 |
 |
| Plant Pathol J > Volume 34(5); 2018 > Article |
|
Abstract
Rice world production is affected due to the growing impact of diseases such as bacterial panicle blight, produced by Burkholderia glumae. The pathogen-induced symptoms include seedling rot, grain rot and leaf-sheath browning in rice plants. It is currently recognized the entrance of this pathogen to the plant, from infected seeds and from environmental sources of the microorganism. However, it is still not fully elucidated the dynamics and permanence of the pathogen in the plant, from its entry until the development of disease symptoms in seedlings or panicles. In this work it was evaluated the infection of B. glumae rice plants, starting from inoculated seeds and substrates, and its subsequent monitoring after infection. Various organs of the plant during the vegetative stage and until the beginning of the reproductive stage, were evaluated. In both inoculation models, the bacteria was maintained in the plant as an endophyte between 1 × 101 and 1 × 105 cfu of B. glumae.g−1 of plant throughout the vegetative stage. An increase of bacterial population towards initiation of the panicle was observed, and in the maturity of the grain, an endophyte population was identified in the flag leaf at 1 × 106 cfu of B. glumae.g−1 fresh weight of rice plant, conducting towards the symptoms of bacterial panicle blight. The results found, suggest that B. glumae in rice plants developed from infected seeds or from the substrate, can colonize seedlings, establishing and maintaining a bacterial population over time, using rice plants as habitat to survive endophyticly until formation of bacterial panicle blight symptoms.
Rice is an important crop worldwide in terms of its high productivity and its status as a staple food for over two billion people on five continents (Juliano et al., 2016). One of the major limiting factors of rice productivity worldwide, is the presence of microbial diseases, among which the bacterial panicle blight of rice caused by Burkholderia glumae, is one of the most persistent and severe (Ham et al., 2011). Burkholderia glumae, attacks rice crops in two different phenological stages of the plant, thereby causing two types of symptoms. Firstly, consists of rice seedling rot that occurs during germination of the seed in the early stages of the crop cycle (Kurita and Tabei, 1967; Uematsu et al., 1976). Later, towards the end of the reproductive phase, during grain filling, arise symptoms that give the name to the disease, known as bacterial panicle blight of rice. This condition causes a reduction of up to 75% of crop yield (Cui et al., 2016; Fory et al., 2014; Francis et al., 2013), because it produces sterility of panicle, grain rot, weight reduction and grain abortion (Chien et al., 1987; Nandakumar et al., 2009; Sayler et al., 2006; Tsushima et al., 1996).
Burkholderia glumae was first reported in Japan (Kurita and Tabei, 1967; Uematsu et al., 1976), however, it is currently distributed in most rice producing countries including Japan, Thailand, Vietnam, South Korea, Malaysia, Philippines, Sri Lanka, United States, Panama, Nicaragua, Costa Rica and Colombia (Devescovi et al., 2007; Fory et al., 2014; Ham et al., 2011; Rush, 1998; Shahjahan et al., 2000; Zhu et al., 2008). Unfortunately, the incidence of B. glumae, has increased in recent years due to variables such as climate change (Schaad, 2008), the high genetic diversity of the species (Karki et al., 2012; Nandakumar et al., 2009; Seo et al., 2015) and a lack of proper management and control strategies (Cui et al., 2016). Despite the importance of the disease, there is still a lack of knowledge of the life cycle of the bacteria, associated with its mechanism of entry to the plant, and the biological events that happen after such entry (Ham et al., 2011). This situation arises because if the pathogen carrier seedlings, manages to survive the postgermination phase, the plant during its vegetative phase is mostly asymptomatic until grain formation, where symptoms of the disease appears again (Hikichi et al., 1993; Li et al., 2016).
It has been suggested that two main reservoirs of the microorganism in nature are asymptomatic seeds (Fory et al., 2014; Hikichi et al., 1993; Zhu et al., 2008), and bulk soil in the crop zones (Stopnisek et al., 2014; Uetmasu et al., 1976). Therefore, there are mainly two hypotheses of the entry of this pathogen to the plant. The first involves bacterial penetration through natural openings and/or wounds on roots, leaves and/or spikelet from an environmental reservoir of the bacteria (Hikichi, 1993; Li et al., 2017; Tsushima et al., 1996). Direct spraying of B. glumae on plant leaves and spikelets has provided support for this mechanism of entry (Tsushima et al., 1996). More recently, Li and colleagues, sprayed directly a bacterial suspension of the B. glumae LMG 2196 strain, expressing the green fluorescent protein (gfp) gene, on leaves of plants at tillering stage using 10 ml at 1 × 109 cfu.ml−1 (Li et al., 2016) and on spikelets using 1 ml at 7 × 108 cfu.ml−1 (Li et al., 2017). These authors successfully showed B. glumae rice plant colonization of the vascular bundle through stomas, epidermal and leaf hairs infection (Li et al., 2016), and colonization of gynoecium and stamens, after bacterial cell proliferation on glumae and penetration through glumae hairs (Li et al., 2017). Similarly, to imitate the transmission of soil-borne of B. glumae, rice seeds have been inoculated using a bacterial suspension of the Pg-4 strain of Pseudomonas glumae (B. glumae) at 108 for 4 h (Hikichi, 1993). That study showed the infection of all plant organs at seedling stage, between 2 × 105 - 107 per gram of fresh weight. More recently, Li et al. (2016), dipped for two hours root seedlings in a bacterial suspension of the gfp-Bg-1 strain, at 109 cfu.ml−1, showing the colonization of the vascular bundle and epidermal cells of the root after 4-5 days after inoculation (Li et al., 2016).
Experiments described above, successfully shows the penetration of B. glumae at vegetative stages from airborne and soil-borne sources; however, this knowledge does not fully elucidate the development of B. glumae infection when bacteria are located within infected asymptomatic seeds. Besides, it is not even clear what happens to bacteria during vegetative growth of the plant after that kind of infection, because those studies do not fully solve the question of whether it remains on the ground, epiphytically or as endophyte in the plant, particularly when rice seeds have been inoculated (Hickichi, 1993). In this context, the aim of this study was to determine the colonization capacity of B. glumae during vegetative and reproductive stage of the rice plant, starting from seed infection, both from asymptomatic seeds, carrying a known population of the bacteria and from B. glumae free rice seeds, planted on water agar as substrate with a known concentration of B. glumae.
A typical field strain of B. glumae, 3200-12 was kindly provided by the Rice Pathology Laboratory of the International Center for Tropical Agriculture (CIAT) from Palmira Colombia. This strain was isolated and identified in previous studies from samples of rice crops located in the department of Córdoba, Colombia, which showed a 75.6% reduction in crop yield according to Fory et al. (2014). B. glumae strain was grown in 5 ml of LB broth, placed in centrifuge tubes of 50 ml of total capacity (Falcón®). It was then incubated in an orbital shaker (Thermoline®) for 24 h at 30°C and 150 rpm. Finally, two washes with 0.85% saline solution were performed, and the microbial biomass was suspended in 20 ml of saline solution to an OD of 0.20 ± 0.05 absorbance at 620 nm, which corresponds to 1.0 × 108 cfu.ml−1 according to plate dilution and the microdroplet count method.
For inoculations with the pathogenic microorganism, rice seeds from a certified batch of the Colombian variety F733 were used as plant material, which had previously been identified as susceptible to infection of B. glumae (Flórez-Zapata and Uribe-Vélez, 2011). Seeds for all trials were washed and surface disinfected using a 6% hypochlorite solution for 6 min. with constant agitation, the disinfectant solution was discarded and three washes were carried out with sterile distilled water (SDW). Surface sterilization of seeds was tested by putting seeds on nutrient agar (Oxoid® UK) and King B (gl−1: Protease peptone, 20; KH2 PO4, 1.5; MgSO4.7H2O, 1.5, anhydrous glycerol, 8.7 ml, bacteriological agar, 14.0), to evaluate persistence of external contaminants.
In order to cover the two most important reservoirs of the pathogenic bacteria, two models of infection were developed, first mimicking when B. glumae is inside seeds and secondly when it is in the seed substrate. For both models, the B. glumae inoculum was prepared as described above, and adjusted to concentrations, between 1.0 × 105 - 1.0 × 108 cfu.ml−1 depending on the assay, to determine the most adequate concentration for the development of disease symptoms in rice seedlings. For the seed infected model, the vacuum inoculation methodology previously described by Devescovi et al. (2007) was used with the modifications of Flórez-Zapata and Uribe-Vélez (2011). Briefly, 20 rice seeds were placed in 5 ml of a suspension of B. glumae at the different concentrations to be evaluated, for 30 min at 150 rpm. Then bacterial suspension was decanted and seeds were placed in Petri dishes, previously prepared with sterile absorbent paper, subsequently surface inoculated seeds were vacuumed at 20 psi for 10 min in order to ensure that bacteria penetrate seeds. Then, samples of 5 seeds were placed in a petri dish with moistened sterile filter paper and left in the dark for 7 days at 28°C ± 2°C and a relative humidity of 70%.
For the inoculation model of B. glumae on substrate, a volume of the bacterial inoculum was mixed with water-agar (4 g agar.l−1), and adjusted for a final concentration of the B. glumae’s strain 3200-12 at three different concentrations 1 × 105, 1 × 106 and 1 × 107 cfu.ml−1. Subsequently, five disinfected rice seeds were placed on an agar previously prepared with the bacterial suspension, then Petri dishes containing the agar were sealed and incubated for 7 days under the same conditions previously described.
In both cases, the stem and root length of the seedlings were measured and assigned a level within the disease severity scale proposed by Flórez-Zapata and Uribe-Vélez (2011). For the tests, a Petri dish with five rice seeds and three replicates per treatment were used as experimental unit, repeating three times in time to confirm the reproducibility of the results. In the case of the seed inoculation model, disinfected seeds not inoculated with the bacteria and subjected to the same vacuum conditions, were used as negative control; for the substrate inoculation, disinfected seeds were used on a water agar without bacterial inoculum. Experiment was repeated twice in time.
Once the most suitable concentration of B. glumae in each of the infection models was determined, seeds were inoculated at the selected concentration, in order to be monitored during the vegetative growth phase. After germination of the seeds under dark conditions, seedlings were transplanted on plant pots of 1 kg of capacity previously prepared with soil, conditioned with 20% of gravel to avoid soil compaction. Plant pots were incubated at 28°C ± 1°C, 4650 ± 100 lux, relative humidity of 75% and a photoperiod of 12:12 light:darkness. Plants were incubated until processing, according to different vegetative stages selected for a total of four evaluation times, the first was at 10 days of inoculation with 1 or 2 unfolded leaves, for the second stage the seedlings had 3 unfolded leaves, for the third stage they already had 7 to 8 unfolded leaves and for the last stage the plants had 9 or more unfolded leaves. For each day of evaluation, three replicates using one plant per replicate was sampled, except for the first evaluation in which 20 seedlings were taken per replicate. At all times of evaluation, stem and root length were determined. Presence of disease symptoms was observed and in the case of the incubation period of 10 days, the degree of infection was determined according to the severity scale proposed by Flórez-Zapata and Uribe-Vélez (2011).
In all sampling times the quantification of B. glumae concentration per gram of fresh plant was done, using semi-selective media for B. glumae (S-PG), described by Tsuchima et al. (1986) and modified for Quesada-Gonzales and Garcia-Santamaria (2014). Briefly, the plants at each evaluation time were surface disinfected, in order to guarantee that the population of B. glumae in the plant was endophytic. Firstly, soil substrate was removed using a gentle wash with tap water, then a 2% hypochlorite solution during 6 min, followed by three washes with sterile distilled water (SDW), were used, and subsequently another wash using 70% ethanol during 6 min., followed by three washes with SDW. To confirm the elimination of epiphytes in each sample, imprints were made on both, nutrient (Oxoid®) and King B (Merk®) agar. After plants were superficially disinfected, they were separated in stem and roots and for the last stages, in roots, stems and leafs, depending on the size of the plant. Subsequently, 0.3 g of fresh weight of each plant organ was macerated with mortar and pestle with 700 μl PBS buffer pH 7.2. Then, serial dilutions (10−2-10−7), of plant extract were plated in semi-selective S-PG medium, incubated for 48 h at 30°C ± 1°C. Then colony forming units of B. glumae.g−1 fresh weight of plant were count. For this, small, opalescent, convex and round (Type B colonies), were counted according to Tsushima et al. (1986), whose morphology corresponded to the growth of B. glumae 3200-12 in this medium. Due that, the medium is not completely selective, for confirmation of counts, presumed B. glumae colonies were grown in solid King B medium. Then after 24 h, the macroscopic characteristics of the typical colonies of this bacterium were observed and putative colonies of B. glumae were used for DNA extraction according to the methodology of Sahoo et al. (2014). Subsequently, its identity was confirmed by PCR using the primers and conditions of Sayler et al. (2006). PCR products were evaluated on 1% agarose gels according to the method of Sambrook and Rusell (2001). Finally, some of the PCR products during the evaluations of the different inoculation models were randomly selected from each of the evaluation times, for sequencing them by the method of Sanger at the Institute of Genetics of the Universidad Nacional de Colombia, to confirm the sequences identity. In order to monitor B. glumae in both infection models (from seed and substrate), un-inoculated plants were used as negative control, which were transplanted, incubated and processed as the pathogen’s treatment. The experiment was repeated twice in time.
In order to determine if B. glumae infected seedlings, are maintained as endophytes throughout the life cycle of the plant, until grain formation, a similar experiment as described in previous section, was done using a growth chamber (Sanyo®, Japan) at 28°C ± 1°C, 4650 ± 100 lux, relative humidity of 75% and a photoperiod of 12:12 light:darkness using different sampling days. First evaluation of the presence of B. glumae was done in plants at 10 days after inoculation (DAI), with 1 or 2 unfolded leaves; for the second stage, the seedlings had 3 to 5 completely unfolded leaves; for the third stage were used plants in the state of tillering (between 2 to 4 tillers); then, plants showing initiation of the panicle were sampled; and finally, plants were sampled at the maturation stage of the grain. Processing of samples for severity of symptoms at seedling stage, root and shoot length and cfu.g−1 of plant tissue was done as described in previous section. Then, at the end of reproductive phase, count of number of full and empty grains per panicle was obtained, and the weight of 100 grains, percentage of full grains/total grains per panicle (%) and weight (g) of 100 grains adjusted to 14% moisture content was recorded. A moisture content analyzer (OHAUS®, model MB45-2AO), was used to determine the percentage of moisture in the grains. Six panicles were used for the evaluation of inoculated and un-inculated treatments. Experiment was repeated twice in time.
Statistical analysis was performed with program R version 3.32. The Shapiro-Wilk Test was used with 95% significance to determine the normality of the data and the Levene Test with 95% confidence to determine the homoscedasticity of the data. In the case of having parametric data, the ANOVA table was used for the analysis of variance homogeneity and in the case of showing differences between treatments, a Duncan multiple comparison test was performed with a 95% confidence interval. When non-parametric data were obtained, data transformation was used according to the kurtosis of the distribution of the data and when this was not possible, the Kuskall-Wallis test was used.
Bacterial infection of rice seedlings by vacuum infected seeds, using different concentrations of B. glumae, showed the development of disease symptoms in 80 to 93% of inoculated plants. The infection showed a dose response effect within evaluated concentrations (1.0 × 106, 1.0 × 107 and 1.0 × 108 cfu.ml−1), showing statistical significant differences in relation to un-inoculated control with respect to the variable degree of infection. All concentrations presented statistically significant different levels of infection. Besides, plants treated with 1.0 × 108 cfu B. glumae.ml−1, presented a severity level of 3 or above, in the disease severity scale described by Florez-Zapata and Uribe-Vélez (2011) (Fig. 1A). Therefore, that concentration was selected for further evaluations. The agronomic variables, stem and root length at 7 DAI, also showed a dose-response reduction effect in relation to B. glumae concentrations, although it was less dramatic with statistical significant differences only for the concentration of 1.0 × 108 cfu.ml−1 (Fig. 1A). This indicates that B. glumae was able to generate disease symptoms and reduce plant vigor when inoculated in seeds by the proposed method.
On the other hand, rice seedlings developed disease symptoms when B. glumae was incorporated in the substrate, in up to 87% of inoculated plants, showing a dose response in relation to an un-inoculated control, with a similar pattern to that obtained in the seed infection model (Fig. 1B). However, in the concentration of 1.0 × 108 cfu. ml−1 there was a mortality of 85% of the seedlings (not shown results), besides, no statistical differences were found between 1.0 × 106 and 1.0 × 107 cfu.ml−1, therefore 1.0 × 107 cfu.ml−1 was selected for subsequent evaluations in order to guarantee the development of the disease symptoms (Fig. 1B). Stem and root length variables showed also a reduction in size, with statistically significant differences for all the concentrations of B. glumae, in comparison with un-inoculated control seedlings. However, no differences were found between them, except for the stem length at the concentration 1.0 × 105 cfu.ml−1. Thus, exposure of rice seeds with B. glumae incorporated into the substrate, indicates that B. glumae can penetrate the plant, developing symptoms of disease, even with loss of plant vigor at least for seedlings of 7DAI.
It is well known that bacterial panicle blight symptoms, under field conditions are evident only until the reproductive phase, when grains are formed. Therefore, a set of experiments were carryout to determine, if bacterial population is detected during the vegetative stage, when infection starts from seeds or substrate, and if so, if bacteria is able to reach until de reproductive stage, showing the typical disease symptoms. The follow-up of bacterial population of B. glumae, artificially incorporated into seeds and placed in the substrate, showed that the phytopathogenic bacteria maintains a basal population in the internal tissues of the plant, during the vegetative phase (Supplementary Table 1, Table 1). Despite of that, infected plants did not show any difference in terms of turgency or color of the plant during the observation window (not shown results). However, evaluation of stem and root length during different plant growth stages, showed that independent of inoculation model there was a significant decrease in terms of stem length with respect to un-inoculated control. Contrary, root length showed a decrease in relation to un-inoculated plants only at the 1-2 unfolded leaves, at 2-4 tillers and at grain ripening stage (Fig. 2A, B). These results showed again, that stem length was an agronomic variable affected by the inoculation of B. glumae in most stages of the vegetative phase and at the beginning of the reproductive phase, independently to the source of inoculum.
In the case of seed inoculations under vacuum conditions, it was possible to force the entry of the inoculum in a concentration of 1 × 105 cfu of B. glumae.g−1 of seed, which is equivalent approximately to 3.00 × 103 cfu of B. glumae. seed−1. In this model, initial inoculum was not only maintained as endophyte during the time of evaluation, but also increased during the phase of 1-2 unfolded leaves, reaching between 3.24 × 105 - 5.00 × 105 cfu of B. glumae.g−1 of root and between 3.17 × 105 - 5.00 × 105 cfu of B. glumae. g−1 of stem (Supplementary Table 1, Table 1). These results suggest that B. glumae is able to mobilize towards aerial and root organs.
For five to six unfolded leaf growth phase, bacterial population was maintained per gram of fresh plant weight at 9.83 × 104 cfu of B. glumae.g−1 of root and 5.00 × 105 cfu of B. glumae.g−1 of stem. However, evaluations of B. glumae population in plants at higher vegetative stages, showed that the endophyte bacterial population begins to decrease over time. For instance, when plant reached 2 to 4 formed tillers, B. glumae decreased by one order of magnitude for both aerial and root organs (2.00 × 104 cfu of B. glumae.g−1, 1.37 × 104 cfu of B. glumae.g−1 and 1.97 × 104 cfu of B. glumae.g−1 of stem, leaf and root respectively) (Table 1). At the initiation of the panicle formation, the bacterial population was maintained for the aerial part of the plant (1.57 × 104 cfu of B. glumae.g−1 of stem and 7.00 × 103 cfu of B. glumae.g−1 of leaf), but was not detected in the root.
Similar results were observed for the inoculation model in the substrate, where seedlings were in contact with the inoculum placed in the agar at 1 × 107 cfu.ml−1, during the initial seven days of incubation. In this case, during the phase of 1 to 2 unfolded leaves, the endophyte population of the phytopathogen reached 1.30 × 105 cfu of B. glumae. g−1 of root and 3.40 × 105 cfu of B. glumae.g−1 of stem, indicating again that bacterium enters the plant and mobilizes to the root and aerial organs of the plant. This endophyte population was constant until the phase of 2 to 4 tillers for the root (1 × 105 cfu.g−1 of root), while in the stem decreased by two orders of magnitude (2.01 × 103 cfu of B. glumae.g−1 of stem and 2.53 × 103 cfu of B. glumae.g−1 of leaf). For the phase of maximum tiller number and initiation of the panicle, population of B. glumae diminished in three orders of magnitude in root (6.02 × 102 cfu of B. glumae.g−1 of root), but increased an order of magnitude in stem (8.89 × 105 cfu of B. glumae.g−1) and leaves (9.56 × 104 cfu of B. glumae.g−1 of leaf) (Table 1). These results show that at the beginning of the reproductive phase, the bacterial population increased towards the aerial organs of the plant, especially towards the stem and leaf pod, and decreased in the root. Plants at the stage of grain ripening showed that bacterial population maintained the size at the root (6.39 × 102 cfu.g−1 of root), at the stem (1.61 × 103 cfu of B. glumae.g−1 of stem) and leaf (4.64 × 103 cfu of B. glumae.g−1 of leaf). However, a clear difference was found with flag leaves that were evaluated separately, finding a significant increase (5.29 × 106 cfu of B. glumae.g−1 of flag leaf) (Table 1). This is important to mention that non-inoculated plants did not show detectable B. glumae population or disease symptoms during all the phases evaluated.
Plants with endophytic B. glumae population maintained throughout vegetative and reproductive growth stages were followed up to grain ripening, and grain filling was recorded as an indication of B. glumae disease symptoms. It was found, that non-inoculated plants showed a significant lower percentage of empty grains (25.72%), in relation to plants inoculated with B. glumae, that showed 58.0% empty grains (Table 2). In the same sense, the weight of 100 grains adjusted to 14% moisture, was higher in control plants with a value of 7.97 g, showing significant statistical differences in relation to inoculated plants, that showed a weight of 4.54 g per 100 grains.
Presumptive B. glumae colonies obtained in semiselective medium S-PG (Tsushima et al., 1986), were confirmed through the isolation of colonies obtained from King B dilution plates, prepared from inoculated and non-inoculated plants, at different phenological stages. As suggested by Tsuschima et al. (1986) all round, small, smooth, convex, creamy colonies that excrete a diffusible yellow pigment in agar, which is the typical morphology of B. glumae species and particularly of strain CIAT3200-12, were selected. Each of those isolated colonies were confirmed through a PCR, using ITS region-specific primers of the 16S gene for B. glumae (Sayler et al., 2006). In all cases presumptive B. glumae colonies, presented the expected molecular size (282 bp) (Fig. 3). An important percentage of PCR products obtained, were confirmed by sequencing, using Sanger technique, in order to determine whether they actually corresponded to the genome of B. glumae CIAT3200-12, finding in all cases a 100% identity. This confirmation was carried out on all evaluated days as well as for all forms of inoculation.
Burkholderia glumae is one of the most limiting pathogens currently present in rice-producing countries, including Colombia, particularly in the climate-warming scenario, since the presence of high temperatures and humidity favor the development of the disease (Shaad, 2008). Despite the importance of the disease worldwide, few works have been designed to follow the infection of B. glumae, from early stages of the proposed reservoirs of plant infection, through vegetative and maturity growth stages of the plant (Hikichi, 1993; Li et al., 2016). In the present work, it was followed the presence of B. glumae in internal tissues of rice plants, during different phenological stages, starting from both sources of inoculum, seeds and substrate.
Pathogen has been detected in naturally and asymptomatic infected grains (Hikichi et al., 1993; Saylor et al., 2006), even up to three years of been stored at room temperature (Tsushima et al., 1989), suggesting that seeds are one of the natural reservoirs of the pathogen. The seedborne infection in this study, was obtained after vacuuming seeds with bacterial inoculum. Surface disinfected seeds showed an internal concentration of approximately 1 × 105 cfu of B. glumae.g−1 of seeds. In this case, germinated seeds after surpassing the initial stage of seedlings, showed that B. glumae continues its growth in internal structures during the vegetative phase, without visible disease symptoms, more than a slight decrease of stem and root length in some growing stages (Fig. 3). There are several studies reporting artificial inoculation of rice seeds with B. glumae trying to mimic natural infection (Devescovi et al., 2007; Flórez-Zapata and Uribe-Vélez, 2011; Hikichi, 1993; Li et al., 2016; Uetmasu et al., 1971). In some of these studies, the development of disease symptoms has been demonstrated from seeds infected with the bacteria. For instance, Hikichi (1993), using seeds inoculated with a bacterial suspension at 1 × 108 cfu.ml−1, reached a 2% of infection in rice seedlings. Nevertheless, such approach did not properly mimic the fate of the B. glumae’s internally infected seeds in rice seedlings, as seeds were externally inoculated. In this work, when applying the vacuum infection strategy, 80 to 93% of seedlings developed symptoms of seedlings rot disease; however, once plantlets surpassed 1-2 unfolded leaves they became asymptomatic. The high infection rate obtained through this methodology, is very important for the subsequent monitoring of the inoculated bacteria over time, since in this way it is guaranteed that seedlings transplanted in soil, had the pathogenic bacteria.
Some authors have suggested that bacterial panicle blight can be a soil borne disease (Uematsu et al., 1976), which is supported by the fact that several species of Burkholderia, including B. glumae, are highly distributed in soil (Stopnisek et al., 2014). For this reason, in this work, we developed a model of infection, using the substrate supporting the plant, with up to 1 × 107 cfu of B. glumae.ml−1 of substrate, as carrier of the pathogen. In this case, 85% of seedlings showed disease symptoms at 7 DAI. Then, seedlings were transplanted to soil free of pathogen, in order to follow the growth and distribution of B. glumae inside the plant. Results showed that after germination, surface sterilized plants have an internal population of B. glumae at a concentration of 1.20 × 105 cfu.g−1 of fresh weight at the stage of 1-2 unfolded leaves. Therefore, bacteria enter the plant from the substrate, maintaining an internal population, which furthermore was kept constant until the phase of 7-8 unfolded leaves (Table 1). In a similar experiment Li et al. (2016), infected two-leaf-period seedlings with 200 mL of a bacterial suspension at 1 × 109 cfu.ml−1, with the aim to imitate the transmission of soil-borne B. glumae in the seedling stage of rice. In this case, it was possible to identify the entrance of B. glumae through lateral root’s hairs, and bacteria were located at the epidermal and endodermal cells of the roots, after five days of inoculation. However, the bacterial population was not followed during the disease cycle; therefore, no conclusions could be obtained in terms of the fate of that inoculum, to generate the bacterial panicle blight symptoms.
After the vegetative growth stage, bacterial population increases towards the beginning of the reproductive phase again and even increased notably in flag leaves, in plants at grain ripening stage. Similar results were found by Tshushima et al. (1996), after spraying plants 10-31 days before heading, under field conditions with a B. glumae suspension. In that study authors reported that population of B. glumae on flag leave sheaths, one week after heading, presented a high correlation (r = 0,78; P = 0,001) with disease incidence, indicating that pathogen on flag leave sheaths, is important in primary infection of the disease. It is important to mention that contrary to Tshushima et al. (1996), in our study, plants were exposed to the pathogen at the beginning of the plant cycle, during seed germination, besides bacterial population was present in internal tissues, not externally sprayed. Therefore, it is speculated that even when infection starts from seeds, flag leaves at the reproductive stage, becomes an important reservoir of the endophyte bacterial population, for the development of disease symptoms during grain ripening.
Very few studies have carried out inoculations of B. glumae in the soil (Tsushima et al., 1996; Uematsu et al., 1976). However, none of those studies followed the infection of the plant through the vegetative phase and up to the development of grain rot disease. For this reason, to the best of our knowledge, this is the first work in which B. glumae is incorporated in the plant substrate, showing to be able to colonize the plant from seeds, to cause disease bacterial panicle blight symptoms at the reproductive phase.
Evaluation of symptoms, in relation to the dynamics of B. glumae population for both inoculation methodologies, showed that the bacterium affected stem length, not only at the seedling level as shown before (Devesconi et al., 2007; Flórez-Zapata and Uribe-Vélez, 2011), but during most of the vegetative stages, including the beginning of the reproductive phase. Although, it may not be a variable that can be distinguished in a high-density field crop, it may suggest that the presence of B. glumae in the plant, have an energetic cost, affecting its vigor during the vegetative phase.
Rice plants, developed from substrate’s inoculated seeds that reached the stage of maturity, presented a remarkable reduction of productivity, with 75% of empty grains at the end of the cycle. These results, coincide with the report of Fory et al. (2014), who found after using same strain as in our study (B. glumae 3200-12), under field conditions, a loss of yield of up to 75%, in plants inoculated by panicle spraying, in comparison to plants inoculated with water. It confirms the virulence of the strain and the biological relevance of our result.
As conclusion, this study contributes to understand the population dynamic and distribution of B. glumae in different plant tissues, starting from artificially infected seeds or plant substrates. It was determined that once the bacterium enters the plant, it can mobilize through different organs and produce disease symptoms during the seedling phase; can be maintained as endophyte in the rice plant within the vegetative growth, even without showing very evident disease symptoms. However, bacterial concentration internally increases subtly when the plant enters the reproductive stage, being more remarkable in the flag leaf, ending with panicle blight symptoms, with production of significant number of empty grains.
Acknowledgments
We thank the financial support given to this work by Departamento Administrativo de Ciencia, Tecnología e Innovación de Colombia (COLCIENCIAS) under the project No. 26650, and by the mean of Young Researchers scholarship given by COLCIENCIAS to Luz Adriana Pedraza y Jessica Bautista.
Fig. 1
Development of B. glumae disease symptoms in rice seedlings (cvF733), at 7 DAI, through two inoculum sources of the pathogen (A) Seed (vacuum inoculation) and (B) Substrate (pathogenic bacteria incorporated into the supporting substrate). Different letters show statistically significant differences between treatments, with each of the variables (stem length, root length and degree of infection), according to the Duncan test, with a level of 95.0% confidence. The graph corresponds to one of at least two repetitions in time, for both infection models, which showed the same trend and statistical differences.

Fig. 2
Effect of inoculation of B. glumae on stem and root length in relation to non-inoculated plants during vegetative and reproductive phases of the plant. (A) Inoculation under the vacuum seed model and (B) Inoculation in substrate (bacteria incorporated in the seed/plantlet substrate). Different letters show statistically significant differences for each of the plant organs between the vegetative stages, according to the Duncan Test with a level of 95.0% confidence. ¶ Phases determined according to the extended BBCH scale for rice according to Hack et al. (1992) modified from Lancashire et al. (1991).
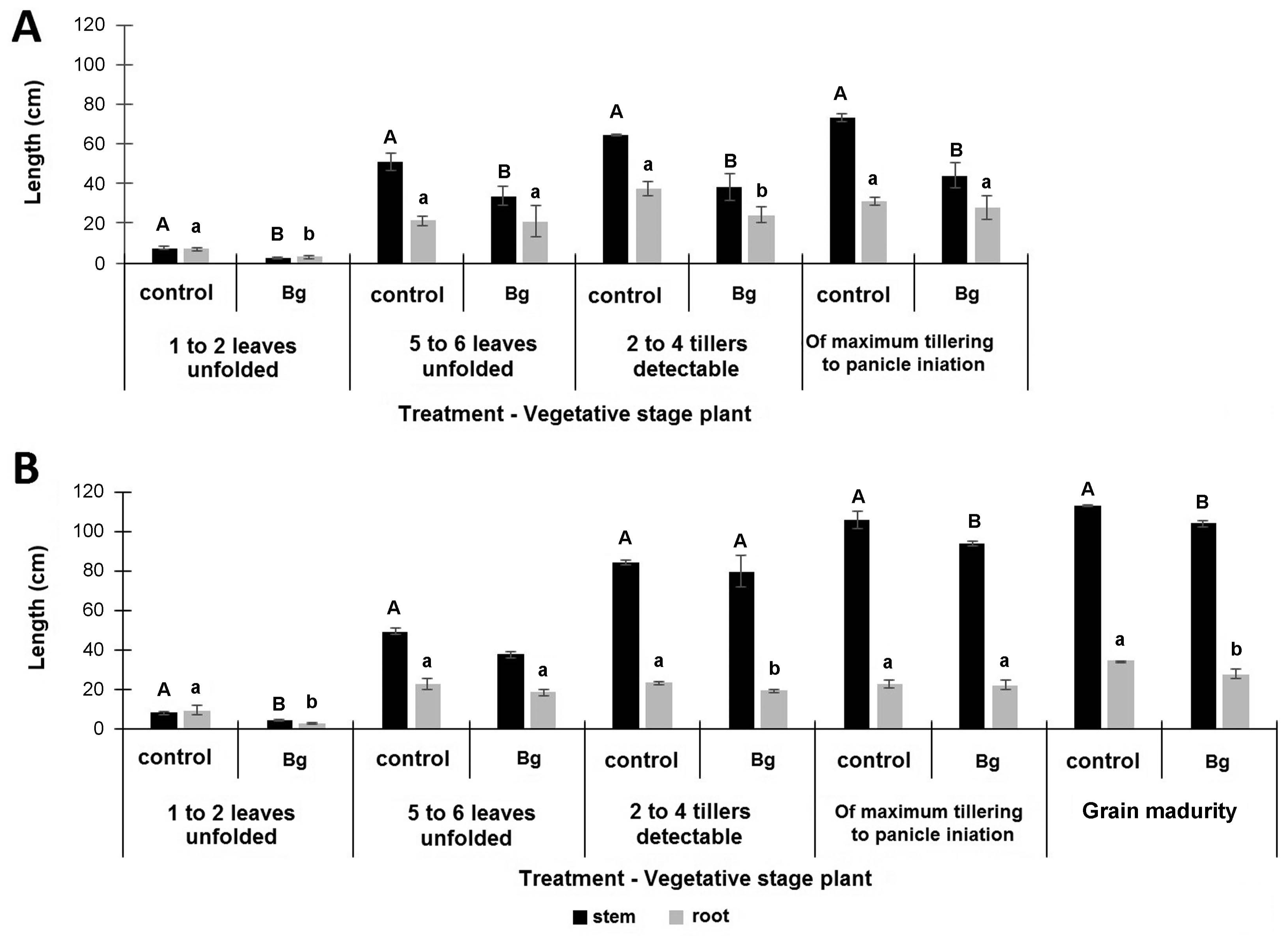
Fig. 3
Agarose gel electrophoresis showing PCR products for presumptive B. glumae colonies of inoculated plants at stage of 2-4 tillers. MWM molecular weight marker. Lines: 1, control reaction without DNA sample; 2, Positive control (pure DNA sample of B. glumae 3200-12); 3-6, Presumptive colonies isolated from leaf; 7-10, Presumptive colonies isolated from stem; 11-12, Presumptive colonies isolates from root; 13, Negative control (non-presumptive colony DNA). The size of the amplicon is 286 bp.

Table 1
B. glumae population (cfu.g−1 of fresh weight of plant) in inoculated plants by vacuumed seeds and seed/plantlet substrate, through different plant growing stages up to the reproductive phase
| CFU of B. glumae g−1 of fresh weight of plant | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| ¶Vegetative phase of plant | Inoculation model in seed | Inoculation model in substrate | ||||
|
|
||||||
| Root | Aerial part | Root | Aerial part | |||
|
|
|
|||||
| Stem | Leaf | Stem | Leaf | |||
| 1-2 unfolded leaves (11) | 5.00 × 105 c | 5.00 × 105 c | 1.30 × 105 b | 3.50 × 105 de | ||
| 5-6 unfolded leaves (15) | 9.83 × 104 b | 3.11 × 105 c | 3.54 × 105 b | 4.67 × 103 bc | ||
| 2 a 4 tilles detectable (22) | 1.97 × 104 a | 2.00 × 104 b | 1.37 × 104 b | 5.00 × 105 c | 2.01 × 103 a | 2.53 × 103 ab |
| Of máximum tillering to panicle initiation | 0.0 | 1.57 × 104 b | 7.00 × 103 a | 6.02 × 102 a | 8.89 × 105 e | 9.56 × 104 cd |
| Fully maturity of grain | ND | ND | ND | 6.39 × 102 a | 1.61 × 103 a |
Flag leaf 5.29 × 106 f Others leaves 4.64 × 103 ab |
* Different letters show statistically significant differences for each of the plant organs between the vegetative stages, according to the Kruskal-wallis Test, with a level of 95.0% confidence.
¶ Phases determined according to the extended BBCH scale for rice according to Hack et al. (1992) modified from Lancashire et al. (1991).
Table 2
Effect of B. glumae on the filling of grains and grain weight in plants inoculated from seed grown during 7 days under the substrate model
References
Chien, CC and Chang, YC 1987. The susceptibility of rice plants at different growth stages nd of 21 commercial rice varieties to Pseudomonas glumae. J Agric Res China. 36:302-310.
Cui, Z, Zhu, B, Xie, G, Li, B and Huang, S 2016. Research status and prospect of Burkholderia glumae, the pathogen causing bacterial panicle blight. Rice Sci. 23:111-118.

Devescovi, G, Bigirimana, J, Degrassi, G, Cabrio, L, LiPuma, JJ, Kim, J, Hwang, I and Venturi, V 2007. Involvement of a quorum sensing-regulated lipase secreted by a clinical isolate of Burkholderia glumae in severe disease symptoms in rice. Appl Environ Microbiol. 73:4950-4958.



Flórez-Zapata, N and Uribe-Vélez, D 2011. Determinación de la infección de Burkholderia glumae en semillas de variedades comerciales Colombianas de arroz. Rev Fac Nac Agron Medellín. 64:6093-6104.
Fory, PA, Triplett, L, Ballen, C, Abello, JF, Duitama, J, Aricapa, MG, Prado, GA, Correa, F, Hamilton, J, Leach, JE, Tohme, J and Mosquera, GM 2014. Comparative analysis of two emerging rice seed bacterial pathogens. Phytopathology. 104:436-444.


Francis, F, Kim, J, Ramaraj, T, Farmer, A, Rush, MC and Ham, J 2013. Comparative genomic analysis of two Burkholderia glumae strains from different geographic origins reveals a high degree of plasticity in genome structure associated with genomic islands. Mol Genet Genomics. 288:195-203.


Hack, H, Bleiholder, H, Buhr, L, Meier, U, Schnock-Fricke, U, Weber, E and Witzenberger, A 1992 Einheitliche codierung der phänologischen entwicklungsstadien mono-und dikotyler pflanzen - erweiterte BBCH-skala, Allgemein-. Nachrichtenbl Deut Pflanzenschutzd. 44:265-270 (in German).
Ham, JH, Melanson, RA and Rush, MC 2011. Burkholderia glumae: next major pathogen of rice? Mol Plant Pathol. 12:329-339.


Hikichi, Y 1993. Relationship between population dynamics of Pseudomonas glumae on rice plants and disease severity of bacterial grain rot of rice. J Pest Sci. 18:319-324.

Hikichi, Y, Okuno, T and Furusawa, I 1993. Immunofluorescent antibody technique for detecting Pseudomonas glumae on rice plants. Japanese J Phytopathol. 59:477-480.

Juliano, B 2016. Rice: Overview. In: Encyclopedia of Food Grains, eds. by C Wrigley, H Corke, K Seetharaman and J Faubion, 125-129. Academic Press, Elsevier, London, UK.

Karki, HS, Shrestha, BK, Han, JW, Groth, DE, Barphagha, IK, Rush, MC, Melanson, RA, Kim, BS and Ham, JH 2012. Diversities in virulence, antifungal activity, pigmentation and DNA fingerprint among strain Burkholderia glumae. PlosOne. 7:e45376

Kurita, T and Tabei, H 1967. On the pathogenic bacterium of bacterial grain rot of rice [abstract in Japanese]. Annals of the Phytopathology Society of Japan. 33:111.
Lancashire, PD, Bleiholder, H, van Den Boom, T, Langelüddecke, P, Stauss, R, Weber, E and Witzen-Berger, A 1991. An uniform decimal code for growth stages of crops and weeds. Ann Appl Biol. 119:561-601.
Li, L, Wang, L, Liu, L, Hou, Y, Li, Q and Huang, S 2016. Infection process of Burkholderia glumae before booting stage of rice. J Phytopathol. 164:825-832.

Li, L, Wang, L, Liu, L, Hou, Y, Huang, S and Li, Q 2017. Infection Process of Burkholderia glumae in Rice Spikelets. J Phytopathol. 165:123-130.

Nandakumar, R, Shahjahan, AKM, Yuan, XL, Dickstein, ER, Groth, DE, Clark, CA, Cartwright, RD and Rush, MC 2009. Burkholderia glumae and B. gladioli Cause Bacterial Panicle Blight in Rice in the Southern United States. Plant Dis. 93:896-905.


Quesada-González, A and García-Santamaría, F 2014 Burkholderia glumae in the rice crop in Costa Rica. Agron Mesoam. 25:371-381 (in Spanish).
Sahoo, RK, Ansari, MW, Pradhan, M, Dangar, TK, Mohanty, S and Tuteja, N 2014. Phenotypic and molecular characterization of efficient native Azospirillum strains from rice fields for crop improvement. Protoplasma. 251:943-953.


Sambrook, J and Rusell, D 2001. Molecular cloning: A laboratory manual. 3rd ed. Cold Spring harbor Laboratory Press, New York, USA. 2344.
Sayler, RJ, Cartwright, RD and Yang, Y 2006. Genetic characterization and real-time PCR detection of Burkholderia glumae, a newly emerging bacterial pathogen of rice in the United States. Plant Dis. 90:603-610.


Schaad, NW 2008. Emerging plant pathogenic bacteria and global warming. In: Pseudomonas syringae Pathovars and Related Pathogens - Identification, Epidemiology and Genomics, eds. by M Fatmi, A Collmer, NS Iacobellis, JW Mansfield, J Murillo, NW Schaad and M Ullrich, 369-379. Springer, Dordrecht, Netherlands.

Seo, Y, Lim, J, Park, J, Kim, S, Lee, H, Cheong, H, Kim, S, Moon, J and Hwang, I 2015. Comparative genome analysis of rice-pathogenic Burkholderia provides insight into capacity to adapt to different environments and hosts. BMC Genomics. 16:349-359.



Stopnisek, N, Bodenhausen, N, Frey, B, Fierer, N, Eberl, L and Weisskoft, L 2014. Genus-wide acid tolerance accounts for the biogeographical distribution of soil Burkholderia populations. Environ Microbiol. 16:1503-1512.


Tsushima, S, Wakimoto, S and Mogi, S 1986 Selective medium for detecting Pseudomonas glumae Kurita et Tabei, the causal bacterium of grain rot of rice. Japanese J Phytopathol. 52:253-259 (in Japanese).

Tsushima, S, Mogi, S, Naito, H and Saito, H 1989 Existence of Pseudomonas glumae on the rice seeds and development of the simple method for detecting P. glumae from the rice seeds. Bull Kyushu Agri Expt Sta. 25:261-270 (in Japanese).
Tsushima, S, Naito, H and Koitabashi, M 1996. Population dynamics of Pseudomonas glumae, the causal agent of bacterial grain rot of rice, on leaf sheaths of rice plants in relation to disease development in the field. Ann Phytopathol Soc Jpn. 62:108-113.

- TOOLS



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Supplement
Supplement Print
Print




