Viruses Associated with Fig Mosaic Disease in Different Fig Varieties in Montenegro
Article information
Abstract
Symptoms of fig mosaic disease have been noticed on leaves of fig (Ficus carica) for several decades, in Montenegro. In 2014, leaf samples were collected from trees of six fig cultivars in a plantation located in the main fig-producing area of Montenegro, to study the disease. After RNA isolation, samples were tested by RT-PCR for detection of nine fig viruses and three viroids. Four viruses were detected: fig leaf mottle-associated virus 1 (FLMaV-1), fig mosaic virus (FMV), fig mild mottle-associated-virus (FMMaV) and fig badnavirus 1 (FBV-1). Most of the viruses were present in mixed infections. The amplicons of the viruses were directly sequenced from both directions. A BLAST search of these sequences revealed sequence identities with their closest counterparts at GenBank of 92, 97, 92 and 100%, for FLMaV-1, FMV, FMMaV and FBV-1, respectively. Different responses in symptom expression due to the various virus combinations detected have been demonstrated. Variety Sušilica had the least symptom expression, with only one virus (FBV-1) found. Considering that the production of figs in Montenegro is increasing and has a substantial relevance in this geographic location, the results indicate that more attention should be given to improving the phytosanitary condition of fig trees in the country.
Introduction
The common fig (Ficus carica) is widely cultivated in the southern and central parts of Montenegro. Until recently, fig was not grown in large areas but mainly as individual trees in family orchards. In the last decade, some fig plantations of several hundred to over 1,000 trees were established. This economic importance of fig in Montenegro is gradually increasing because it exhibits a high potential for export, touristic purposes and organic production. However, the enlargement in production has led to the emergence of certain diseases (caused by fungi and viruses) that previously had either a limited or no major economic impact on yield. In 2012, fig fruit rot caused by Alternaria alternata was detected for the first time in Montenegro (Latinović et al., 2014). Later, in 2014, there was a severe infection of figs caused by fig rust pathogen Cerotelium fici that was favoured by a humid and warm summer (Latinović et al., 2015). Beside these fungi, symptoms resembling fig mosaic disease (FMD) have been observed in fig orchards (Mijušković, 1999; Perišić, 1952).
Fig mosaic is an important disease that affects figs worldwide. The symptoms were recorded for the first time in the early 1930s, in California (Condit and Horne, 1933). However, detailed information about the aetiological agents of the disease has only been reported relatively recently (Elbeaino et al., 2006, 2007b, 2009, 2010). Some viruses and viroids were detected in several fig-producing areas. In fig orchards in Syria, in 2010, six viruses and one viroid were found (Elbeaino et al., 2012). These included fig mosaic virus (FMV; family Fimoviridae, genus Emaravirus), fig fleck-associated virus (FFkaV; family Tymoviridae, genus Maculavirus), fig leaf mottle-associated virus 1 (FLMaV-1; family Closteroviridae, genus Closterovirus), fig leaf mottle-associated virus 2 (FLMaV-2; family Closteroviridae, genus Closterovirus), fig mild mottle-associated virus (FMMaV; family Closteroviridae, genus Closterovirus), fig latent virus 1 (FLV-1; family, Betaflexiviridae genus Trichovirus), and hop stunt viroid (HSVd; family Pospiviroidae, genus Hostuviroid). In Iran, based on a 2012 survey, approximately 14.7% of the tested fig trees were infected with fig cryptic virus (FCV; family Partitiviridae, genus Alphacryptovirus), FFkaV and FMV (Ale-Agha and Rakhshandehroo, 2014). In Saudi Arabia, Alhudaib (2012) noted that FMD is caused by FLMaV-1 and FMV. Conversely, in Egypt, Elbeshehy and Elbeaino (2011) detected four viruses: FLMaV-1 (predominant), FMV, FLMaV-2 and FMMaV. In Tunisia, El Air et al. (2013) observed the presence of FMMaV and FLV-1 in fig trees, with FLV-1 detected in all surveyed areas in symptomless, as well as symptomatic trees. In the United States, fig badnavirus-1 (FBV-1; family Caulimoviridae, genus Badnavirus) was widely detected by Laney et al. (2012) in various fig cultivars. In an Iranian fig orchard, Norozian et al. (2014) observed the presence of fig leaf mottle-associated virus 3 (FLMaV-3; family Closteroviridae, genus Closterovirus). Beside these viruses, several viroids have also been reported in figs. Yakoubi et al. (2007) discovered fig infected with HSVd and citrus exocortis viroid (CEVd; family Pospiviroidae, genus Pospiviroid), presenting symptoms of FMD. Also, Chiumenti et al. (2014) identified a viroid resembling apple dimple fruit viroid (ADFVd; family Pospiviroidae, genus Apscaviroid) in a fig accession.
In Montenegro, FMD was initially noticed in 1952 (Perišić, 1952). Later, Mijušković (1999) observed the disease on figs every year, with symptoms that varied according to the season and fig variety. Bandelj et al. (2009) also noticed the symptoms of mosaic in figs and stated that over the last decades of the 20th century, the disease has significantly decreased fig tree numbers in Montenegro. In 2015, Perović et al. (2016) analysed fig leaf samples to assess the presence of five fig viruses and revealed that infections are caused mainly by FLMaV-1, FMV and FMMaV. Delić et al. (2017) reported the prevalence of FBV-1, followed by FLMaV-1, FMV, FMMaV and FFkaV in Montenegrin fig orchards, together with their phylogenetic analyses.
The present study aimed to investigate the presence of nine viruses and three viroids that infect fig trees and establish possible association of symptoms with the viruses observed that influence on different responses of several Montenegrin fig varieties.
Material and Methods
Field survey and plant material
FMD symptoms were observed in 2012, on different local fig varieties in a 10-year-old commercial orchard (1,100 plants) near Podgorica (Fig. 1). However, the monitoring and sample collection were conducted in September 2014. During monitoring, leaf samples were collected from 28 fig trees from 6 different local varieties (Izraelka, Jesenka, Trojka, Sušilica, Kalamata and Sultanija). Leaves were collected from five trees per variety, except Trojka, where the leaves were taken from three trees. Each sample consisted of 10–15 leaves per tree.
RNA isolation, RT-PCR and sequencing
Leaf samples were subjected to RNA isolation using a SpectrumTM Plant Total RNA kit (Sigma-Aldrich, St. Luis, MO, USA). The total RNAs were used as templates for RT-PCR (Qiagen One-Step RT-PCR kit), carried out with specific pairs of primers for nine viruses and three viroids, which were previously reported on figs (Table 1). The one-step RT-PCR reaction was performed at 50°C for 30 min (reverse transcription), 95°C for 15 min (initial PCR activation step) and followed by 40 cycles at 94°C for 30 s, 55°C for 30 s, 72°C for 1 min, and a final extension step at 72°C for 10 min. PCR products were analysed by agarose gel electrophoresis and ethidium bromide staining. For each detected amplicon, at least two representative amplified products were selected, purified (QIAquick PCR Purification kit) and subjected to Sanger sequencing by the commercial service GATC Biotech, Germany.
Processing of images with leaf symptoms
Ten leaves from each tree of each fig variety tested for viruses and viroids were taken to assess symptom severity, according to the percentage of the leaf area with symptoms. Fully developed leaves were randomly collected from the middle part of the tree crown (height from 1.5 to 2.0 m). Most of the collected leaves showed symptoms presumed to be of viral origin and not to have been caused by any other factor. Each leaf (upper leaf surface) was scanned (HP Scanjet 200) and saved in TIF format. These RGB images were subjected to the algorithm written in MATLAB programming language, for further processing.
The input of the algorithm (written in MATLAB) is an RGB image of a fig leaf. The output of the algorithm is a binary image (an image whose pixels assume a value of 0 or 1, representing black and white, respectively), where white areas indicate the regions of the image where the leaf symptoms are present. Additionally, the algorithm computes the percentage of the infected area relative to the total area of the leaf. The algorithm begins by extracting two channels: blue for creating the mask (used for extracting the leaf from the background) and green for the actual processing. Mask creation is performed using the Otsu algorithm (Otsu, 1979), and then the mask is applied to the green channel image, to separate the leaf from the background. The extracted leaf image contains a significant amount of impulse noise, which is why it is necessary to filter the image with a median filter before the further processing. In this instance, a 3-by-3 median filter is used. After the pre-processing, the data is ready for the application of k-means algorithm (Likas et al., 2003). In this paper, the k-means algorithm with two centroids has been used, with each centroid representing each of the two classes: healthy region and infected region. This algorithm aims to classify each of the image pixels into one of the two classes, i.e., to determine if the analysed pixel belongs to the healthy or infected region. The output of the algorithm is a binary image on which further postprocessing techniques are applied. In this example, a morphological opening operation has been used to eliminate various types of noise, namely, byproducts of the k-means algorithm (Gonzalez and Woods, 2002). The morphological opening is done using a seven-pixel-wide “square” structural element.
Results
Symptoms of the disease
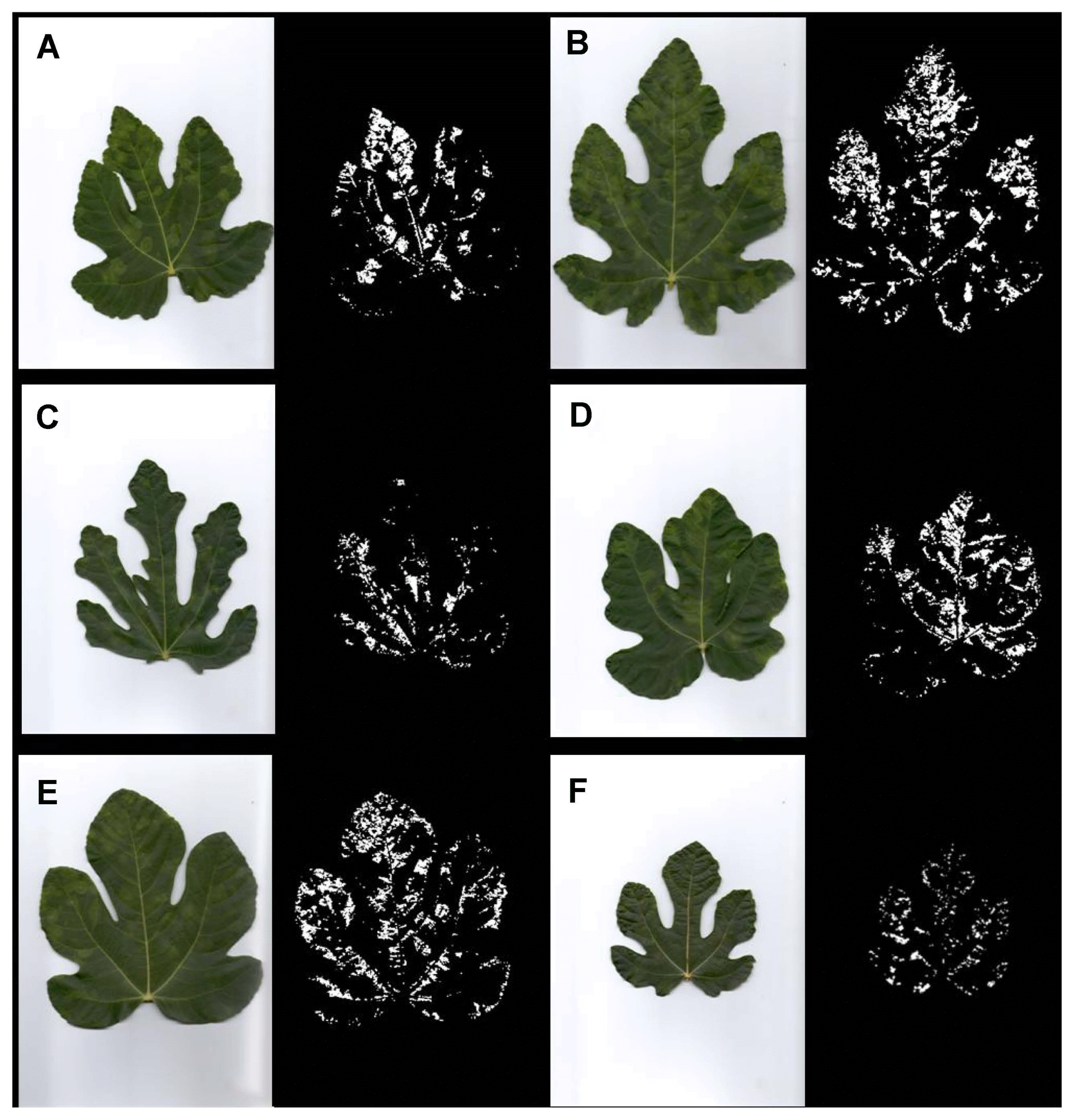
The visual disease monitoring in September 2014 revealed around 20% disease incidence, depending on the variety. The symptoms included poor tree growth with a lower yield, distorted leaves and foliar discolorations. However, symptom types varied in regard to different viruses detected in different fig varieties. Trees of all examined varieties were symptomatic except in Trojka and Sušilica where one tree and three trees were symptom-less, respectively. Out of nine fig viruses and three viroids tested, only four viruses were detected and no viroids, while at least one virus was detected in each symptomatic plant, and in four symptom-less plants (Table 2). Various symptom types in studied fig varieties are presented in Fig. 2. Symptom expressions are different among the varieties and even among the trees of each variety, probably due to particular viruses or virus combinations found. In regard to this statement, predominant symptoms noted in the study are presented in Fig. 2. In variety Izraelka there were mainly well-defined rings on the leaves; in Jesenka and Trojka most of the symptoms were chlorotic mottling and blotching, rarely mosaic; Sušilica were mainly symptom-less but some leaves showed mild fig mosaic symptoms; symptoms of chlorotic ringspots dominated in Kalamata variety, accompanied by mottling and mosaic; the most diverse symptoms were noted in variety Sultanija such as ringspots, chlorotic mottling, blotching and mosaic, even vein banding.

Incidence of fig viruses detected in leaves of the sampled trees of different fig varieties in the orchard of Podgorica region in Montenegro and presence of symptoms
RT-PCR detection and sequencing
Testing of leaf samples for the presence of nine fig viruses and three viroids revealed the presence of four viruses: FLMaV-1, FMV, FMMaV and FBV-1. In all six fig varieties, FBV-1 was detected (out of 28 tested trees, 27 were positive for FBV-1). The results correspond with Laney et al. (2012), who demonstrated that FBV-1 is widely present in fig germplasm of the United States and furthermore, the full viral sequence can be integrated into the fig genome. The virus was the only one detected in variety Sušilica. FLMaV-1 was detected in all tested varieties (23 trees), except Sušilica. FMV was detected in 11 and FMMaV in 8 fig trees, respectively.
Most of the viruses were present in mixed infections (22 trees were infected with more than one virus). Mixed infections were as follows: seven trees had FLMaV-1 + FBV-1, seven had FLMaV-1 + FMV + FBV-1, four had FLMaV-1 + FMMaV + FBV1, and four trees were infected with all four detected viruses. FMV and FMMaV were each found only in mixed infections with at least one other virus. One symptomatic tree of variety Jesenka was positive only for FLMaV-1. In all varieties, 3–4 different viruses were detected, except variety Sušilica, where only FBV-1 was found. The highest number of positive RT-PCR signals out of total expressed as a percentage was detected in varieties Kalamata (80%), Izraelka (75%) and Sultanija (70%). In comparison, Trojka (66%), Jesenka (55%) and, especially, Sušilica (25%), show a lower number of positive signals. None of the trees had positive signals for the other tested viruses, FLV-1, FLMaV-2, FLMaV-3, FCV and FFkaV and viroids, HSVd, CEVd and ADFVd. It is worth mentioning that FLMaV-1 and FBV-1 were detected in an asymptomatic tree of variety Trojka and FBV-1 was found in three symptomless trees of Sušilica (Table 2).
For each detected virus RT-PCR amplicons obtained from a minimum of six samples representing at least one tree per infected variety (Table 2) were merged in one bulk sample and were directly sequenced from both directions in two repetitions. No differences were observed between repetitions. The sequences were submitted to GenBank as Accession No. KX397035 (FLMaV-1), KX397036 (FMV), KX397034 (FMMaV) and MG584625 (FBV-1), respectively. BLAST search of these sequences revealed sequence identities of 92, 97, 92 and 100%, for FLMaV-1, FMV, FMMaV, and FBV-1 respectively, with their closest counterparts at GenBank.
Processing of images with leaf symptoms
Data obtained in the processed leaf images, which indicate the leaf area with symptoms relative to the total leaf area, provided differences of symptom severity in regard to different viruses detected and different fig varieties.
Based on the binary images (Fig. 2), obtained as the output of the algorithm, the percentage of the leaf area with symptoms relative to the total leaf area was determined (Table 3). Accordingly, the calculated data showed that Sušilica had the lowest percentage of the leaf area with fig mosaic disease symptoms (3.3%). Varieties with moderate expression of symptoms were Jesenka, Trojka and Kalamata (10.3, 11.8 and 12.2%, respectively). Izraelka and Sultanija were the varieties with the most intensive symptom expression (15.9 and 16.3%, respectively).
Discussion
FMD, a complex disease associated with mixed virus infections, has been found worldwide. However, the association of particular viruses or virus combinations with FMD symptoms is still not clear, except for FMV, which has been recognized as the principal causal agent of FMD (Elbeaino et al., 2009, 2012).
Among nine viruses and three viroids tested that could be associated with FMD, the study confirmed the presence of four viruses: FLMaV-1, FMV, FMMaV and FBV-1. The results corroborate those reported by Perović et al. (2016) and Delić et al. (2017), on diseased figs in Montenegro, but also with results obtained in other Mediterranean countries. Perović et al. (2016) detected FLMaV-1, FMV and FMMaV in Montenegro during a survey of two localities–Podgorica (central part of Montenegro) and Bar (southern part of Montenegro). However, in that study, FMMaV was found only in Bar, in mixed infection with two other viruses, whereas, in our research, FMMaV has been identified in Podgorica, thereby confirming the presence of the virus in this Montenegrin locality as well. Delić et al. (2017) noted the presence of FBV-1, FLMaV-1, FMV, FMMaV and FFkaV in fig leaf samples collected from Bar and Podgorica. In comparison with these findings, in our study, the most widespread virus was FBV-1, followed by FLMaV-1, FMV and FMMaV, in agreement with the results published by Delić et al. (2017). Therefore, it can be summarized that so far five fig viruses have been found - FBV-1, FLMaV-1, FMV, FMMaV and FFkaV - among nine viruses and three viroids tested in Montenegro.
In other countries there are several similar findings. In Bosnia and Herzegovina out of eight viruses tested, FBV-1, FLMaV-1, FMV, FMMaV, FFkaV and FLMaV-2 were detected (Delić et al., 2017). In Egypt Elbeshehy and Elbeaino (2011) assessed the presence of FLMaV-1, FLMaV-2, FMMaV and FMV and detected all of them. Elbeaino et al. (2012) studied the presence of FLMaV-1, FLMaV-2, FMMaV, FMV, FLV-1, FCV, FFkaV and HSVd in Syria and determined that FMV was the prevailing virus, whereas FCV was not found. In Saudi Arabia, mixed infection of FLMaV-1 and FMV has been found while FLMaV-2 has not been detected (Alhudaib, 2012). In Lebanon the presence of FLMaV-1 and FLMaV-2 was studied and both viruses were detected (Elbeaino et al., 2007a).
FMV is widely distributed on fig trees. It is found as a rule in symptomatic plants, causing mosaic symptoms on fig leaves (Elbeaino et al., 2009). In our study, FMV is present in all tested varieties except in Sušilica and always in mixed infection with FLMaV-1 and FBV-1, sometimes even with FMMaV. Its incidence was the highest in Izraelka, followed by Sultanija and Kalamata. It is always detected in symptomatic trees with various symptoms (probably due to mixed infection), mostly related with mosaic pattern and well-defined rings. The pathogenicity of other viruses found in plants with FMD is still unclear. FLMaV-1 is the first closterovirus found to be associated with FMD (Elbeaino et al., 2006). According to the authors, it occurs predominantly in symptomatic trees, but also in trees with no apparent symptoms, and the virus has failed to be transmitted mechanically to different inoculated herbaceous plants. Similarly, in our findings FLMaV-1 was mostly detected in symptomatic trees with leaves showing chlorotic mottling and blotches; However, a tree of variety Trojka, with confirmed presence of FLMaV-1 and FBV-1 showed no symptoms. According to Alhudaib (2012) who studied the incidence of FLMaV-1 and FMV in Saudi Arabia, absence of symptoms can be explained by high temperature that plays role in the development of these viruses.
Elbeaino et al. (2010) were the first to describe FMMaV as a novel closterovirus infecting fig. They stated that FMMaV is found mostly in mixed infections, and attempts at mechanical transmission to various test plants have been unsuccessful. In our study FMMaV is found always in mixed infection and it was the virus with the lowest incidence. It was not detected in varieties Sušilica and Izraelka. Elbeaino et al. (2010) suggested that singly FMMaVinfected plants show light mottling symptom, which cannot be confirmed in our study since the virus was present always in combination with at least two other viruses.
Regarding FBV-1, in our study, the virus was widespread, appearing in 96.4% of the samples (it was absent from only 1 of 28 trees). The wide distribution of FBV-1 in fig has already been revealed by Laney et al. (2012), in the United States, in a large number of trees of diverse origins and at a 98% detection rate. A similar situation was noticed in New Zealand, where Minafra et al. (2012a) detected the virus in 100% of the samples analysed. As stated by Minafra et al. (2012b), FBV-1 has been identified in numerous countries, including a fig sample from Montenegro. Delić et al. (2017) also found FBV-1 as the most prevailing with an infection rate of 100%. A lower percentage (48.4%) of infection by FBV-1 was mentioned by Mijit et al. (2017), in China, but, even then, the virus was the most abundant among the tested fig samples. Concerning the relation of FBV-1 presence and appropriate symptom induction, it can be concluded that the virus was found in our study both in symptomatic and asymptomatic trees of variety Sušilica as the only virus detected. In symptomatic trees, mild mosaic symptoms were noticed. These findings are in accordance with results of Laney et al. (2012), who connected these findings with the distinct possibility that FBV-1 infection alters the internal physiology of the host as has been previously shown with FLMaV-1. The same authors stated that FMD is more complex than originally thought and symptoms may not only be caused by FMV but also by mixed virus infections. Similarly, Martin et al. (2013) reported that Blackberry yellow vein disease is caused by a complex of various viruses and symptom severity is closely associated with the number of viruses infecting plants. It seems that FMD has similar etiology and that FMV is not absolutely required for induction of FMD.
Literature data on differences in susceptibility of fig varieties to FMD is quite limited. Elbeshehy and Elbeaino (2011) reported that FLMaV-1 was the prevailing virus in Egypt (similar to our research) and its incidence was particularly high in cv. Sultany (Sultanija). The same authors commented that there could be differences in the biological response of certain fig varieties to FMV infection. El Air et al. (2015) observed that variety Soltani (Sultanija) belongs to the fig varieties with the highest infection rates, which is confirmed in this study as well.
Therefore, the obtained results indicate that there could be important differences in susceptibility to viral infections among various fig varieties. However, in future research susceptibility must be confirmed by using single and mixed virus inoculation tests which is particularly important for variety Sušilica where we have found only FBV-1 infection.
The reason for the presence of these four viruses infecting fig trees in the orchard is likely because the orchard was set up by cuttings obtained from fig trees located elsewhere in Montenegro. The issue regarding the use of cuttings to establish new orchards was also commented by Bandelj et al. (2009). In the study, the authors suggested introducing micropropagation protocols into the production of planting material and protocols for virus elimination.
In conclusion, FMD was noticed on leaves in all studied fig varieties in Montenegro but with different intensity. After RT-PCR and sequencing, among the nine viruses and three viroids tested, confirmation was obtained for the presence of four viruses: FLMaV-1, FMV, FMMaV and FBV-1. FBV-1 was the most frequent, and it was detected in all the studied fig varieties. The second most frequent was FLMaV-1, which was found in all tested varieties, except Sušilica. In comparison, FMV and FMMaV occurred in fig trees at a lower incidence. Most of the viruses were present in mixed infections. Based on processed binary images of the leaf area with symptoms, there were differences in susceptibility of the fig varieties: Sultanija showed the highest susceptibility (all four viruses detected) while Sušilica had the smallest leaf area with symptoms (only one virus detected). Given that fig planting material is obtained by cuttings, it is necessary to improve its health condition by implementing certification program which will include virus and viroid testing, sanitary selection, vector control and susceptibility testing of fig varieties.
Acknowledgements
This research was supported by the bilateral project between Slovenia and Montenegro “Forecasting and detection of grapevine and fruit tree pathogens”, and the BIOICT Centre of Excellence Montenegro.