plants, due to the presence of physical barriers (e.g., hairs, waxes) that prevent insects from probing or feeding on them and delivering the virus. Resistance could also be attributed to volatile emissions that repel insects or stronger antiviral resistances that are active against the delivered viruses (Chung et al., 2015; Fajinmi and Fajinmi, 2010). In addition, in older plants, virus spread into already developed tissues is slower or completely blocked as it follows the source-to-sink route (Roberts et al., 1997), whereas in younger developing plants, systemic spread into new developing tissues occurs quickly.
Temperature is an environmental parameter that differentially affects the interaction of compatible hosts with different RNA viruses (Del Toro et al., 2015). Elevated temperatures have been proposed to increase the efficacy of the gene silencing-based antiviral defense (Chellappan et al., 2005; Szittya et al., 2003), whereas in potatoes, higher efficiency of a hypersensitive resistance to PVY was observed at lower temperatures (Valkonen, 1997). In the case of infection of the Solanaceous host Nicotiana benthamiana by a PVY isolate, it was found that, at relatively elevated temperatures (30°C), the antiviral silencing defense could be suppressed by the virus.
Systemic viral titers could affect the transmission efficiency of the virus to new hosts by the insect vector, although this has not been tested. Therefore, other processes in the viral cycle, including replication and spread, must be affected by temperature in this plant-virus interaction (Del Toro et al., 2015; Glasa et al., 2003; Syller, 1987). Whatever the cause, temperature is a major factor affecting the systemic infection of compatible host plants by some positive-sense RNA viruses (Chung et al., 2015; Del Toro et al., 2015). In the case of the PVY-potato system, a model that predicts when, after infection, a potato plant becomes a new source of virus for a vector—or when the plant becomes fully systemically infected by the virus at a certain temperature—would be an important tool to forecast the spread of PVY infection in a crop. In this study, therefore, we developed a systemic infection model that explained and predicted the proportion of systemically-infected plants in a potato population at an ambient growth temperature.
An aphid transmissible PVY-O Korean isolate (PVY-Jeju) was used as inoculum to infect potatoes. A Korean potato cultivar Chu-Baek was used in the systemic infection experiments. Because aphid is poikilotherm and their role as a PVY vector can be affected by various other factors, PVY virus was inoculated mechanically following the description of Valkonen et al. (1995). Fifty microliters of an extract from PVY-infected potato leaves was inoculated on their two lower leaves of a potted potato plant, 10-15 cm in height and with 4-5 expanded leaves that had been grown for 4 weeks after the rooting of cuttings. The extract was prepared in phosphate-buffered saline (PBS) at a pH of 6.8 (weight:volume, 1:1). This extract was aliquoted and then kept frozen until inoculation, to ensure that all plants in the experiments were challenged with the same inoculum. The inoculation was performed by applying the extract onto carborundum-dusted leaves, followed by gentle rubbing with gloved fingers.
To establish how temperature affected the systemic infection of potatoes, plants were grown at 12°C, 16°C, 20°C, 24°C, 28°C, 32°C, and 35 ± 1°C, in a 16:8 (daylight:darkness) photoperiod in controlled environment growth chambers (MLR-350H; Sanyo Electric Co., Osaka, Japan). Twenty plants were used for each temperature treatment. Sample discs were collected with an Eppendorf cap (disc diameter of 8 mm) from upper expanding leaves of each potato plant at 2 d intervals from the third day (72 h) after the initial inoculation, for a total of 17 days (eight discs collected per plant). Discs were frozen in liquid N and stored at −80°C until the time for analysis.
The presence or absence of PVY in discs was determined by the direct enzyme-linked immunosorbent assay (Direct-ELISA). Each disc was ground inside an Eppendorf tube (1.5 ml) in 150 μl of PBS at a pH of 6.8, and centrifuged for 1 min at 5,000 rpm for clarification. One hundred microliters of clear supernatant were mixed with coating buffer (0.1 M sodium-carbonate buffer, pH 9.6) at a 1:1 ratio and poured into the wells of ELISA plates. The plates were stored overnight at 4°C for coating. Extract-coated plates were then washed twice with PBS containing 5% Tween-20 (PBS-T), and 200 μl of a primary rabbit polyclonal antibody against the coat protein of PVY (Llave et al., 1999) was added, diluted to 1/1,000 in PBS with 0.6% nonfat milk for 2 h. Plates were then washed twice with PBS-T and incubated with 200 μl of a solution of a commercial alkaline phosphatase-conjugated secondary antibody (Cat. A3687; Sigma Aldrich, St. Louis, MO, USA) diluted to 1/10,000 in PBS containing 0.6% non-fat milk. After 2 h of incubation at 37°C, the wells were washed and 200 μl of the para-nitrophenol phosphate substrate solution was added to each well. One hour later, colorimetric reactivity was measured at 405 nm by UV-Spectroscopy (Versamax Microplate Reader, Sunnyvale, CA, USA). Readings were considered a positive indication of infection with PVY if they were at least two times higher than the readings obtained from discs of non-inoculated potatoes (control) in the same plate.
Completion rate to systemic infection (1/mean day) were expressed as the reciprocal of the days required for systemic infection by PVY-O to be detected. A linear relationship was observed in the range of 16°C to 28°C. The lower temperature threshold and the thermal constant were calculated by solving the intercept/slope and 1/slope of the fitted equation for each stage, respectively. The completion rate curve to systemic infection was also fitted to a nonlinear model. The Lactin-2 model (Lactin et al., 1995) is
where r(T) is the development rate at T °C, Tmax is a thermal maximum, Δ is the temperature range over which “thermal breakdown” becomes the overriding influence, and ρ is a composite value for critical enzyme-catalyzed biochemical reactions (Damos and Savopoulou-Soultani, 2008). The λ allows the curve to intersect the abscissa at suboptimal temperatures (Lactin et al., 1995). These parameters were estimated using the TableCurve 2D program (Jandel Scientific, 1996).
A cumulative frequency distribution for completion of systemic PVY infection was constructed by the thermal constant derived from adding the frequencies of successive ages (expressed as days) of the plants. The curve was scaled to “1” by dividing the frequency at each plant age by the total frequency. The standardized cumulative proportion curve, which showed the variation in the systemic infection time, was obtained by transforming the day-based plant age into a standardized age (i.e., physiological age) at various temperatures. Physiological age is used as an index of relative age, dependent on the physiological or behavioral history of the individual instead of chronological age (reviewed by Hayes and Wall, 1999). The cumulative proportion curve was fitted by a three-parameter Weibull function (Wagner et al., 1984)
where F(Pxi) is the cumulative proportion of systemic infection completion of physiological age (Pxi) at the ith day. The parameters, γ, η, and β, were estimated using the TableCurve 2D program (Jandel Scientific, 1996). The physiological age (Pxi) at the ith day was calculated according to the rate summation method (Curry and Feldman, 1987)
where r (Tj) is the completion rate to systemic infection at temperature T (°C) at the jth day after infection. The Δt is the time interval and is set to 1, with 1 day intervals. Therefore, the daily probability of systemic infection in the PVY-infected potato plant at the ith day could be calculated as
Data are expressed as means ± standard error, and ANOVA analysis was conducted with the SAS program (SAS Institute, 1999). Means were separated by Tukey’s studentized range test at P = 0.05.
Temperature affected the timing of appearance of systemic infection of PVY in upper leaves of Chu-Baek potatoes and, therefore, the rate of spread of the infection. The times of systemic infection at different temperatures are shown in Table 1. No systemic infection was detected for 12°C, 16°C, and 35°C at 17 days after inoculation. The length of time for the appearance of systemic infection decreased steadily with increasing temperatures, from 14.0 days at 20°C to 5.7 days at 28°C, and then increased sharply at higher temperatures (F = 18.37, degree of freedom [df] = 3, 36, P < 0.0001). Systemic infection in all the plants was observed only at 24°C and 28°C. The percentage of plants infected systemically decreased to 90% at 32°C, and it dropped to only 20% at 20°C (Table 1).
The parameters, a and b, of the linear model (Y = a·X + b) were 0.01525 and −0.2, respectively (r2 = 0.985). The estimated lower thermal threshold was 15.6°C. The thermal constant, the thermal amount needed to achieve systemic infection, was 65.6 degree days (DD) for systemic infection of PVY in potato (Fig. 1A). For example, systemic infection can be estimated when the cumulative degree days of air temperature with base temperature 15.6°C is greater than 65.6°C.
Concerning the completion rate to systemic infection model, the Lactin-2 model (Lactin et al., 1995) was statistically significant (F = 104.187; df = 3, 2; P = 0.0095). This model produced an infection rate curve with a high r2 value for nonlinear regression and low standard errors for all parameters (Fig. 1A, Table 2). The infection rate increased with increasing temperature, to a maximum at 28°C, after which it decreased (Fig. 1A).
The cumulative distribution of completion of systemic PVY infection against the physiological plant age is shown in Fig. 1B. The three-parameter Weibull function (Wagner et al., 1984) was applied to fit to the curve. The regression model was statistically significant (F = 269.286; df = 2, 24; P < 0.0001), and the estimated values for parameters, γ, η, and β, were 0.4706, 0.6202, and 2.4891, respectively (Table 2). Among these parameters, γ indicates the expected the first occurring time of systemic, and β indicates the steepness of curve for cumulative completion rate of incubation time. A fifty percent of systemic infection will occur at the physiological time of γ plus η (Choi and Kim, 2014; Wagner et al., 1984).
The predicted probability curve of a plant becoming systemically infected, relative to temperature and days after infection by PVY in the potato population, is shown in the vertical axis of the plot (Fig. 2). The combined pattern of curves had a ‘U’ shape within the temperature range of 16°C to 35°C, with a maximum systemic infection probability peak at 28°C.
In this study, temperature affected the systemic infection of PVY in young potato plants (Table 1). The time needed to detect systemic infection decreased steadily with increasing temperature, from 14.0 days at 20°C to just 5.7 days at 28°C, only to increase sharply at higher temperatures. These results are similar to those reported for PVY-infected tobacco (Nicotiana tabacum L. [Close, 1964; Shepard and Uyemoto, 1976]). Systemic infection of PVY was not detected by ELISA as well as external symptoms in potato plants maintained at less than 16°C or at 35°C (Fig. 1A). In addition, the proportion of potato plants that became systemically infected dropped to only 20% at 20°C, whereas 100% of potato plants became systemically infected at 24°C and 28°C. Considering the sharply decreased completion rate to systemic infection and the lower thermal threshold, estimated at 16°C, our PVY isolate could not systemically infect these potatoes under such low temperature. This was perhaps attributable to the low replication rate, the inability to overcome plant defense responses, or the limited progress of viral systemic spread after infection. In this regard, a hypersensitive resistance to PVY in some potato cultivars is more efficient at lower temperatures (16-18°C) than at higher temperatures (19-24°C; Valkonen, 1997).
We constructed a model that reflects the systemic infection of PVY in potato using a completion rate to systemic infection model, and a systemic infection distribution model to describe the occurrence of systemic infection complete, based on the stage transition approach of multiple cohorts in insect populations (Curry and Feldman, 1987; Wagner et al., 1984, 1985). The completion rate curve to systemic infection within a temperature range of 15.7-35.1°C showed that PVY systemic infection increased linearly, to an optimum temperature, and decreased thereafter (Fig. 1A). Physiological age was calculated after summing the rates from the Lactin-2 model for a given temperature.
Three-parameter Weibull functions were used to create a systemic infection distribution model that incorporated the stochastic aspects of systemic infection. Variable time of appearance of systemic infection was normalized at the different temperatures to the PVY-infected plant age (Wagner et al., 1984). This was useful for predicting the first day of appearance of systemic infection compete, 50% of infection of the potato population, and the day at which systemic infection affected the entire potato population (Choi and Kim, 2014). With these procedures (Wagner et al., 1985), the probability of completion of systemic infection in the PVY-infected potato population could be simulated (Fig. 2). Although systemic infection model is not validated with field data, it describes the systemic infection of potato population well at a various temperature range.
In this study, we showed that temperature affects the systemic infection of potato plant by PVY, implying that systemic infection must be an important step to decide the time when the potato plant is fully infected after transmission of PVY by aphid in nature. Also, there were individual variations in systemic infection. Those aspects are well incorporated into our systemic infection model based on the concept of insect model (Curry and Feldman, 1987; Wagner et al., 1984, 1985). Therefore, the systemic infection model of this study, alone or together with other models, could be useful in predicting the occurrence and prevalence of systemic infection by a PVY outbreak in a potato population, at different temperatures. In addition, the model could be useful for the diagnosis and control of disease, and for breeders and biotechnologists when producing and evaluating resistance in potato.
Acknowledgments
This work was supported by the “Fundamental Research Program for Agriculture Science & Technology Development (Project No. PJ01024602)” Rural Development Administration, Republic of Korea, and in cooperation with the Spanish Council for Scientific Research (CSIC).
Fig. 1
Systemic infection model of Potato virus Y (PVY) in Chu-Baek potato plants. (A) Linear and non-linear model were fitted to the completion rate curve to systemic infection (1/mean day). (B) Cumulative proportion when the systemic infection in the PVY-infected potato plant occurs, as a function of the plant physiological age. A three-parameter Weibull function (Wagner et al., 1984) was used.

Fig. 2
Predicted curve of the probability of systemic infection by Potato virus Y in Chu-Baek potato plants relative to the ambient temperature and the time after the initial local inoculation.
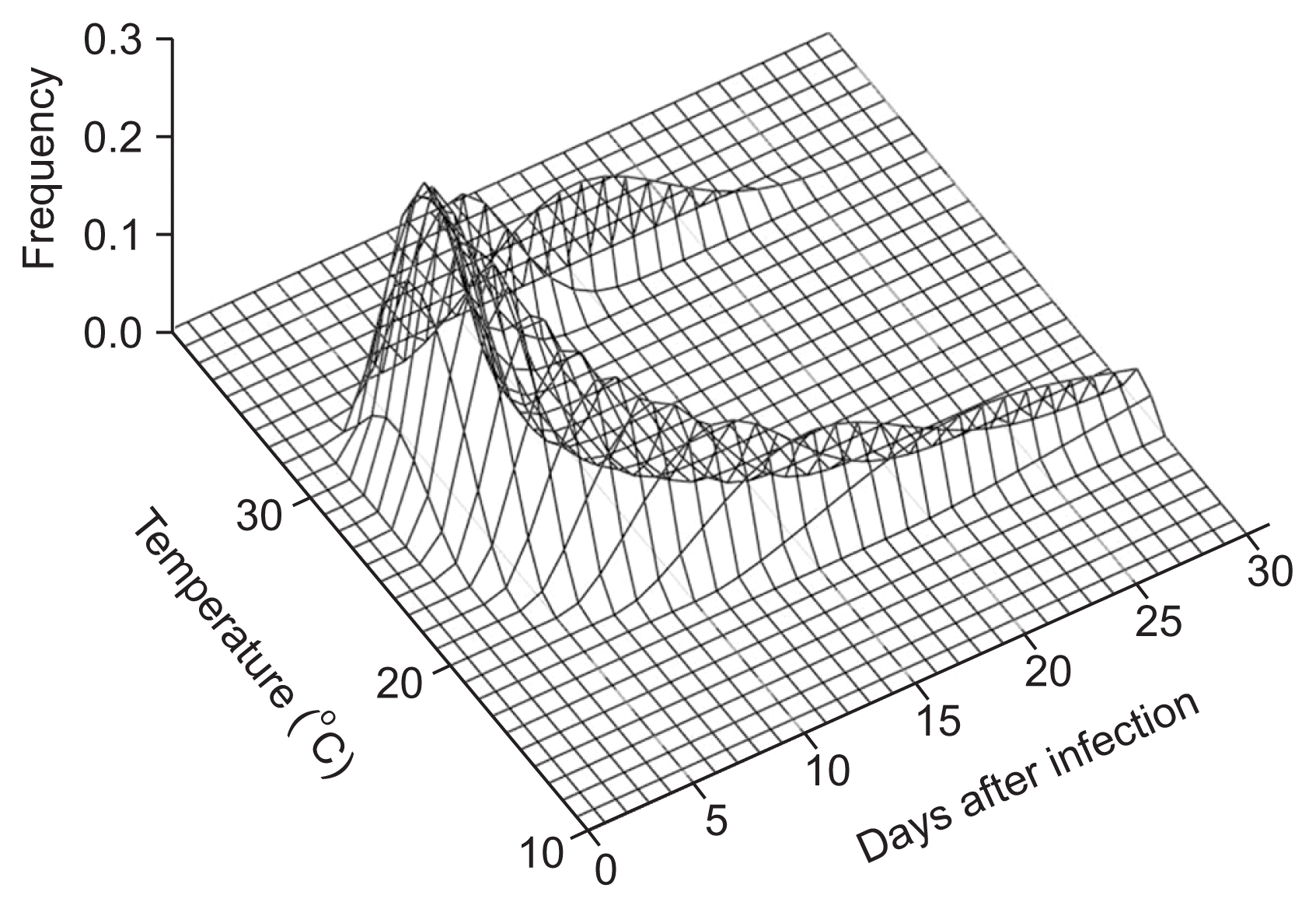
Table 1
Time of appearance of systemic infection by Potato virus Y in Chu-Baek potatoes at different constant temperatures
| Temperature (°C) | No. of potato plants | Infection time (day) | % of plants infected | |
|---|---|---|---|---|
|
|
||||
| Mean ± standard error* | Median | systemically | ||
| 12 | 20 | - | - | 0 |
| 16 | 20 | - | - | 0 |
| 20 | 20 | 14.0 ± 0.07 a* | 14.0 | 20 |
| 24 | 20 | 7.0 ± 0.35 b | 6.2 | 100 |
| 28 | 20 | 5.7 ± 0.33 b | 4.5 | 100 |
| 32 | 20 | 7.8 ± 0.67 b | 8.5 | 90 |
| 35 | 20 | - | - | 0 |
Table 2
Parameters of the non-linear model of the systemic infection of Chu-Baek potato plants by Potato virus Y
| Model | Parameter | Estimated value | r2 |
|---|---|---|---|
| Infection rate model* | ρ | 0.0160 ± 0.00245 | 0.994 |
| Tmax | 42.2707 ± 1.18834 | ||
| Δ | 5.0511 ± 1.15696 | ||
| λ | −1.2745 ± 0.04539 | ||
| Infection distribution model† | γ | 0.4706 ± 0.21544 | 0.957 |
| η | 0.6202 ± 0.22568 | ||
| β | 2.4891 ± 1.06413 |
* Lactin-2 model (Lactin et al., 1995) was applied.
† Three-parameter Weibull function (Wagner et al., 1984) was applied.
References
Broadbent, L 1948. Aphis migration and the efficiency of the trapping method. Ann Appl Biol. 35:379-394.


Chellappan, P, Vanitharani, R, Ogbe, F and Fauquet, CM 2005. Effect of temperature on geminivirus-induced RNA silencing in plants. Plant Physiol. 138:1828-1841.




Choi, KS and Kim, DS 2014. Temperature-dependent development of Ascotis selenaria (Lepidoptera: Geometridae) and its stage emergence models with field validation. Crop Prot. 66:72-79.

Chung, BN, Choi, KS, Ahn, JJ, Joa, JH, Do, KS and Park, KS 2015. Effects of temperature on systemic infection and symptom expression of turnip mosaic virus in Chinese cabbage (Brassica campestris). Plant Pathol J. 31:363-370.



Close, R 1964. Some effects of other viruses and of temperature on the multiplication of potato virus X. Ann Appl Biol. 53:151-164.

Curry, GL and Feldman, RM 1987. Mathematical foundations of population dynamics. Texas Engineering Experiment Station monograph series, no. 3. Texas A & M University Press, College Station, TX, USA.
Damos, PT and Savopoulou-Soultani, M 2008. Temperature-dependent bionomics and modeling of Anarsia lineatella (Lepidoptera: Gelechiidae) in the laboratory. J Econ Entomol. 101:1557-1567.


Del Toro, FJ, Aguilar, E, Hernández-Walias, FJ, Tenllado, F, Chung, BN and Canto, T 2015. High temperature, high ambient CO2 affect the interactions between three positive-sense RNA viruses and a compatible host differentially, but not their silencing suppression efficiencies. PLoS One. 10:e0136062



Fajinmi, AA and Fajinmi, OB 2010. Incidence of okra mosaic virus at different growth stages of okra plants ([Abelmoschus esculentus (L.) Moench) under tropical condition. J Gen Mol Virol. 2:28-31.
Glasa, M, Labonne, G and Quiot, JB 2003. Effect of temperature on plum pox virus infection. Acta Virol. 47:49-52.

Hayes, EJ and Wall, R 1999. Age-grading adult insects: a review of techniques. Physiol Entomol. 24:1-10.


Hull, R 2002. Matthews’ plant virology. 4th ed. Academic Press, San Diego, CA, USA.
Jandel Scientific. 1996. TableCurve 2D. Automated curve fitting and equation discovery: version 4.0. Jandel Scientific, Sam Rafael, CA, USA.
Lactin, DJ, Holliday, NJ, Johnson, DL and Craigen, R 1995. Improved rate model of temperature-dependent development by arthropods. Environ Entomol. 24:68-75.

Llave, C, Martínez, B, Díaz-Ruíz, JR and López-Abella, D 1999. Serological analysis and coat protein sequence determination of Potato virus Y (PVY) pepper pathotypes and differentiation from other PVY strains. Eur J Plant Pathol. 105:847-857.
Robert, Y, Woodford, JA and Ducray-Bourdin, DG 2000. Some epidemiological approaches to the control of aphid-borne virus diseases in seed potato crops in northern Europe. Virus Res. 71:33-47.


Roberts, AG, Cruz, SS, Roberts, IM, Prior, D, Turgeon, R and Oparka, KJ 1997. Phloem unloading in sink leaves of Nicotiana benthamiana: comparison of a fluorescent solute with a fluorescent virus. Plant Cell. 9:1381-1396.



SAS Institute. 1999. SAS OnlineDoc Version 8. SAS Institute, Cary, NC, USA.
Shepard, JF and Uyemoto, JK 1976. Influence of elevated temperatures on the isolation and proliferation of mesophyll protoplasts from PVX-and PVY-infected tobacco tissue. Virology. 70:558-560.


Syller, J 1987. The influence of temperature on transmission of potato leaf roll virus by Myzus persicae Sulz. Potato Res. 30:47-58.


Szittya, G, Silhavy, D, Molnár, A, Havelda, Z, Lovas, Á, Lakatos, L, Bánfalvi, Z and Burgyán, J 2003. Low temperature inhibits RNA silencing-mediated defence by the control of siRNA generation. EMBO J. 22:633-640.



Valkonen, JPT 1997. Novel resistances to four potyviruses in tuber-bearing potato species, and temperature-sensitive expression of hypersensitive resistance to potato virus Y. Ann Appl Biol. 130:91-104.

Valkonen, JPT, Puurand, Ü, Slack, SA, Mäkinen, K and Saarma, M 1995. Three strain groups of potato A potyvirus based on the hypersensitive responses in potato, serological properties, and coat protein sequences. Plant Dis. 79:748-753.

Wagner, TL, Wu, HI, Feldman, RM, Sharpe, PJH and Coulson, RN 1985. Multiple-cohort approach for simulating development of insect populations under variable temperatures. Ann Entomol Soc Am. 78:691-704.

Wagner, TL, Wu, HI, Sharpe, PJH and Coulson, RN 1984. Modeling distribution of insect development time: a literature review and application of Weibull function. Ann Entomol Soc Am. 77:475-483.



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




