 |
 |
| Plant Pathol J > Volume 33(3); 2017 > Article |
Abstract
Pokkah Boeng is a serious disease of sugarcane, which can lead to devastating yield losses in crop-producing regions, including southern China. However, there is still uncertainty about the causal agent of the disease. Our aim was to isolate and characterize the pathogen through morphological, physiological, and molecular analyses. We isolated sugarcane-colonizing fungi in Fujian, China. Isolated fungi were first assessed for their cell wall degrading enzyme capabilities, and five isolates were identified for further analysis. Internal transcribed spacer sequencing revealed that these five strains are Fusarium, Alternaria, Phoma, Phomopsis, and Epicoccum. The Fusarium isolate was further identified as F. verticillioides after Calmodulin and EF-1α gene sequencing and microscopic morphology study. Pathogenicity assay confirmed that F. verticillioides was directly responsible for disease on sugarcane. Co-inoculation of F. verticillioides with other isolated fungi did not lead to a significant difference in disease severity, refuting the idea that other cellulolytic fungi can increase disease severity as an endophyte. This is the first report characterizing pathogenic F. verticillioides on sugarcane in southern China.
Sugarcane is an economically important crop worldwide with many agricultural and industrial uses including animal feed, sweeteners, biofuels, and bagasse. Sugarcane is produced in tropical and subtropical countries, including southern regions of People’s Republic of China, which is now the fourth largest producer of sugarcane worldwide (Anderson-Sprecher and Wei, 2015; Koo and Taylor, 2014). China produced over 126 million ton of sugarcane in 2013, but due to recent profit losses, increasing production costs, unfavorable climatic events and disease pressures, production levels are now decreasing up to 7% each year (Anderson-Sprecher and Wei, 2015; FAO, 2015). Sugarcane is vulnerable to over 100 diseases, which can cause major declines in the annual yield of sugarcane (Matsuoka and Maccheroni, 2015). Despite the production decline, the demand for sugarcane still outweighs the annual yield making China the number one importer of sugarcane (Koo and Taylor, 2014). It is therefore critical to address the ongoing threats to the sugarcane industry in China and around the world.
Pokkah Boeng is a re-emerging disease of sugarcane which has been found recently to cause major yield losses in most sugarcane producing regions, including South Africa, Malaysia, India, and China (Lin et al., 2014; McFarlane and Rutherford, 2005; Sidique and Nordahliawate, 2007; Singh et al., 2006; Vishwakarma et al., 2013). Pokkah Boeng disease was first described by Walker and Went in 1896, and the name originates from a Javanese for ‘malformed top’ (Martin et al., 1989). The usual symptoms include red streaking, chlorosis, malformation, and stalk rot. The disease is known to cause 10-38% yield losses in the susceptible sugarcane variety POJ 2878 (Ricaud et al., 2012). Research in India revealed overall increase in disease incidence in all sugarcane varieties from 2007 to 2013, particularly up to 90% infection in variety S224/20 (Lin et al., 2014; Moretti, 2009). Historically, the incidence of Pokkah Boeng increase following major rain events or post monsoon season. However, the disease is now present throughout the growing season during both wet and dry periods (Lin et al., 2014).
The causal agent of Pokkah Boeng is still ambiguous and disputed amongst researchers. In older literature, Fusarium moniliforme is named as the causal pathogen. However, Fusarium classification and nomenclature system has gone through its turbulent transformation, and researchers have not been able to come to a consensus on which Fusarium species is the appropriate forma specialis (f. sp.) for Pokkah Boeng pathogen. Recent studies have suggested F. sacchari, F. fujikuroi, F. verticillioides, F. andiyazi, and F. proliferatum as the causal agents (Lin et al., 2014; McFarlane and Rutherford, 2005; Sidique and Nordahliawate, 2007; Singh et al., 2006; Vishwakarma et al., 2013). This is in part due to the ambiguity of Fusarium taxonomy particularly in the Liseola section, which includes F. verticillioides, F. proliferatum and F. fujikuroi (Leslie and Summerell, 2011). Gibberella fujikuroi, or section Liseola, includes eight known mating populations, which are capable of causing disease on a wide range of crops such as maize, sugarcane, and sorghum (Bandyopadhyay et al., 2008; Kerényi et al., 1999; Zakaria et al., 2011). These species are also known to produce a variety of mycotoxins that are detrimental to human and animal health, making the study of these pathogens economically and socially significant. The classification of species falling under section Liseola has gone through both expansion and contraction since the first description of Fusarium by Wollenweber and Reinking in 1935 (Waalwijk et al., 1996; Wollenweber and Reinking, 1935). With current ambiguity surrounding the causal agent of Pokkah Boeng, one of the important needs is to characterize the etiology of pathogenic Fusarium species in sugarcane.
In terms of virulence, we still have a limited understanding of how Fusarium pathogens cause disease in sugarcane. When we consider rot diseases in crops, it is reasonable to anticipate that fungal enzymes play a significant role. Many plant pathogenic fungi require cell wall degrading enzymes (CWDE) for successful invasion of plant cell walls and ultimately cause disease (Kubicek et al., 2014). For example, a pectate lyase gene PL1332 in Alternaria brassicicola is required for pathogenicity in Brassica (Cho et al., 2015). In Leptosphaeria maculans, causal agent of Blackleg of canola, LmSNF1 is a major regulator of CWDE genes (Feng et al., 2014). CWDE production was downregulated in LmSNF1 knockout mutants, and strains were avirulent with a reduction in fitness. Furthermore, Jorge et al. (2006) measured the production of polygalacturonase, pectate lyase and xylanase CWDEs by Fusarium oxysporum f. sp. ciceris during disease development in chickpea. The production of CWDEs was positively correlated to symptom development in leaves and stems, indicating that CWDEs are important for F. oxysporum f. sp. ciceris pathogenicity. Sugarcane is a row crop with high recalcitrant lignocellulosic content, therefore it would be reasonable to hypothesize that the causal agent of Pokkah Boeng produces a high level of cellulolytic CWDE during plant pathogenesis.
The aim of this study was to isolate and characterize the causal agent of Pokkah Boeng through morphological, physiological, and molecular genetic analyses. We isolated the fungal strains from diseased crops sampled from a sugarcane breeding research field in Fujian, China. Isolates were assessed for the production of cellulase, a prominent CWDE. In addition, the isolates were inoculated into sugarcane stalks to assess for pathogenicity. Co-inoculations were also performed to gain an understanding of microbial interactions in situ. We performed molecular analyses of the isolated strains to determine the identity of Pokkah Boeng causal agent. Our goal was to gain a better understanding of this critical disease and how the pathogen is able to produce such devastating outcomes on sugarcane crops.
Diseased sugarcane samples were collected in the Sugarcane Breeding Research Field at the Fujian Agriculture and Forestry University (FAFU) in China. The area of the FAFU sugarcane research field (approximately 7 hectares) was separated into two 3.5-hectare sections, and one of the sections was chosen for sampling. The sugarcane cultivar grown in the 3.5-hectare field is ROC22, a hybrid of Saccharum officinarum and Saccharum spontaneum. The sampled area was further divided into four different quadrants and an equal number of diseased plants were randomly taken from each quadrant. Diseased plants were observed for the following symptoms: leaf malformation, leaf curling, stalk rot, red streaking, and necrosis. Samples were cut, placed in plastic bags and labeled before returning to the lab for surface sterilization. A total of 74 diseased plants were sampled in June of 2014. Images of the sampled sugarcanes are provided in Fig. 1.
Following field sampling, diseased plants were subjected to surface sterilization followed by standard pathogen isolation in the lab (Harborne, 1998). Four subsamples of 1-2 cm2 plant tissue were cut out from each sample approximately 2-5 cm away from diseased lesions. These subsamples were placed on sterilized petri dishes for surface sterilization. The surface sterilization process consisted of washing subsamples three times with sterile ddH2O to remove soil and other surface contaminants. A 10% sodium hypochlorite solution was then applied for 5 min on a shaker at 100 rpm. The samples were washed again with sterile ddH2O before a 70% ethanol (EtOH) solution was applied for 30 s. The subsamples were then washed three times with sterile ddH2O. After surface sterilization, the subsamples were inoculated onto CMI (6% yeast extract, 6% casamino acids, 10% glucose, and 20% agar powder) plates. The CMI plates were then overlayed with CMI supplemented with 0.1% ampicillin and 0.1% streptomycin antibiotics. The inoculated plates were placed in an incubator at 25°C with a 12-h light/dark cycle. Once fungal growth was observed, the mycelia was collected and transferred onto new CMI plates supplemented with 0.1% ampicillin and 0.1% streptomycin. Colonies were single spore isolated to maintain purity of the culture (Choi et al., 1999). A total of 137 fungal strains were isolated, and fungal isolates exhibiting analogous phenotypic and morphological markers were grouped, thus arriving at 87 isolates, prior to cellulose/amylase production analysis.
A total of 87 fungal isolates were screened for cellulase and amylase production using the 3,5-dinitrosalicylic acid (DNS)-based enzyme assay (Kim et al., 2014; Percival Zhang et al., 2006). The fungal isolates were grown in CMI liquid culture with 2.5% glucose and 0.5% wheat bran for 5 days in a shaker at 28°C and 150 rpm. A 1-ml aliquot was extracted from the top supernatant of the fungal culture and transferred in a 1.5 ml Eppendorf tube. The Eppendorf tubes were centrifuged at 12,000 rpm for 10 min at room temperature. Approximately 700 μl of the supernatant was transferred into a new 1.5-ml Eppendorf tube. For the cellulase assay, 20 μl of a 1% Avicel (Sigma, St. Louis, MO, USA) solution was mixed with 20 μl of fungal supernatant. For the amylase assay, 20 μl of a 1% soluble starch solution was mixed with 20 μl of fungal supernatant. Controls were prepared by mixing sterile ddH2O and serial dilutions of dextrose. Negative controls with 1% Avicel and 1% soluble starch in sterile ddH2O were also prepared. The mixtures were then incubated at 45°C for 3 h. Following the incubation, a 160-μl DNS solution was added to each tube and incubated at 95-100°C for 20 min. The tubes were then monitored for a color change, and ultimately selected ten tubes with most significant color changes. These ten fungal samples were tested again for cellulase production using the DNS assay with three biological replicates per trial and three technical trials. The optical density of each sample was recorded for quantitative analysis using a spectrophotometer at 570 nm.
To determine the identity of fungal isolates, we amplified genes encoding the internal transcribed spacer (ITS) region, the Elongation Factor 1-Alpha (ELF), the calmodulin, and the MAT1-2-1 mating type genes using the primers listed in Supplementary data (Supplementary Fig. 1, 2, Supplementary Table 1, 2). Following the polymerase chain reaction, the amplicons were sequenced (Gene Technology Lab, Texas A&M University, College Station, TX, USA), and alignments were performed using BLASTN algorithm. Reference sequences with the highest identity were selected and imported into the open source software MEGA 7 (Kumar et al., 2016). Phylogenetic trees were calculated by maximum likelihood method with 1,000 bootstrap replications and those with the highest log likelihood were reported. A neighborjoining method and BioNJ algorithms were used to obtain the trees (Hall, 2013).
Fungal strains were grown on CMI medium for morphological study. All image acquisition was performed using an Olympus BX51 fluorescence microscope outfitted with an Olympus DSU (http://www.olympus-ims.com/en/microscope/) and a Hamamatsu Orca ER camera (http://sales.hamamatsu.com/). The agar block method was used to mount slides for microscopic observation.
The three identified fungal isolates, Fusarium, Phomopsis, and Phoma species, were grown on Complete Media (CMII) plates (Hicks et al., 1997). The fungal spores were collected and enumerated using a haemocytometer. The concentration of conidia was adjusted to 1 × 108 per 10-μl sterile ddH2O and spot inoculated onto CMII, CMI, CMI + 200 mg/ml Congo Red, CMI + 0.01% sodium dodecyl sulfate (SDS), and CMI + 2.5 mM hydrogen peroxide (H2O2). The diameter of the mycelium was measured after 5 days of growth in a 25°C incubator with a 12-h light/dark cycle. A total of three biological replicates per trial in three independently repeated trials were measured. Conidia were collected from the CMII plates after the 5-day incubation period using a borer tube with a 1.3-cm diameter and then counted using a haemocytometer. For the growth assays in liquid culture, 1 × 108/ml conidia were inoculated into 50-ml liquid CMI medium. After 5 days at 25°C in a shaker at 150 rpm, fungal mycelia were filtered and dried before weighing on a scale.
Mature sugarcane stalks were ordered from the Hainan Province, China. The stalks were surface sterilized with 70% ETOH before being cut and separated at the nodes. The sugarcane samples were wounded and then inoculated with 100 μl of a 1 × 108 spore suspension of Fusarium, Phomopsis, and Phoma species. Combinatorial pathogenicity assays were also performed by inoculating 100 μl of a 1:1 mixture of 1 × 104 spore suspension of the following combination of fungi: Fusarium and Phomopsis, Fusarium and Phoma, Fusarium and Alternaria, Fusarium and Epicoccum nigrum, Phomopsis and Phoma, Phomopsis and Alternaria, Phomopsis and E. nigrum, Phoma and Alternaria, and Phoma and E. nigrum. The inoculated sugarcane samples were then placed in humidity chambers and incubated for 7 days at approximately 25°C. Following the incubation, sugarcane samples were cut longitudinally at the wound site to observe the internal rot disease symptoms. A total of three biological replicates per trial and three technical trials were performed.
Diseased sugarcane tissues collected from experimental fields in Fuzhou, China were analyzed for the presence of fungal pathogens associated with Pokkah Boeng symptoms. We collected a total of 74 diseased plant samples, and followed standard surface sterilization and pathogen isolation procedures. Not surprisingly, initial culture plates contained a heavy mixture of fungal species and needed further single spore isolation. Ultimately we isolated a total of 137 fungal strains that showed a wide range of morphological phenotypes on CMI agar plates (Supplementary Fig. 1, 2, Supplementary Table 1, 2). To facilitate our screening effort, we sorted fungal strains into groups by visually inspecting on-plate phenotypes, e.g., growth rate, colony morphology, pigmentation, and aerial mycelia abundance. Ultimately, we arrived at a total of 87 isolates as the representative collection of strains for further analysis.
We first tested 87 fungal isolates for the ability to produce CWDEs, based on the premise that CWDE is an important characteristic of Pokkah Boeng pathogen. We cultured fungal isolates in synthetic media with wheat bran as the sole carbon source. Wheat bran has been used to promote cellulase, xylanase, and amylase production in a variety of fungal species (Lee et al., 2015; Pandey et al., 1999). Fungal culture extracts were harvested and used to test for the hydrolysis of Avicel (cellulosic fiber) and starch in vitro using a standard DNS method as previously described (Kim et al., 2014; Percival Zhang et al., 2006). From our preliminary screening, we identified ten fungal isolates with high enzymatic activity for further testing. We repeated our experiments in biological triplicates, focusing on their ability to hydrolyze Avicel into glucose (Fig. 2). To create a standard curve, serial dilutions of Avicel and glucose were prepared and then analyzed using a spectrophotometer. The negative control containing 100% Avicel was used as a blank before analyzing the samples. In this assay, fungal strains 22, 37, and 47 showed significantly higher levels of glucose after the treatment with fungal culture extracts. All other strains showed cellulase production that resulted in detectable glucose conversion. Notably, strains 20 and 27, which were visually diagnosed and predicted as Fusarium and Alternaria species, respectively, were not one of the highest cellulase producers.
Once we selected five sugarcane-associated fungi for further study, we performed DNA sequencing and microscopy for species identification. We isolated genomic DNA samples from five fungal strains and sequenced the amplified ITS regions (Fig. 3). We determined that 475-bp ITS from strain 20 (GenBank KU508286) shared 100% match with F. verticillioides 7600 (teleomorph Gibberella moniliformis) (GenBank NW 017387867.1), F. begoniae CBS 452.97 (NR 111864.1), F. bactridioides CBS 100057 (NR 120262.1). However, it is important to note that we found more than 20 sequences showing 100% alignments, and all were Fusarium species (data not shown). The 545-bp ITS from strain 22 (GenBank KU508289) best matched those of Metarhizium brunneum ARSEF 3297 (NW 014574660.1), Alternaria alternata CBS 916.96 (GenBank NR 131316.1), and A. destruens ATCC 204363 (GenBank NR 137143.1). We also determined strain 27 (GenBank KU508290) as Diaporthe endophytica CBS 133811 (GenBank NR 111847.1) or D. novem CBS 127270 (GeneBank NR 111855.1). Strain 37 (GenBank KU508291) was highly related to Phoma prosopidis (GenBank NR 137386.1). And lastly, strain 47 (GenBank KU508292) was determined as Ascochyta phacae (GenBank NR_135942.1) or Epicoccum huancayense (GenBank NR_135977.1).
The fact that several Fusarium species have been suspected as the causal agent of Pokkah Boeng prompted us to perform additional DNA analyses of strain 20 with calmodulin (GenBank KU508287), ELF-1 (GenBank KU508288), and MAT1-2-1 genes (Lin et al., 2014; McFarlane and Rutherford, 2005; Sidique and Nordahliawate, 2007; Singh et al., 2006; Vishwakarma et al., 2013). Sequence analysis showed that ELF-1, calmodulin, and MAT1-2-1 genes matched type species, F. verticillioides 7600 (GenBank NC_031675.1).
Subsequently, we gathered morphological images that can help our classification effort. We concluded that the five isolates are members of the Phylum Ascomycota or Deuteromycota. Hyphae possessed simple septa and each of the fungi produced asexual conidia. In particular, strain 20, the Fusarium species, produced microconidia that lacked septa and had monophialic conidiophores (Fig. 4). This species description is consistent with that of F. verticillioides (teleomorph G. moniliformis) (Leslie et al., 2008).
Our species identification was followed by vegetative growth assays, particularly focusing on fungal tolerance to stress agents. Fungal plant pathogens are exposed to a wide range of stress agents both from the ambient environment and in planta which can affect virulence and fitness. Congo Red, SDS and H2O2 have been used in previous studies to imitate oxidative stress on fungal cells (Gerik et al., 2008; Roncero and Durán, 1985). Here we focused on Fusarium, Phomopsis, and Phoma isolates since these fungal species are recognized as aggressive phytopathogens in crops (Fig. 2). When we measured the colony diameter on agar plates, the Fusarium isolate grew at approximately equal rates in comparison to the Phomopsis strain but grew significantly faster than Phoma on the CMI and CMI + H2O2 media. The addition of Congo Red inhibited the growth of Fusarium but did not significantly affect the growth of Phoma or Phomopsis strains. Interestingly, the addition of SDS drastically inhibited the growth of Phomopsis and Phoma. While the growth of Fusarium strain was negatively affected by SDS, the fungus exhibited a drastically vigorous growth on CMI + SDS in comparison to Phoma and Phomopsis strains. Other than the growth rate, the addition of the H2O2, SDS or Congo Red did not have any detrimental effect on the overall phenotype of fungal strains when visually inspected.
In CMI liquid medium, Phomopsis produced the highest mycelial mass followed by Phoma and Fusarium. The only significant difference in the production of mycelia was between the Phomopsis and Fusarium isolate. After collecting conidia, it was found that Phoma produced the highest number of conidia, followed by Phomopsis and Fusarium strains. The production of conidia was significant in comparison to Fusarium but not in comparison to Phomopsis. Fusarium strain produced both macroconidia and microconidia in CMI culture medium.
The three pathogenic fungi, Phoma, Phomopsis and Fusarium, were independently inoculated into mature sugarcane stalks (Fig. 5A). Phoma and Phomopsis strains produced little to no lesions and were comparable to the negative water control. However, inoculation of the Fusarium led to extensive tissue maceration and rot. The experiment was conducted in three biological replicates, and showed consistent outcomes. This outcome, along with our morphological and phylogenetic studies, strongly suggests that F. verticillioides is a key pathogen of sugarcane fields in Fujian, China.
The next question we asked was whether four other fungal isolates could influence the severity of lesions caused by F. verticillioides when coinoculated into sugarcane stalks. In literature, Phomopsis, Phoma, Alternaria, and Epicoccum are recognized as pathogens, saprophytes, or endophytes. More importantly, we have determined that these isolates can produce a high level of CWDE under laboratory conditions (Fig. 2). Therefore, F. verticillioides isolate was co-inoculated into sugarcane stalks with either Phoma, Phomopsis, Epicoccum, or Alternaria (Fig. 5B). Results showed that the individual F. verticillioides inoculation led to a significantly larger lesion than the co-inoculation of F. verticillioides and Phomopsis based on a 95% confidence interval. We did not find significant difference between the individual inoculation of F. verticillioides and other co-inoculated samples. Also, ANOVA revealed no significant difference between the co-inoculations and the individual inoculation of F. verticillioides (Supplementary Fig. 1, 2, Supplementary Table 1, 2).
Sugarcane is produced throughout tropical and subtropical regions of the world. Pokkah Boeng is a disease of sugarcane that can lead to 38% yield losses and 90% infection rate in susceptible varieties (Lin et al., 2014; Ricaud et al., 2012; Vishwakarma et al., 2013). Sugarcane producing regions are experiencing an increase in severity and incidence of Pokkah Boeng throughout the growing season (Lin et al., 2014). The aim of this study was to identify the causal agent of Pokkah Boeng by characterizing samples collected in Fujian, China. Proper identification of the causal agent for Pokkah Beong is important to gain clearer understanding of the disease and the subsequent development of breeding and management practices to minimize future losses.
F. moniliforme species complex under section Liseola has been reclassified since the 1990s, and there is still on-going discussion amongst researchers to clarify the nomenclature. Recently, several species of Fusarium have been reported as the causal agents of Pokkah Boeng, including F. sacchari, F. fujikuroi, F. verticillioides, F. andiyazi, and F. proliferatum (Lin et al., 2014; McFarlane and Rutherford, 2005; Sidique and Nordahliawate, 2007; Singh et al., 2006; Vishwakarma et al., 2013). Classification of Fusarium species relies on morphological characteristics along with phylogenetic and molecular techniques (Moretti, 2009; O’Donnell et al., 1998). In this study, we used a combination of morphological, molecular and phylogenetic techniques to identify the isolated causal agent of Pokkah Boeng disease. The isolate was found to possess morphological characteristics consistent with F. verticillioides, and phylogenetic analysis of four different genes confirms these findings. Here, we are concluding that the causal agent of Pokkah Boeng that we isolated from Fujian, China is F. verticillioides.
CWDE are known as key virulence factors associated with a number of pathogenic microbes (Kubicek et al., 2014). Fusarium graminearum, a species closely related to F. verticillioides, encodes at least 30 diverse xylanase genes that are known to be involved in head scab disease of wheat (Hatsch et al., 2006; Mary Wanjiru et al., 2002). When initiating our screening for the putative pathogen of Pokkah Boeng our premise was that high CWDE production would be associated with pathogenesis. Our study showed that the isolated F. verticillioides was determined as one of the highest producers of cellulolytic enzymes from the pool of 87 fungal isolates. Genomic analysis of carbohydrate-active enzymes (CAZymes) across a wide range of fungi revealed that F. verticillioides has the highest number of CAZymes (Zhao et al., 2013). F. verticillioides genome encodes for over 800 CAZymes, and approximately 28 different types of cellulases (Kubicek et al., 2014; Zhao et al., 2013). However, the fact that the isolated F. verticillioides was not the highest CWDE producer suggests that there are likely other virulence factors contributing to the infection of sugarcane. Another explanation would be a difference in the production of cellulolytic enzymes in vitro versus in vivo. For example, CWDE production in Botrytis cinerea is variable dependent on the degree of infection and pathogen localization in the host (Verhoeff and Warren, 1972). Furthermore, Zhao et al. (2013) found that Rhizoctonia solani produces polygalacturonase and polymethylgalacturonase at higher concentrations in vitro, while the activity of β-glucosidase and carboxymethyl cellulase is highest in vivo. Further analysis of cellulase production by the isolated Fusarium isolate in vivo will need to be carried out in order to make additional conclusions.
During our screening, we discovered that four fungal isolates other than the pathogenic F. verticillioides, i.e., Alternaria, Phoma, Phomopsis, and Epicoccum, exhibited high levels of cellulase production, albeit the fact that none were able to independently cause disease in sugarcane. This outcome was unexpected since Alternaria, Phoma, and Phomopsis species are well recognized as aggressive plant pathogens, causing lesions and rots in a variety of crops (Agrios, 2005). Subsequently, we asked whether these fungal isolates play supplementary role in Pokkah Boeng pathogenesis, perhaps enhancing the aggressiveness of F. verticillioides during colonization and rot. However, our co-inoculation experiments showed that these four species have no impact on the severity of the disease and are likely endophytic co-inhabitants in sugarcane.
Recently, Shrestha et al. (2015) isolated over one hundred fungi from sugarcane and silvergrass (Miscanthus) in the United States to study fungal species for enzymatic activity, which included Alternaria, Phoma, Epicoccum, and Fusarium species. Amongst the tested, E. nigrum was one of the top producers of endocellulase, glucosidase, and xylanase, whereas F. proliferatum strain isolated from silvergrass was found to be only a moderate producer of CWDEs. However, it is unclear whether Shrestha et al. (2015) tested these fungal isolates for pathogenicity. de Lima Fávaro et al. (2011) isolated 112 Epicoccum strains from sugarcane in Brazil, separated them into two genotypic groups (Group I, E. nigrum; Group II, novel Epicoccum species) and subsequently analyzed these strains for CWDE production. Over 90% of isolates in Group I were capable of producing polygalacturonase, pectinlyase and lipases, while the isolates classified in Group II were less capable of producing the CWDEs. One symbiotic isolate, E. nigrum isolate P16, was determined to induce root growth in sugarcane and inhibit the growth of the pathogens in vitro, in particular F. verticillioides (de Lima Fávaro et al., 2011, 2012). We questioned whether our E. nigrum strain possessed antifungal properties, but the E. nigrum strain did not exhibit any inhibitory effect on the pathogenic F. verticillioides strain isolated in this study (data not shown).
With previous studies supporting the role of E. nigrum as a beneficial co-inhabiting fungus, it raises an interesting possibility that E. nigrum uses its arsenal of CWDE during a saprophytic shift the in the fungal lifestyle (de Lima Fávaro et al., 2012). This outcome coupled with the CWDE producing capabilities of the fungus further provides an insight into the diversity of E. nigrum throughout sugarcane growing regions of the world, as well as symbiotic interactions of this particular species with other pathogenic fungi. There are several commercial products containing fungal biocontrol agents, which can reduce disease severity directly through the production of antifungal compounds or by inducing the host plant defense responses (Butts and Krysan, 2012; Schulz et al., 2002). For example, Acremonium isolated from date palm reduced wilt symptoms by up to 87% when antagonized with Fusarium albedinis in vivo (El-Deeb and Arab, 2013). In contrast, there is also evidence that the interaction between some fungi and other pathogens may lead to an increase in disease severity. Ridout and Newcombe (2015) co-inoculated blight pathogen Dothistroma with six different endophytic isolates onto pine needle. Four fungi, Sydowia polyspora, Bionectria ochroleuca, Penicillium raistrickii, and a culturable species of Elytroderma, increased the disease severity of Dothistroma by 4.7%, 4.2%, 3.6%, and 2.5%, respectively. In our current study, we co-inoculated F. verticillioides with Phoma, Phomopsis, Alternaria and Epicoccum into the sugarcane hybrid. Given the evidence for an arsenal of CWDEs in the Epicoccum, Phoma, and Alternaria strains, and the literature supporting the role of E. nigrum as a potential biocontrol, we hypothesized that interactions between fungi would lead to a difference in pathogenesis of the Fusarium pathogen. However after analyzing the lesion size amongst each of the replicates, no significant difference was found in the disease severity.
Acknowledgments
Funding for this study was provided in part by the College of Agriculture & Life Sciences (COALS) and the Department of Plant Pathology & Microbiology at Texas A&M University and the College of Plant Protection at Fujian Agricultural & Forestry University. We would also like to thank the Fujian Agricultural and Forestry University faculty and staff for providing excellent technical and logistical support for this study.
Fig. 1
Characteristic Pokkah Boeng disease symptoms observed in diseased sugarcanes. (A) Red streaking and chlorosis. (B) Stalk rot. (C) Knife cut and deformed top.
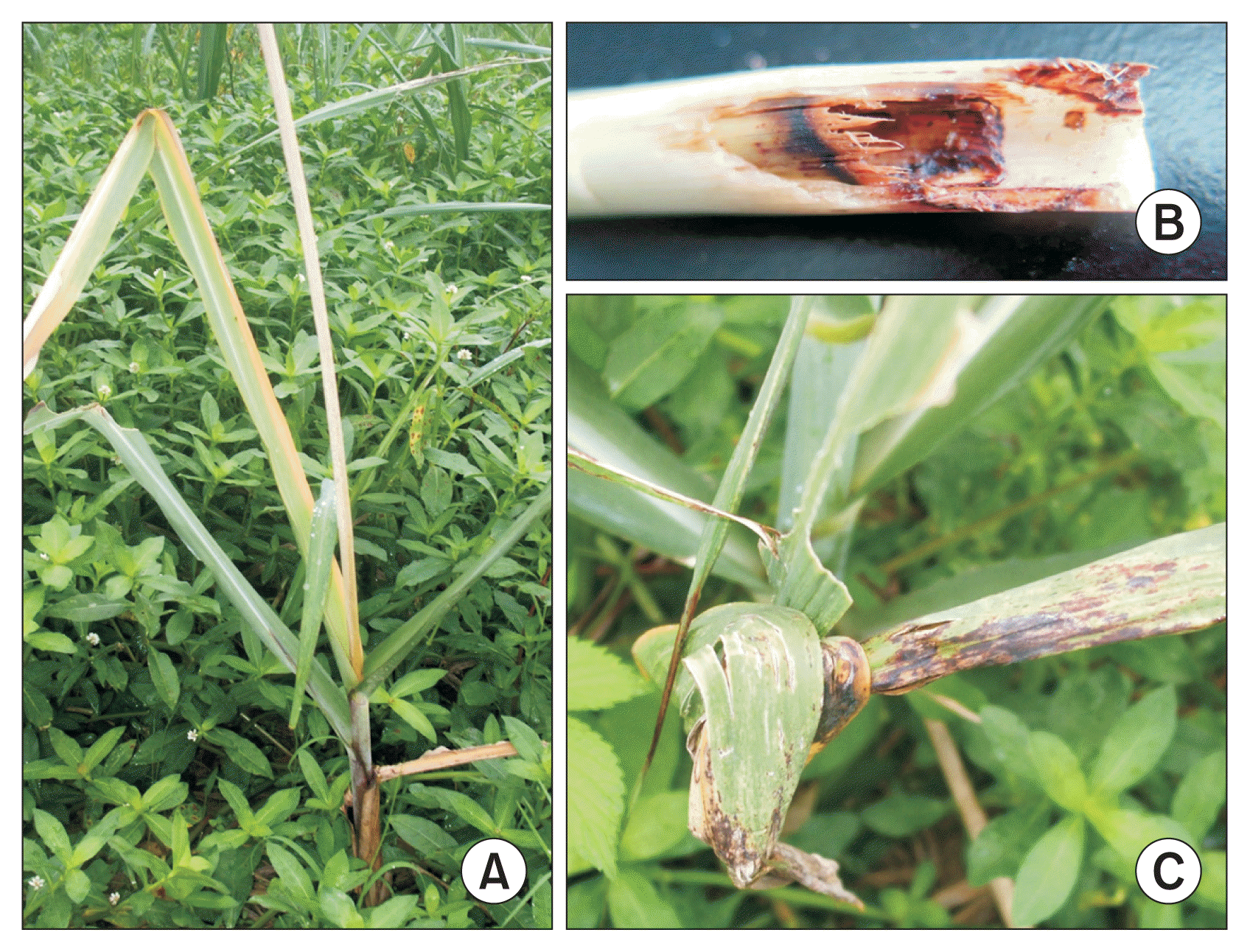
Fig. 2
Phenotypic characteristics of fungal strains isolated from diseased sugarcane. (A) Average glucose conversion from Avicel (Sigma, USA) by the fungal isolates strain 20 (Fusarium), strain 22 (Alternaria), strain 27 (Phomopsis), strain 37 (Phoma), and strain 47 (Epicoccum). (B) Growth rate analysis of strain 20 (Fusarium), strain 27 (Phomopsis), and strain 37 (Phoma) on different synthetic media. SDS, sodium dodecyl sulfate. (C) Average weight of fungal biomass of strain 20 (Fusarium), strain 27 (Phomopsis), and strain 37 (Phoma) in CMI broth. (D) Production of conidia by strain 27 (Phomopsis), strain 20 (Fusarium), and strain 37 (Phoma) in CMII media.
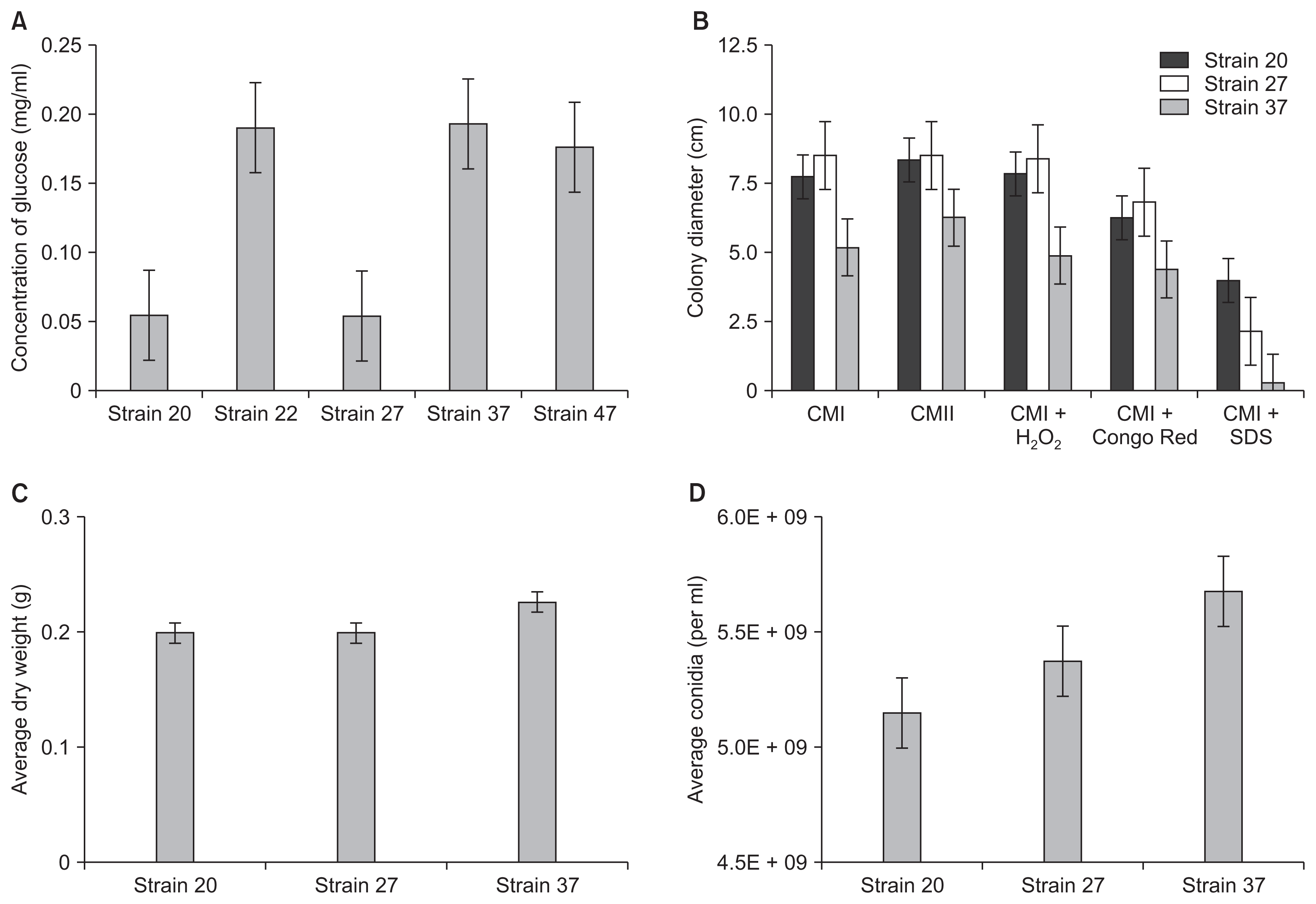
Fig. 3
Phylogenetic analyses of fungal strains isolated from diseased sugarcanes. Phylogenetic analysis was performed by maximum likelihood method with 1,000 bootstrap replications with MEGA 7 software (Kumar et al., 2016). The trees, obtained by neighbor-joining method and BioNJ algorithms, is drawn to scale, with branch lengths measured in the number of substitutions per site. (A) Five fungal strains were analyzed based on internal transcribed spacer DNA sequence. (B) Fusarium strain analysis with translation elongation factor 1-alpha DNA sequence. (C) Fusarium strain analysis with Calmodulin DNA sequence.

Fig. 4
Microscopic observation of strain 20 Fusarium species. (A) Hyphae possess simple septa and produce conidiogenous monophialide cells (scale bar = 10 μm). (B) Microconidia are oval to club shaped and lacks septa (scale bar = 100 μm).
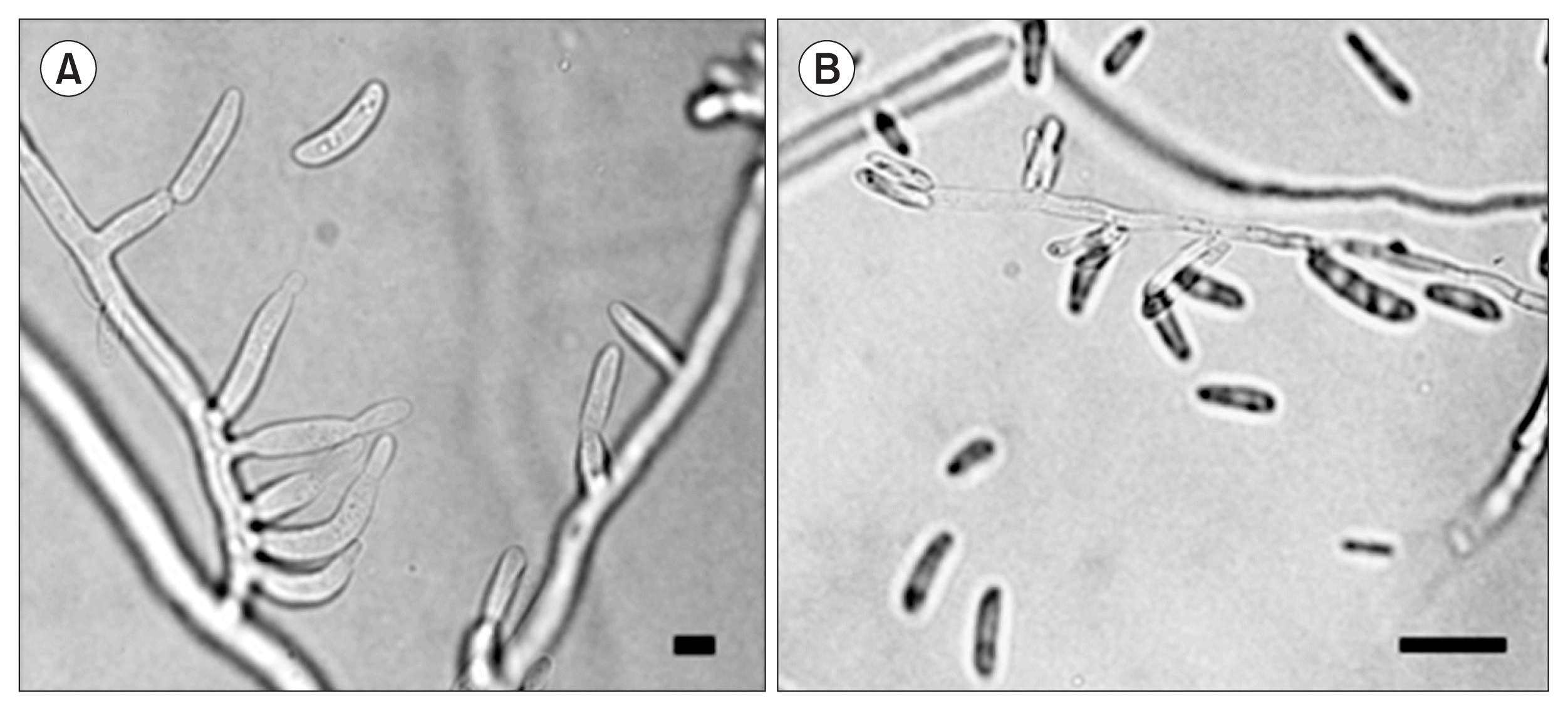
Fig. 5
Pathogenicity assay. Fungal isolates were inoculated into sugarcane stalk and cut longitudinally at the wound site after a 7-day incubation to assess disease severity. (A) a: negative control, b: strain 20 (Fusarium), c: strain 27 (Phomopsis), d: strain 37 (Phoma). (B) a: strain 20 (Fusarium), b: strain 20 (Fusarium) and strain 27 (Phomopsis), c: strain 20 (Fusarium) and strain 37 (Phoma), d: strain 20 (Fusarium) and strain 22 (Alternaria), e: strain 20 (Fusarium) and strain 47 (Epicoccum). (C) Average lesion size produced by fungal strains in sugarcane stock when inoculated independently and in combination.

References
Agrios, GN 2005. Chapter 11: plant diseases caused by fungi. In: Plant pathology, 5th ed. eds. by GN Agrios, 385-614. Academic Press, San Diego, CA, USA.
Anderson-Sprecher, A and Wei, J (2015 onwards). China 2015 sugar annual. USDA Foreign Agricultural Service, Global Agricultural Information Network (GAIN) Report number: CH15015. URL https://gain.fas.usda.gov/Recent%20GAIN%20Publications/Sugar%20Annual_Beijing_China%20-%20Peoples%20Republic%20of_5-14-2015.pdf. 1 October 2015.
Bandyopadhyay, R, Little, CR, Waniska, RD and Butler, DR 2008. Sorghum grain mold: through the 1990s into the new millennium. In: Sorghum and millets diseases, eds. by JF Leslie, 171-183. Iowa State Press, Ames, IA, USA.

Butts, A and Krysan, DJ 2012. Antifungal drug discovery: something old and something new. PLoS Pathog. 8:e1002870



Cho, Y, Jang, M, Srivastava, A, Jang, JH, Soung, NK, Ko, SK, Kang, DO, Ahn, JS and Kim, BY 2015. A pectate lyase-coding gene abundantly expressed during early stages of infection is required for full virulence in Alternaria brassicicola. PLoS One. 10:e0127140



Choi, YW, Hyde, KD and Ho, WH 1999. Single spore isolation of fungi. Fungal Diversity. 3:29-38.
de Lima Fávaro, LC, de Melo, FL, Aguilar-Vildoso, CI and Araújo, WL 2011. Polyphasic analysis of intraspecific diversity in Epicoccum nigrum warrants reclassification into separate species. PLoS One. 6:e14828



de Lima Fávaro, LC, de Souza Sebastianes, FL and Araújo, WL 2012. Epicoccum nigrum p16, a sugarcane endophyte, produces antifungal compounds and induces root growth. PLoS One. 7:e36826



El-Deeb, HM and Arab, YA 2013. Acremonium as an endophytic bioagent against date palm fusarium wilt. Arch Phytopathol Plant Protect. 46:1214-1221.

[FAO] Food and Agriculture Organization of the United Nations. 2015. FAOSTAT database. Sugarcane production. FAO, Rome, Italy.
Feng, J, Zhang, H, Strelkov, SE and Hwang, SF 2014. The LmSNF1 gene is required for pathogenicity in the canola blackleg pathogen Leptosphaeria maculans. PLoS One. 9:e92503



Gerik, KJ, Bhimireddy, SR, Ryerse, JS, Specht, CA and Lodge, JK 2008. PKC1 is essential for protection against both oxidative and nitrosative stresses, cell integrity, and normal manifestation of virulence factors in the pathogenic fungus Cryptococcus neoformans. Eukaryot Cell. 7:1685-1698.




Hall, BG 2013. Building phylogenetic trees from molecular data with MEGA. Mol Biol Evol. 30:1229-1235.


Harborne, JB 1998. Chapter 1: methods of plant analysis. In: Phytochemical methods: a guide to modern techniques of plant analysis, eds. by JB Harborne, 1-39. Chapman and Hall, New York, NY, USA.
Hatsch, D, Phalip, V, Petkovski, E and Jeltsch, JM 2006. Fusarium graminearum on plant cell wall: no fewer than 30 xylanase genes transcribed. Biochem Biophys Res Commun. 345:959-966.


Hicks, JK, Yu, JH, Keller, NP and Adams, TH 1997. Aspergillus sporulation and mycotoxin production both require inactivation of the FadA G alpha protein-dependent signaling pathway. EMBO J. 16:4916-4923.



Jorge, I, Navas-Cortés, JA, Jiménez-Díaz, RM and Tena, M 2006. Cell wall degrading enzymes in fusarium wilt of chickpea: correlation between pectinase and xylanase activities and disease development in plants infected with two pathogenic races of Fusarium oxysporum f. sp. ciceris Can J Bot. 84:1395-1404.
Kerényi, Z, Zeller, K, Hornok, L and Leslie, JF 1999. Molecular standardization of mating type terminology in the Gibberella fujikuroi species complex. Appl Environ Microbiol. 65:4071-4076.




Kim, JJ, Kwon, YK, Kim, JH, Heo, SJ, Lee, Y, Lee, SJ, Shim, WB, Jung, WK, Hyun, JH, Kwon, KK, Kang, DH and Oh, C 2014. Effective microwell plate-based screening method for microbes producing cellulase and xylanase and its application. J Microbiol Biotechnol. 24:1559-1565.


Koo, WW and Taylor, RD 2014. 2014 Outlook of the U.S. and world sugar markets, 2013-2023. Agribusiness & Applied Economics Report No. 725. August;2014. Center for Agricultural Policy and Trade Studies, Department of Agribusiness and Applied Economics, North Dakota State University, Fargo, ND, USA.
Kubicek, CP, Starr, TL and Glass, NL 2014. Plant cell wall-degrading enzymes and their secretion in plant-pathogenic fungi. Annu Rev Phytopathol. 52:427-451.


Kumar, S, Stecher, G and Tamura, K 2016. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol. 33:1870-1874.




Lee, Y, Oh, CH, Heo, SJ, Kang, DH and Shim, WB 2015. Highly potent saccharification of Arthrospira maxima glycogen by fungal amylolytic enzyme from Trichoderma species J113. BioEnerg Res. 8:1868-1876.


Leslie, J and Summerell, B 2011. In search of new Fusarium species. Plant Breed Seed Sci. 63:94-101.

Leslie, JF, Summerell, BA and Bullock, S 2008. The Fusarium laboratory manual. John Wiley & Sons, Hoboken, NJ, USA.
Lin, Z, Xu, S, Que, Y, Wang, J, Comstock, JC, Wei, J, McCord, PH, Chen, B, Chen, R and Zhang, M 2014. Species-specific detection and identification of fusarium species complex, the causal agent of sugarcane pokkah boeng in China. PLoS One. 9:e104195



Martin, J, Handojo, H and Wismer, C 1989. Chapter 11: pokkah boeng. In: Diseases of sugarcane: major diseases, eds. by C Ricaud, 157-168. Elsevier, New York, NY, USA.
Mary Wanjiru, W, Zhensheng, K and Buchenauer, H 2002. Importance of cell wall degrading enzymes produced by Fusarium graminearum during infection of wheat heads. Eur J Plant Pathol. 108:803-810.
Matsuoka, S and Maccheroni, W 2015. Chapter 6: disease management. In: Sugarcane: agricultural production, bioenergy, and ethanol, eds. by CA Santos, C Borém and C Caldas, 115-132. Academic Press, San Diego, CA, USA.
McFarlane, SA and Rutherford, RS 2005. Fusarium species isolated from sugarcane in kwazulu-natal and their effect on Eldana saccharina (Lepidoptera: Pyralidae) development in vitro. Proc S Afr Sugar Technol Assoc. 79:120-123.
Moretti, AN 2009. Taxonomy of Fusarium genus: a continuous fight between lumpers and splitters. Zbornik Matice srpske za prirodne nauke. (117):7-13.

O’Donnell, K, Cigelnik, E and Nirenberg, HI 1998. Molecular systematics and phylogeography of the Gibberella fujikuroi species complex. Mycologia. 90:465-493.

Pandey, A, Selvakumar, P, Soccol, CR and Nigam, P 1999. Solid state fermentation for the production of industrial enzymes. Curr Sci. 77:149-162.
Percival Zhang, YH, Himmel, ME and Mielenz, JR 2006. Outlook for cellulase improvement: screening and selection strategies. Biotechnol Adv. 24:452-481.


Ricaud, C, Egan, B, Gillaspie, A and Hughes, C 2012. Diseases of sugarcane: major diseases. Elsevier, Amsterdam, the Netherlands.
Ridout, M and Newcombe, G 2015. The frequency of modification of Dothistroma pine needle blight severity by fungi within the native range. For Ecol Manag. 337:153-160.

Roncero, C and Durán, A 1985. Effect of Calcofluor white and Congo red on fungal cell wall morphogenesis: in vivo activation of chitin polymerization. J Bacteriol. 163:1180-1185.




Schulz, B, Boyle, C, Draeger, S, Römmert, AK and Krohn, K 2002. Endophytic fungi: a source of novel biologically active secondary metabolites. Mycol Res. 106:996-1004.

Shrestha, P, Ibáñez, AB, Bauer, S, Glassman, SI, Szaro, TM, Bruns, TD and Taylor, JW 2015. Fungi isolated from Miscanthus and sugarcane: biomass conversion, fungal enzymes, and hydrolysis of plant cell wall polymers. Biotechnol Biofuels. 8:38




Sidique, M and Nordahliawate, S 2007. Pathogenicity and aethiology of Fusarium species associated with pokkah boeng disease on sugarcane [sb741. F9 s623 2007 frb]. Master’s thesis. Universiti Sains Malaysia, Pulau Pinang, Malaysia.
Singh, A, Chauhan, SS, Singh, A and Singh, SB 2006. Deterioration in sugarcane due to pokkah boeng disease. Sugar Tech. 8:187-190.


Verhoeff, K and Warren, JM 1972. In vitro and in vivo production of cell wall degrading enzymes by Botrytis cinerea from tomato. Neth J Plant Pathol. 78:179-185.


Vishwakarma, SK, Kumar, P, Nigam, A, Singh, A and Kumar, A 2013. Pokkah boeng: an emerging disease of sugarcane. J Plant Pathol Microbiol. 4:2-7.
Waalwijk, C, Jacq, RAdK, Baayen, RP and Gams, W 1996. Discordant groupings of Fusarium spp. From sections Elegans, Liseola and Dlaminia based on ribosomal ITS1 and ITS2 sequences. Mycologia. 88:361-368.

Wollenweber, HW and Reinking, OA 1935. Die fusarien, ihre beschreibung, schadwirkung und bekämpfung. P. Parey, Berlin, Germany.



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Supplement
Supplement Print
Print




