Diffusible and Volatile Antifungal Compounds Produced by an Antagonistic Bacillus velezensis G341 against Various Phytopathogenic Fungi
Article information
Abstract
The aim of this study was to identify volatile and agar-diffusible antifungal metabolites produced by Bacillus sp. G341 with strong antifungal activity against various phytopathogenic fungi. Strain G341 isolated from four-year-old roots of Korean ginseng with rot symptoms was identified as Bacillus velezensis based on 16S rDNA and gyrA sequences. Strain G341 inhibited mycelial growth of all phytopathogenic fungi tested. In vivo experiment results revealed that n-butanol extract of fermentation broth effectively controlled the development of rice sheath blight, tomato gray mold, tomato late blight, wheat leaf rust, barley powdery mildew, and red pepper anthracnose. Two antifungal compounds were isolated from strain G341 and identified as bacillomycin L and fengycin A by MS/MS analysis. Moreover, volatile compounds emitted from strain G341 were found to be able to inhibit mycelial growth of various phytopathogenic fungi. Based on volatile compound profiles of strain G341 obtained through headspace collection and analysis on GC-MS, dimethylsulfoxide, 1-butanol, and 3-hydroxy-2-butanone (acetoin) were identified. Taken together, these results suggest that B. valezensis G341 can be used as a biocontrol agent for various plant diseases caused by phytopathogenic fungi.
Introduction
Severe crop loss remains inevitable due to plant diseases, particularly those caused by pathogenic fungi. Alternaria, Botrytis, Fusarium, Geotrichum, Phytophthora, and Rhizoctonia are common and damaging plant pathogenic fungi (Liu et al., 2007). Phytopathogenic fungi reduce both yield and quality of crops. They are major restraints in sustainable agriculture production, especially in intensive cropping systems. Synthetic fungicides have been extensively used to control diseases caused by these pathogenic agents. Application of synthetic fungicides is one of the cheapest and the most effective approaches for the control of plant diseases (Fletcher et al., 2006). However, these chemicals may lead to toxic residues in treated products (Barnard et al., 1997; Isman, 2000). Synthetic pesticides can also cause environmental pollution due to their slow biodegradation (Kordali et al., 2008; Misra and Pavlostathis, 1997). Thus, many researchers have focused on the use of biological methods to protect crops from invasion and spread of pathogens. Indeed, management of pathogens by using antagonistic microorganisms or their secondary metabolites is now considered as a viable method for disease control (Han et al., 2005; Liu et al., 2007).
As biocontrol agents, many antagonistic microorganisms have been shown to be effective against various pathogens (Cazorla et al., 2007; Stein, 2005). One representative candidate is Bacillus species belonging to Gram-positive bacteria. Bacillus species can produce various kinds of diffusible and volatile compounds with strong inhibitory activity against plant pathogens (Arrebola et al., 2010; Almenar et al., 2007; Hossain et al., 2016; Kim et al., 2015; Nam et al., 2016; Ongena and Jacques, 2008). Among various diffusible compounds, cyclolipopeptides have many advantages compared to chemical surfactants, including low toxicity, high biodegradability, environmentally friendly characteristics (Ongena and Jacques, 2008; Peypoux et al., 1999; Yu et al., 2002), and low foaming (Compant et al., 2005). Furthermore, cyclolipopeptides are produced by biocontrol agents to protect cells from attacks by microorganisms (Baehler et al., 2006; Cazorla et al., 2006; Fogliano et al., 2002; Shoda, 2000).
Moreover, volatile compounds can promote plant growth (Ryu et al., 2003) with antifungal activity (Arrebola et al., 2010). They can induce systemic resistance in crops (Farag et al., 2006). As for antifungal activity of volatile compounds produced by Bacillus species, they can inhibit mycelial growth of Fusarium oxysporum that causes Fusarium wilt of onion (Sharifi and Ramezani, 2003). They can also reduce postharvest decay in citrus (Arrebola et al., 2010). In order to facilitate multi-applications of diffusible and volatile compounds, a rapid and efficient approach to isolate and identify these useful compounds from Bacillus species needs to be established.
In view of this, the objectives of this study were: 1) to characterize bacterial antagonist with strong antifungal activity, 2) to identify volatile and agar-diffusible antifungal metabolites, and 3) to investigate in vivo antifungal activity of agar-diffusible metabolites against various plant diseases caused by fungal pathogens.
Materials and Methods
Identification of G341
Bacterial strain G341 was isolated from four-year-old roots of Korean ginseng with rot symptoms as described previously (Son et al., 2009). This strain was identified by sequence analyses of 16S rRNA and gyrA genes. Isolation of genomic DNA and PCR amplification of 16S rRNA gene sequence were performed using previously described methods (Park et al., 2005). PCR amplification for gyrA gene was performed using primer pairs p-gyrA-f/p-gyrA-r as described previously (Chun and Bae, 2000).
PCR fragments were purified using Wizard PCR prep Kit (Promega, Madison, WI, USA) and directly sequenced using BigDye terminator cycle sequencing kit (Applied Biosystems, USA) according to the manufacturer’s instruction. The same primers used for PCR amplification were used for sequencing. These sequences were compared to 16S rRNA and gyrA gene sequences available in public databases of GenBank. A neighbor-joining tree was inferred with Kimura’s 2-parameter distance model (Kimura, 1980). The resultant neighbor-joining tree topology was evaluated by bootstrap analysis (Felsenstein, 1985) based on 1000 resampled datasets. Alignment with representative sequence of B. subtilis complex and subsequent phylogenetic analyses were carried out using PHYDIT program available at http://plaza.snu.ac.kr/~jchun/phydit/ (Chun, 2001).
In vitro antifungal activity
Using in vitro dual-culture analysis, G341 was subjected to in vitro antifungal activity assay against the following nine phytopathogenic fungi: A. panax causing ginseng Alternaria blight, B. cinerea causing tomato gray mold, Colletotrichum coccodes causing red pepper anthracnose, F. oxysporum f. sp. lycopersici causing tomato Fusarium wilt, Magnaporthe oryzae causing rice blast, P. infestans causing tomato late blight, Pythium ultimum causing cucumber damping-off, R. solani causing rice sheath blight, and Sclerotinia sclerotiorum causing cucumber sclerotinia rot. Potato dextrose agar medium was used as basal medium. A plug (0.6-cm in diameter) containing mycelium was taken from 6-day-old target fungi and placed at the centre of PDA dual plates. Single G341 colonies were patched at a distance of about 3 cm from the fungus. After 3–7 days, the width of inhibition zone between bacterial colony and fungal pathogen was measured. Each treatment was repeated with five replicates. The experiment was also repeated twice.
Evaluation of in vivo control efficacy
One-day protective activities of the fermentation broth of G341 strain and purified substances were evaluated against seven plant pathogenic fungi on plants: M. oryzae on rice plants, R. solani on rice plants, B. cinerea on tomato plants, P. infestans on tomato plants, Puccinia recondita on wheat plants, Blumeria graminis f. sp. hordei on barley plants, and C. coccodes on red pepper plants. These in vivo antifungal bioassays were performed as described previously (Kim et al., 2001, 2004). The strain G341 was cultured in tryptic soy broth (TSB; BD, Sparks, MD, USA) medium at 37°C and 150 rpm for 3 days and then the fermentation broth was used for in vivo assay. Pots were arranged in a randomized complete block design (three replicates per treatment). All experiments for in vivo antifungal activities of the purified substances were conducted twice. Six estimates for each treatment were converted into percentage (± standard deviation) compared to control treatments.
Blasticidin-S (50 μg mL−1) for rice blast, validamycin (50 μg mL−1) for rice sheath blight, fludioxonil (50 μg mL−1) for tomato gray mold, dimethomorph (10 μg mL−1) for wheat leaf rust, benomyl (100 μg mL−1) for barley powdery mildew and dithianon (50 μg mL−1) for red pepper anthracnose were applied as positive controls. Pots were arranged in a randomized complete block design (three replicates per treatment). All experiments were conducted twice. Six estimates for each treatment were converted into a percentage (± standard deviation) compared to control treatments using the following equation:
Where A was area of infection (%) on leaves or sheaths sprayed with Tween-20 solution alone and B was area of infection (%) on treated leaves or sheaths.
Purification of antifungal antibiotics
In preliminary experiment, TSB medium was used as the optimal medium for the production of antifungal substances. Strain G341 was cultured in TSB medium (4 l) at 37°C and 150 rpm for 3 days. Bacterial cells were removed after centrifugation at 8,000 rpm for 6 min. The culture supernatant was successively partitioned twice with equal volumes of ethyl acetate (EtOAc) and n-butanol (BuOH). Each layer was concentrated by drying and then subjected to in vitro antifungal activity against M. oryzae. Among four organic fractions obtained from culture broth of strain G341, BuOH layer (17.2 g) was found to be the most active layer, followed by the EtOAc layer (10.2 g). The BuOH layer was applied onto a silica-gel column (3.6 (i.d.) × 60 cm, Kiesel gel 60, 400 g, 70–230 mesh; E. Merck, Darmstadt, Germany). It was then eluted with a mixture of chloroform-methanol-water (30:9:1 and 65:25:4, v/v/v). Active fractions showing inhibitory activity against M. oryzae were collected and applied onto a Sephadex LH-20 column (2.8 (i.d.) × 45 cm, 200 g; Sigma, MO, USA) after concentration. Active fractions eluted from the Sephadex LH-20 column with methanol were evaporated to dryness and loaded onto a Sep-Pak C18 cartridge (10 g) (Waters, Milford, MA, USA) with methanol-water. Using this protocol, 220 mg of compound 1 and 24 mg of compound 2 were obtained from 4 l of culture supernatant.
Electrospray ionization mass and tandem mass spectrometry
Purified antifungal substances were analyzed on a Hybrid Quadrupole-Time-Of-Flight (Q-TOF) mass spectrometer (QSTAR XL, AB Sciex Insturments, CA, USA) operated in electrospray ionization (ESI) positive ion mode. These samples were dissolved in 50% MeOH/49% H2O/1% AcOH (v/v/v) and applied to nano-spray tip of Q-TOF MS. Compound 1 was analyzed by ESI-MS/MS without ring-opening. In contrast, compound 2 was ring-opened by cleavage of the lactone bond (Williams et al., 2002) and analyzed. Amino acid sequences were determined from series of bn obtained from de-novo sequencing of lipopeptide. Amino acid compositions were obtained as described previously (Razafindralambo et al., 1998).
Analysis of antifungal activities of volatile compounds
Strain G341 culture broth (50 μl) was spread onto half of a divided plate containing TSA. Following 24 h of incubation at 25°C, 5 mm mycelia plugs of M. oryzae, R. solani, B. cinerea, P. infestans, F. oxysporum, S. sclerotiorum, P. capsici, and C. coccodes were placed on the other half of the divided plate containing PDA. Plates were wrapped immediately in Parafilm to seal in volatiles. Measurements of radial mycelia growth were taken at 48 h to 72 h post incubation of the pathogen on both bacterial and control plates. The experiment was repeated twice.
Collection of volatile compounds
Headspace volatile compounds produced by strain G341 were collected using a setup recommended by DeMilo et al. (1996) with slight modifications. Briefly, strain G341 was cultured at 37°C with shaking (150 rpm) in 200 ml of TSB in 500 ml of Erlenmeyer flasks fitted by a two-way rubber cork with insertion of glass tubes. One of the inserted glass tubes was placed just 1 cm above the culture of strain G341. The other end of the tube was connected to a nitrogen supply system to remove headspace volatiles. One end of the second tube was placed near the neck of the flask while the other end was connected to a volatile trap made of glass tube (7 cm in length and 0.4 cm in diameter) containing 150 mg of activated charcoal (Darco, 20–40 mesh, Aldrich, Milwaukee, WI, USA). Before use, these traps were placed in 150 mm Petri plate wrapped in aluminum foil and sterilized in 350°C oven for 24–36 h. The neck of the conical flask was tightly sealed with Parafilm to prevent the escape of volatile compounds. TSB medium without bacteria was used as control. The stream of dry nitrogen flow was started at 12 h post inoculation of the bacterial strain and maintained at 300 ml/min for up to 48 h. Flasks were shaken throughout the collection process at 150 rpm. Volatile compounds in the activated charcoal trap were eluted into glass vials with 0.5 ml of methylene chloride.
GC-MS analysis of volatile compounds produced by G341
Volatile organic compounds produced by G341 strain were analyzed by GC-MS (Shimadzu GC-MS QP5050, Shimadzu co., Kyoto, Japan). A 1-μl aliquot of methylene chloride solution containing volatile compounds was injected into the injection port of the GC-MS. A capillary column SPB-5 (30 m × 0.25 mm in i.d., 0.25 μm in film thickness; PA, USA) was used. The initial temperature of the column was held at 30°C for 2 min and increased to 220°C at 5°C min−1. The injection port and interface were set at 240°C and 200°C, respectively. Helium carrier gas was used at a flow rate of 2.2 ml min−1. Mass spectra of unknown compounds were compared to those deposited in the NIST/EPA/NIH Mass Spec. Library (Version 2.0).
Statistical analysis
Analysis of variance was performed using PROC GLM procedure (SAS institute, Cary, NC, USA). If P > F was less than 0.01, means were separated with Duncan’s multiple range test at P = 0.05 level.
Results
Antifunagl activity
Strain G341 inhibited mycelial growth of all fungal pathogens tested. Mycelial growth of A. panax, B. cinerea, C. coccodes, F. oxysporum, M. oryzae, and P. capsici was significantly inhibited by G341. However, the antagonistic bacterium was weakly active to mycelial growth of P. ultimum, R. solani and S. aclerotiorum (Fig. 1).
Identification of G341 strain
Strain G341 exhibited phenotypic similarity with Bacillus spp. based on biochemical, morphological, and cultural characteristics (data not shown). Analysis of 16S rRNA gene sequence (MF167634) showed that strain G341 formed a monophyletic group with species of B. subtilis complex, sharing 99% sequence similarity (Fig. 2A) and the sequence analysis of gyrA genes revealed that strain G341 shared 96.6% similarity with type strain of B. velezensis LMG 22478T. Based on the phylogenetic analysis of gyrA gene of G341 (MF167633) with Bacillus spp. belonging to the monophyletic group, strain G341 was identified as B. velezensis (Fig. 2B).
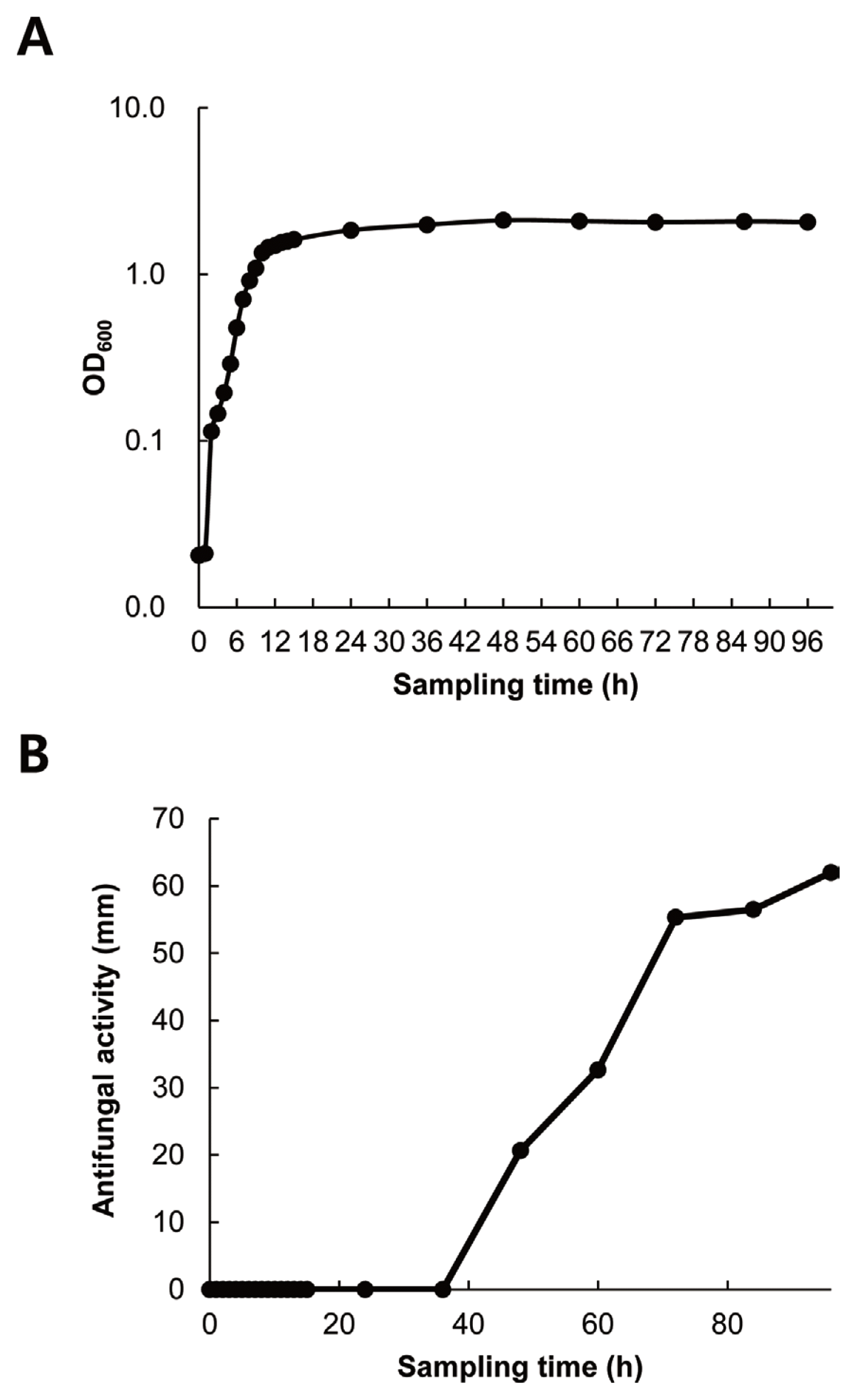
Growth curve of strain G341 and its production of antifungal metabolites
As shown in Fig. 3, strain G341 could grow relatively quickly. It reached stationary phase at 12 h after inoculation. There was almost no lag phase. The antifungal activity of its culture broth sampled at different time intervals was significantly correlated with cell growth over the four-day growth period. The strongest antifungal activity against M. oryzae was obtained at 96 h after incubation. Therefore, the optimal harvest time for antifungal metabolites of strain G341 might be at 96 h after inoculation under the culture conditions used in this study.
In vivo antifungal activity and structure determination
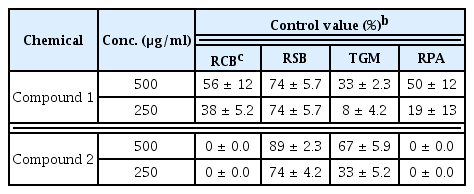
The fermentation broth of G341 showed potent antifungal activity against M. oryzae and R. solani on rice plants, B. cinerea on tomato plants, and C. coccodes on red pepper plants. It reduced the development of the four diseases by more than 50% even at a 9-fold dilution (Table 1).
Among four organic fractions obtained from culture broth of strain G341, BuOH fraction showed the strongest in vivo antifungal activity. The BuOH fraction also exhibited strong in vivo antifungal activity against rice blast, rice sheath blight, tomato gray mold, and red pepper anthracnose (data not shown). Because the BuOH fraction was the most active one, its constituents were separated by bioassay-lead fractionation. Two antifungal substances were isolated from the BuOH fraction by repeated column chromatography such as silica-gel, Sephadex LH-20, and Sep-Pack C18. Finally, two active compounds (1 and 2) were isolated.
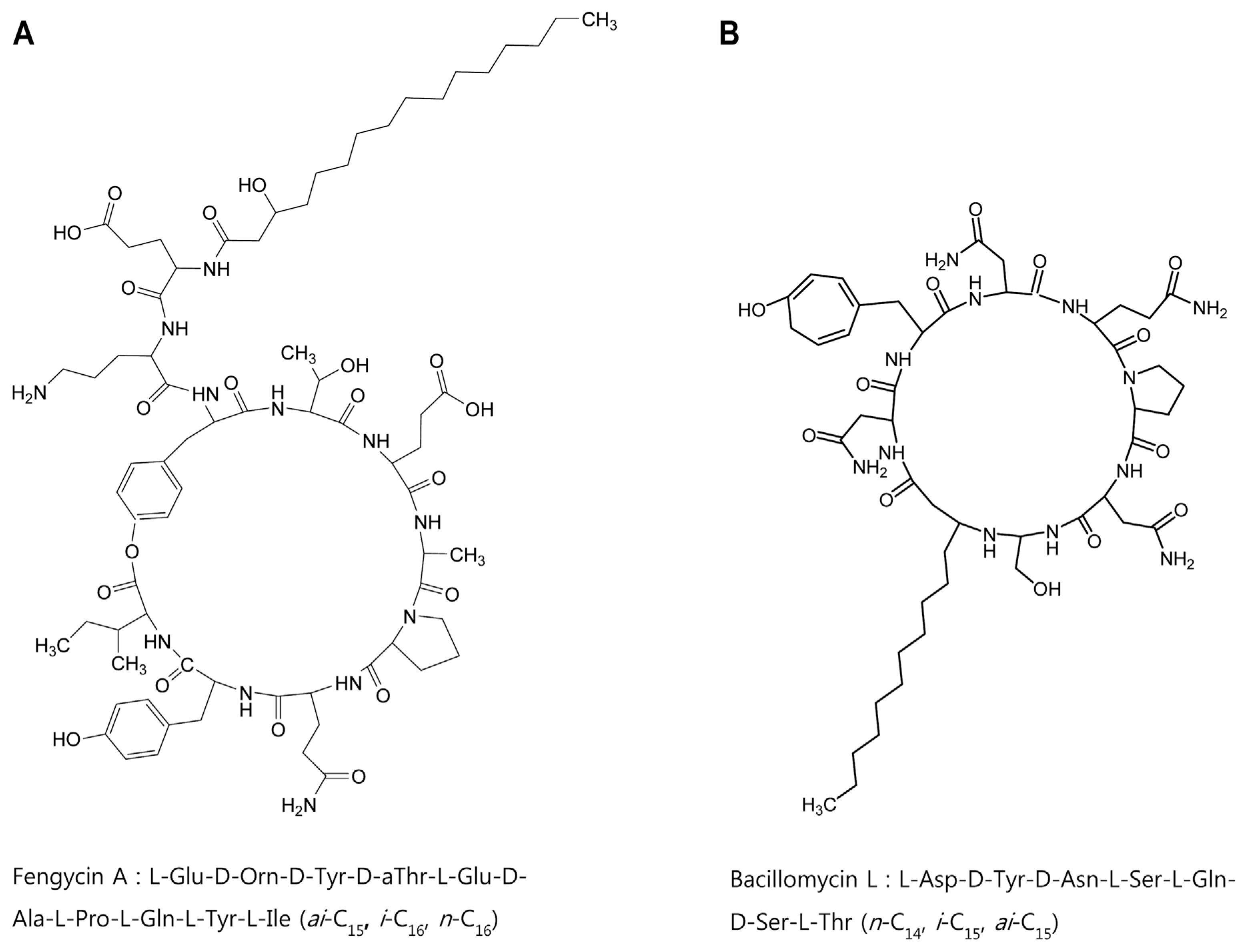
In order to identify chemical structures of the two isolated metabolites, their mass spectra were recorded by ESI-TOF mass spectrometry. The mass spectra of compound 1 showed a series of mass number (m/z 1021.5, 1035.5, and 1049.5). It revealed differences of 14 Da, suggesting that the purified compound had a different carbon chain length (-CH2-). Based on tandem mass spectrometry, the amino acid composition of compound 1 was determined to be Asp, Tyr, Ser, Glu, and Thr in a ratio of 2:1:2:1:1. The sequence deduced from MS/MS spectrum obtained from m/z 1035.5 ion peak of compound 1 was identical to that of bacillomycin L (Fig. 4A). Thus, compound 1 was identified as bacillomycin L with fatty acid moieties of C14–C16.
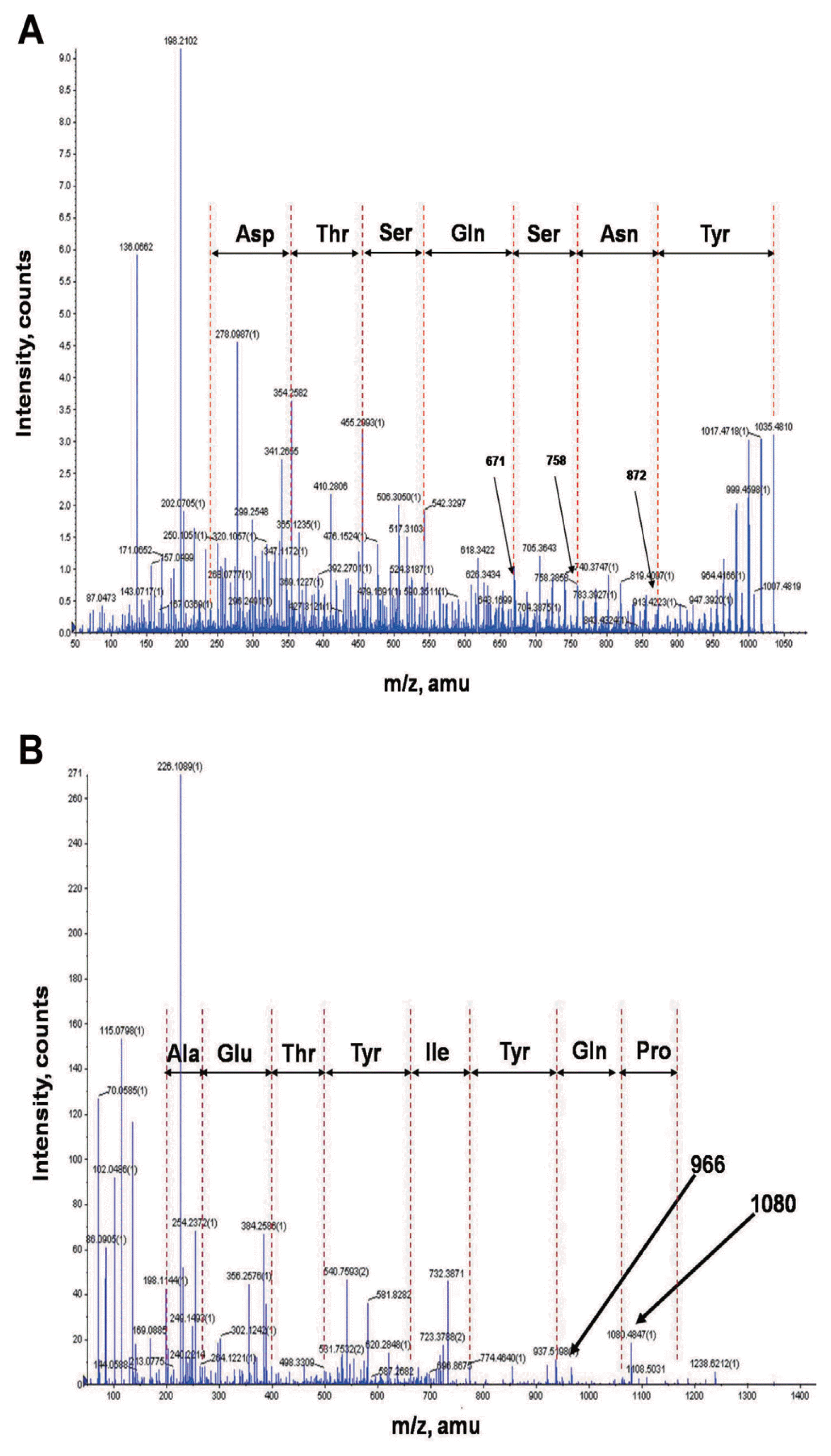
Electrospray ionization (ESI)-tandem mass spectrometry spectra of bacillomycin L (A) and fengycin A (B) isolated from liquid culture of Bacillus velezensis G341.
ESI-TOF mass spectrum of compound 2 showed [M+H]+ ion peaks at m/z 1449.7, 1463.7, 1477.7, 1491.7, and 1505.7. Amino acid analysis revealed that compound 2 comprised Glu, Orn, Tyr, Thr, Ala, Pro, and Ile in a ratio of 3:1:2:1:1:1:1. ESI-MS of hydrolysate of compound 2 showed a protonated peak at m/z 1481.7. The mass gain of 18 Da from m/z 1463.7 of compound 2 could be due to hydrolysis of a lactone bond. MS/MS spectrum of m/z 1481.7 ion obtained from hydrolysate of compound 2 revealed that the amino acid sequence of compound 2 was identical to that of fengycin A (Fig. 4B). Thus, compound 2 was identified as fengycin A with fatty acid moieties of C15–C19. Based on the above results, chemicals of lipopeptides produced by G341 were found to be bacillomycin L and fengycin A (Fig. 5).
In vivo antifungal activity
Results of in vivo antifungal activities of the two lipopeptides isolated from strain G341 are summarized in Table 2. Out of four plant diseases tested, the two lipopeptides effectively suppressed the development of rice sheath blight. The in vivo antifungal spectra of the two lipopeptides were found to be different from each other. Bacillomycin L was active against rice blast, rice sheath blight, and red pepper anthracnose. However, it was virtually inactive against tomato gray mold. In comparison, fengycin A showed in vivo antifungal activity against rice sheath blight and tomato gray mold, but not against rice blast or red pepper anthracnose.
In vitro antifungal activity and analysis of volatile compounds of G341
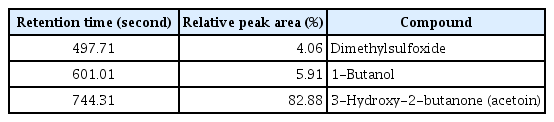
Strain G341 produced antifungal volatiles and inhibited mycelial growth of various fungal pathogens. It significantly inhibited mycelial growth of S. sclerotiorum, R. solani, and B. cinerea (Table 3). P. infestans, P. capsici, C. coccodes, and M. oryzae were relatively resistant to these volatile compounds produced by G341. Volatile profiles indicated that strain G341 produced 3 volatile compound: dimethylsulfoxide, 1-butanol, and 3-hydroxy-2-butanone (acetoin). Among them, acetoin was found to be the major compound (Table 4, Supplementary Fig. 1).
Mycelia growth of phytopathogenic fungi inhibited by volatile compounds produced by Bacillus velezensis G341
Discussion
One of the biggest ecological challenges facing microbiologists and plant pathologists in the near future is the development of environmentally friendly alternatives to chemical pesticides for combating crop diseases. The use of beneficial microorganisms is considered as one of the most promising methods for more rational and safe crop-management practices (Ongena and Jacques, 2008). In this respect, we attempted to isolate antagonistic bacteria with strong antifungal activity against various phytopathogenic fungi. As a result, we found that G341 strain inhibited mycelial growth of various plant pathogenic fungi tested. These preliminary results suggested that B. velezensis G341 strain could produce antifungals. It is well-known that bacteria can commonly produce cell wall-degrading enzymes and secondary metabolites to hinder the growth of other microorganisms (Shoda, 2000).
Identification of B. subtilis complex has been difficult due to their almost identical phenotypic characteristics and 16S rRNA gene sequences. Recently, partial gyrA sequences coding for DNA gyrase subunit A have been found to be able to provide rapid and accurate classification and identification for B. subtilis and closely related taxa (Chun and Bae, 2000). In this study, strain G341 was found to share 99% sequence similarity with species of the B. subtilis complex based on sequence analysis of 16S rRNA gene. It shared 96.6% of sequence similarity with type strain B. velezensis LMG 22478T based on sequence analysis of partial gyrA gene (Fig. 1). Thus, it is reasonable to identify the strain G341 as B. velezensis.
B. velezensis sp. nov. was recently isolated during a research focusing on discovering novel bacterial strains capable of synthesizing new lipopeptides with surfactant and/or antimicrobial activity (Ruiz-Garcia et al., 2005). Several research groups have reported that B. velezensis have potential to control Fusarium wilt in strawberries (Nam et al., 2009), wheat powdery mildew (Cai et al., 2017), Fusarium head blight (Palazzini et al., 2016). Recently, Gao et al. (2017) reported that one endophytic B. velezensis ZSY-1 strain produces volatile compounds having antifungal activity against phytopathogenic fungi. However, to the best of our knowledge, there has been no report that B. velezensis can produce both diffusible and volatile antifungal compounds. Accordingly, we investigated the control efficacy of diffusible and volatile compounds produced by strain G341 against plant diseases caused by phytopathogenic fungi.
In in vivo experiment, the fermentation broth of strain G341effectively controlled the development of rice blast, sheath blight, tomato gray mold, and red pepper anthracnose. In order to identify antifungal principles produced by this strain, we isolated two antifungal compounds by partitioning with BuOH, silica-gel column chromatography, Sephadex LH-20 column chromatography, and C18 column chromatography. The two compounds were identified as bacillomycin L and fengycin A based on MS/MS analyses (Fig. 3, 4). Bacillomycin L is a member of the iturin group produced by B. subtilis. It is one of the extensively studied peptide antibiotics. Bacillomycin D, F, and L have been reported as antifungal peptides produced by B. subtilis (Besson et al., 1978; Mhammedi et al., 1982; Moyne et al. 2001; Qian et al., 2016). Fengycin A is a biologically active lipopeptide produced by several B. subtilis (Vanittanakom et al., 1986). Its structure is composed of a β-hydroxyl fatty acid linked to a peptide part comprising 10 amino acids, with 8 of them being organized in a cyclic structure. Loeffler et al. (1986) have also proved that fengycin is less toxic to tested plants. It protected these plants from some filamentous pathogenic fungi better than several peptide antibiotics such as iturin (Loeffler et al., 1986). In this study, bacillomycin L was found to be active against rice blast, rice sheath blight, and red pepper anthracnose while fengycin was found to be active against rice sheath blight and tomato gray mold (Table 2). Up to date, various types of lipopeptides isolated from B. subtilis have been reported to possess antifungal activity against plant diseases (Ongena and Jacques, 2008). Among iturin group, iturin A produced by B. subtilis RB14 can reduce the development of damping-off of tomato (a seedling disease) caused by R. solasni (Asaka and Shoda, 1996). Fengycin is known to possess antifungal activity against filamentous fungi (Kulimushi et al., 2017). Its hemolytic activity is 40-fold lower than that of surfactin (Schneider et al., 1999; Vanittanakom et al., 1986). Cazorla et al. (2007) have reported that iturin, fengycin A, and surfactin isolated from B. subtilis strains have antifungal activity against soil-borne phytopathogenic fungi from avocado rhizoplane.
Strain G341 also produced volatile antifungal compounds that could inhibit mycelial growth of phytopathogenic fungi in sealed plates (Table 3). Antifungal volatile compounds have been demonstrated previously in several pathogen systems. For example, trimethylamine has been shown to be able to inhibit hyphal extension and formation of arthrospore in Geotrichum candidum (Robinson et al., 1989). Allyl alcohol an also inhibit carpogenic germination of sclerotia of S. sclerotiorum in bean (Huang et al., 1997). Hydrogen cyanide produced by Pseudomonas has been used to control root rot of tobacco (Voisard et al., 1989). Our data indicate that strain G341 could inhibit mycelial growth of various phytopathogenic fungi by producing dimethylsulfoxide, 1-butanol, and acetoin (Table 4). Among them, acetoin was found to be a major volatile metabolite. Fernando et al. (2005) have reported that sulfur-based compounds benzothiazole and dimethyl trisulfide possess high fungicidal activity. Many commercially used fungicides and soil fumigants are sulfur-based. Alcohols such as 1-hexanol also have antifungal activity which can be used to prevent diseases (Archibold et al., 1997). Acetoin can significantly reduce symptomatic leaves inoculated with soft rot causing pathogen Erwinia carotovora by inducing systemic resistance (Ryu et al., 2003). Arrebola et al. (2010) have reported that B. amyloliquefaciens PPCB004 can produce acetoin as a major volatile compound. Radial growth of several Penicillium species has been found to be inhibited in vitro in the presence of volatile compound of PPCB004. They have also reported that antagonist PPCB004 could significantly reduce decay incidence and severity in Valencia inoculated with P. crustosum (Arrebola et al., 2010). Gao et al. (2017) reported that B. velezensis ZSY-1 strain can produce various volatile and antifungal metabolites such as 2-tridecanone, pyrazine (2,5-dimethyl), benzothiazole, and phenol (4-chloro-3-methyl).
Taken together, our results suggest that antagonist B. velezensis G341 can be used as a good biocontrol agent candidate, although how effective this antagonist would be under field conditions is currently unclear. Diffusible and volatile compounds produced by B. velezensis G341 might be used in agriculture as a direct contact biofungicide and biofumigant.
Supplementary Information
Acknowledgments
This research was supported by a grant (315007-03) of Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through Advanced Production Technology Development Program funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA), Republic of Korea.