 |
 |
| Plant Pathol J > Volume 35(4); 2019 > Article |
Abstract
In our previous study, pyrrolnitrin produced in Pseudomonas chlororaphis G05 plays more critical role in suppression of mycelial growth of some fungal pathogens that cause plant diseases in agriculture. Although some regulators for pyrrolnitrin biosynthesis were identified, the pyrrolnitrin regulation pathway was not fully constructed. During our screening novel regulator candidates, we obtained a white conjugant G05W02 while transposon mutagenesis was carried out between a fusion mutant G05ΔphzΔprn::lacZ and E. coli S17-1 (pUT/mini-Tn5Kan). By cloning and sequencing of the transposon-flanking DNA fragment, we found that a vfr gene in the conjugant G05W02 was disrupted with mini-Tn5Kan. In one other previous study on P. fluorescens, however, it was reported that the deletion of the vfr caused increased production of pyrrolnitrin and other antifungal metabolites. To confirm its regulatory function, we constructed the vfr-knockout mutant G05Δvfr and G05ΔphzΔprn::lacZΔvfr. By quantifying β-galactosidase activities, we found that deletion of the vfr decreased the prn operon expression dramatically. Meanwhile, by quantifying pyrrolnitrin production in the mutant G05Δvfr, we found that deficiency of the Vfr caused decreased pyrrolnitrin production. However, production of phenazine-1-carboxylic acid was same to that in the wild-type strain G05. Taken together, Vfr is required for pyrrolnitrin but not for phenazine-1-carboxylic acid biosynthesis in P. chlororaphis G05.
Now, some soil-borne fungal pathogens often cause diseases that lead to heavy yield losses in agriculture (Haas and Keel, 2003). Although some fungicides are effectively employed in protecting crops, their intensive applications are not permitted duo to concern for the environment and public health (Chen et al., 2018; D’Mello et al., 1998). Therefore, more and more fluorescent Pseudomonas sp. are paid great attention because they can alleviate plant diseases and increase crop productivity (Baehler et al., 2005; Haas and Defago, 2005; Haas and Keel, 2003). Pseudomonas chlororaphis G05 is a root-colonizing biocontrol agent that bioprotects some plants from the diseases caused by fungal phytopathogens, such as Fusarium oxysporum, Rhizoctonia solani, and F. graminearum (Chi et al., 2017; Ge et al., 2008; Huang et al., 2018). It has been demonstrated that antifungal compounds, phenazine-1-carboxylic acid and pyrrolnitrin that are produced in this bacterium, mainly contribute to suppression of mycelial growth of these phytopathogenic fungi (Chi et al., 2017; Huang et al., 2018). Up to date, besides phenazines and pyrrolnitrin, more and more antifungal compounds, including pyoleuteorin (PLT), hydrogen cyanide (HCN), 2,4-diacetylphloroglucinol (DAPG), lipopeptides, furanomycin and so on, have been identified in Pseudomonads’ strains and exhibited a remarkable biocontrol ability (Fenton et al., 1992; Ge et al., 2004; Laville et al., 1992; Mavrodi et al., 1998; Thomashow and Weller, 1988; Trippe et al., 2013; Voisard et al., 1989).
In our previous study, we found that pyrrolnitrin played a more essential role than phenazines in growth suppression of F. graminearum and bioprotection of wheat crops against Fusarium head blight (FHB) disease (Huang et al., 2018). The production of pyrrolnitrin, however, is not high in the wild-type strain G05. Therefore, to increase pyrrolnitrin production and expand its application in agriculture, we should screen and identify more novel regulators and create regulatory pathway of pyrrolnitrin in detail. In P. chlororaphis PA23, ANR and PtrA were identified to mediate pyrrolnitrin production (Nandi et al., 2016; Shah et al., 2016). In P. fluorescens FD6, RetS and Vfr were reported to regulate pyrrolnitrin biosynthesis (Zhang et al., 2015, 2016). In P. chlororaphis O6, RpoS and GacS deficiency could change the production of pyrrolnitrin (Oh et al., 2013; Park et al., 2018). Although pyrrolnitrin can be biosynthesized in many different genera of bacteria and some regulators that mediate its biosynthesis have been identified, its regulatory pathway in detail is not fully made clear. To identify more novel regulatory candidate genes involving in pyrrolnitrin biosynthesis, in our study with P. chlororaphis G05, We first constructed the fusion mutant G05ΔphzΔprn::lacZ (Luo et al., 2018). In this mutant, the phz operon (phz-ABCDEFG, phenazine biosynthetic loci) was knocked out and the prn operon (prnABCD, pyrrolnitrin biosynthetic loci) was deleted and its promoter zone was in-frame fused with the truncated lacZ reporter gene (Minton, 1984). With the fusion mutant G05ΔphzΔprn::lacZ as recipient cell, conjugation mating was then carried out with random insertion of transposonMini-Tn5Kan (de Lorenzo et al., 1990). One white colony was fortunately found and isolated in an LB agar plate supplemented with 5-bromo-4-chloro-3-indolyl β-D-galactopyranoside (X-gal). By inverse PCR, we cloned and identified the site of transposon insertion. vfr, a novel candidate gene mediating the pyrrolnitrin biosynthesis was then identified. In this study, we confirmed that vfr was indeed required for pyrrolnitrin, but not for phenazine-1-carboxylic acid biosynthesis in P. chlororaphis G05.
All strains and plasmids employed in this work are listed in Table 1. All oligonucleotide primers used for regular PCRs or RT-qPCRs in this study are showed in Table 2. Escherichia coli strains were routinely cultivated in Luria-Bertani (LB) medium at 37°C (Sambrook and Russell, 2001). P. chlororaphis strains were regularly grown in LB medium at 30°C (Ge et al., 2008), or in glycerol-alanine medium (GA) at 30°C for phenazine assays (Chieda et al., 2005). If required, ampicillin (Amp, 100 μg/ml), chloramphenicol (Chl, 30 μg/ml), spectinomycin (Spe, 100 μg/ml), kanamycin (Kan, 50 μg/ml), and gentamicin (Gen, 20 μg/ml) were supplemented in medium for E. coli growth. For P. chlororaphis growth, tetracycline (Tet, 125 μg/ml), gentamicin (40 μg/ml) were used in its medium.
Standard techniques were employed for gel electrophoresis, restriction endonuclease digestion, and ligation (Sambrook and Russell, 2001). Plasmid DNA isolation from E. coli and P. chlororaphis strains was carried out with alkaline lysis method or with the recommended protocols provided by Plasmid DNA Extraction Kit (Sangon, Shanghai, China). Chromosomal DNA was isolated from P. chlororaphis using the Genomic DNA Extraction Kit (Solarbio, Beijing, China) or the regular method as described by Chen and Kuo (1993). Regular PCR amplifications were carried out with a 25 μl reaction mixture containing 1 × LA with GC buffer, 2 mM MgSO4, 200 μM (each) dATP, dGTP, dCTP, and dTTP, 10 pmol of each primer, 0.2 μl LA DNA polymerase (Takara Bio, Dalian, China), and 10 ng of purified genomic DNA of the strain G05 or its derivative mutants. All the amplifications were performed in T100TM thermal cycler (Bio-Rad Laboratory, Hercules, CA, USA). The cycling program started with a 2-min pre-denaturation at 94°C, followed by 33 cycles (30-sec denaturation at 94°C, 30 s anneal at 60-66°C, 2-min extension at 72°C), and ended with 7-min final extension at 72°C. PCR amplicons were routinely purified using PCR Purification Kit (Sangon, Shanghai, China). To do transformation, P. chlororaphis competent cells were first prepared and electroporation was then performed as described by Smith and Iglewski (1989).
Random mutagenesis was performed using transposon mini-Tn5Kan which contains a kanamycin resistance marker (de Lorenzo et al., 1990). Bacterial conjugations were carried out to introduce mini-Tn5Kan into the P. chlororaphis chromosome. Briefly, a 500 μl sample of each of two overnight cultures, E. coli S17-1(λpir)/pUT/mini-Tn5 Kan and P. chlororaphis G05ΔphzΔprn::lacZ, was harvested, washed twice with LB medium, mixed together into a 100 μl aliquot, then transferred onto a 25-mm-diameter filter (0.22 μm pore size) that was placed on the surface of an LB agar plate, and grown for at least 12 h at 30°C. The cells grown on the filter surface were then suspended in 1 ml of LB broth, diluted and spread on LB agar plates that contained Kanamycin, chloramphenicol, and X-gal. Plates were kept in an incubator at 30°C till blue colonies developed. A white colony named G05W02 developed around many blue colonies after 3 days of growth, and was then isolated.
To identify the localization of transposon insertion, chromosomal DNA from the white conjugant G05W02 was isolated, digested with SalI, purified, and self-ligated. With the purified ligation as a template, inverse PCR amplification was then carried out using a pair of primers TN5-inF and TN5-inR. The PCR amplicons were finally cloned into pUCm-T (T-vector), and sequenced using primers M13-F and M13-R.
To confirm the vfr function in P. chlororaphis G05, we constructed a vfr-defective mutant G05Δvfr using a homologous recombination strategy (Hoang et al., 1998), in which the vfr DNA region was deleted and replaced with the gentamicin resistance genes (aacC1) in chromosome. Firstly, two PCR amplifications were performed with two pairs of primers (V-1F/V-1RXba and V-2FXba/V-2R), obtaining two 1.0 kb amplicons: one is a 1007 bp portion of the G05 genome upstream of the vfr; another is a 1100 bp region downstream of the vfr. Two amplicons were pooled, purified, digested with XbaI, re-purified, and finally ligated. The purified ligation was used as the template and the nested PCR was performed with a pair of primers (V-3FAcc/V-3RHin) to obtain 2.0 kb PCR products.
After simultaneous digestion with Acc65I and HindIII, the digested PCR products were cloned into the suicide plasmid pEX18Tc, resulting in pEXV (Hoang et al., 1998). Secondly, an 878 bp gentamicin resistance gene (aacC1) was purified with XbaI-digestion of pUCGm (Schweizer, 1993), then inserted into the XbaI site in pEXV to yield pEXVG. After sequence confirmation, biparental mating was carried out and the derivative pEXVG was mobilized to P. chlororaphis G05 from E. coli SM10. The potential mutant G05Δvfr was screened and isolated on LB medium plates supplemented with 10% sucrose and gentamicin, suggesting that a double-crossover event had occurred (Ge et al., 2007; Hoang et al., 1998). In addition, biparental mating was also performed between E. coli SM10/pEXV and G05ΔphzΔprn::lacZ, generating the mutant G05ΔphzΔprn::lacZΔvfr. All mutants were verified by PCR using the primers G-F/G-R and G-LF/G-LR that annealed in the gentamicin resistance cassette specifically (data now shown).
To complement the mutant G05Δvfr, pME10V was constructed as follows. The 1.0 kb DNA amplicons containing the whole vfr amplified by PCR with primers V-WFEco and V-WRXho were cleaved with EcoRI and XhoI, and then cloned into the same sites of a low-copy shuttle vector pME6010, creating pME10V (Heeb et al., 2000). After sequence confirmation, pME10V and pME6010 were respectively transformed into G05Δvfr and other derivatives for complementation assay.
Pseudomonas strains were cultivated in GA broth similarly to genomic DNA preparation. The prnA was selected for qRT-PCR analysis. Cells grown for 24 h, 48 h, and 72 h were havested. The total RNAs was isolated from cells of the strain G05 and G05Δvfr using a TRIzol reagent (Takara, Dailian, China) according to manufacturer’s instructions. The trace of genomic DNA in total RNA samples was removed with digestion using RNase-free DNase I. Reverse transcription to cDNA was performed at 42°C for 60 min using random hexamer primer with a RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific). The resulting cDNA was amplified and quantified by RT-qPCR with a ChamQTM SYBR qPCR Master Mix (Vazyme) on ABI Q6 Flex PCR system. The rpoD gene was used as a reference (Liu et al., 2018; Mulet et al., 2009). The primers RT-prnAF/RT-prnAR were designed to amplify 125-bp DNA fragment in prnA. The qPCR amplifications were carried out at 95°C for 30 s, followed by 40 cycles of 95°C for 10 s, and 58°C for 30 s, and a final dissociation curve analysis step from 58 to 95°C. The transcriptional level of prnA between G05 and G05Δvfr was compared by the 2−ΔΔCt method (Livak and Schmittgen, 2001).
To quantify phenazine-1-carboxylic acid, the wild-type strain G05 and its derivatives were respectively inoculated in 150 ml GA broth at 30°C for 72 h. Samples of each cultures were collected and quantified once every 12 h. Samples were prepared with previously established methods and PCA was quantified spectrophotometrically at 252 nm (Cui et al., 2016; Kim, 2000).
To quantify pyrrolnitrin, bacterial strains were cultivated with same methods above. Samples were prepared with previously created methods (Huang et al., 2018). Pyrrolnitrin quantified by high performance liquid chromatography (HPLC) with reverse phase C18 column (Ovadis et al., 2004). Standard sample of pyrrolnitrin was purchased from Sigma-Aldrich (St. Louis, MO, the U.S.A.).
For β-galactosidase enzyme assay, the wild-type strain G05 and its derivative were grown in 150 ml of GA or LB medium at 30°C. Samples were harvested after a specified period of growth. After treated with SDS and chloroform in appropriate amounts, β-galactosidase activities were released and quantified with standard methods (Miller, 1972).
All statistical data in this work were analyzed and processed with an analysis of variance test (ANOVA) or a two-tailed paired Student t-test using the statistical software package SPSS (Chicago, IL, USA), and Duncan’s multiple range test was employed for means separation of antifungal compound production and β-galactosidase activities. Values of P < 0.05 were considered statistically significant, and values of P < 0.01 were extremely significant.
To identify more novel regulators that modulate the prn expression, mini-Tn5-mediated mutagenesis was carried out between E. coli and P. chlororaphis G05ΔphzΔprn::lacZ. In an LB medium plate containing X-gal and kanamycin, a white colony, called G05W02, was screened and picked up. To confirm its color change and mutagenesis, we streaked it in another X-gal-supplemented LB medium plate again, using its parental strain G05ΔphzΔprn::lacZ as a control. As shown in Fig. 1A, the exconjugant G05W02 totally differed from its parental strain G05ΔphzΔprn::lacZ with white color. Meanwhile, we quantified its β-galactosidase activities while it grown in GA medium for 72 h. As shown in Fig. 1B, in comparison with the fusion mutant G05ΔphzΔprn::lacZ, β-galactosidase activities produced by the transposon-mediated mutant G05W02 were extremely low, suggesting that the expression of the prn operon was suppressed in this white exconjugant.
To clone the flanking DNA fragment of transposon insertion, we employed inverse-PCR to amplify and identify the transposon-disrupted gene. Before PCR, the template of the genomic DNA of the conjugant G05W02 was prepared as described in Material and methods. After inverse PCR, 3.0 kb amplicon was cloned into the pUCm-T (T-vector) and created pUCTW02 for sequencing. Sequencing results verified that the transposon mini-Tn5Kan was actually inserted the vfr gene in the conjugant G05W02. According to the sequence of the vfr, the predicted Vfr in the strain P. chlororaphis G05 contains 214 amino acid residues with a molecular mass of 24 KDa, showing closest similarity to that in the strain P. chlororaphis (99%), P. fluorescens (98%), P. aeruginosa PAO1 (83%), and E. coli K12 (63%).
To examine regulatory effects of Vfr on the expression of the prn operon, we first created the vfr-knockout mutant G05ΔphzΔprn::lacZΔvfr. As shown in Fig. 2A, the mutant G05ΔphzΔprn::lacZΔvfr turned out to be white on a LB medium plate supplemented with X-gal. As it was complemented with bearing the shuttle plasmid pME10V, the transformant could turn blue again. The transformant harboring the original plasmid pME6010, however, did not turn blue. Meanwhile, we inoculated the fusion mutant G05ΔphzΔprn::lacZ and its derivatives in GA medium, and then quantified their β-galactosidase activities. As shown in Fig. 2B, β-galactosidase activities produced in the vfr-knockout mutant G05ΔphzΔprn::lacZΔvfr were much lower than those in the parental strain G05ΔphzΔprn::lacZ. When the mutated vfr gene was complemented with introduction of pME10V, however, the transformant G05ΔphzΔprn::lacZΔvfr/pME10V produced almost same β-galactosidase activities as the parental strain G05ΔphzΔprn::lacZ did. In addition, we also found that the transformant G05ΔphzΔprn::lacZΔvfr/pME6010 expressed same β-galactosidase activities as the vfr-knockout mutant G05ΔphzΔprn::lacZΔvfr did.
These results indicated that the expression of the prn operon was indeed decreased in the absence of the vfr gene, suggesting that the expression of the prn operon requires the presence of Vfr in the wild-type strain G05.
To assess regulatory effects of Vfr on pyrrolnitrin production, we also created the mutant G05Δvfr. For quantifying their pyrrolnitrin production, the wild-type strain G05, the mutant G05Δvfr and its derivative transformants were respectively grown in GA medium. As shown in Fig. 3A, in comparison with the wild-type strain G05, the production of pyrrolnitrin in the mutant G05Δvfr was remarkably decreased. When the mutant G05Δvfr was introduced with pME10V, pyrrolnitrin produced in the transformant G05Δvfr/pME10V was almost same to that in the wild-type strain G05. The tranformant G05Δvfr/pME6010, however, looked like its parental strain G05Δvfr and produced a tiny amount of pyrrolnitrin. These results indicated that deletion of the vfr caused much less pyrrolnitrin production in P. chlororaphis G05.
In addition, we also determined phenazine-1-carboxylic acid production while they were inoculated and grown in GA medium. According to the Fig. 3B, it was shown that phenazine-1-carboxylic acid produced in the mutant G05Δvfr was same to that in the wild-type strain G05, suggesting that Vfr did not exert any effects on the biosynthesis of phenazine-1-carboxylic acid.
To further confirm the results above, we also employed the translational fusions (phzA′-′lacZ and prnA′-′lacZ) (Heeb et al., 2000; Zhang et al., 2018) and transcriptional fusions (phzA-lacZ and prnA-lacZ) (Blumer et al., 1999; Zhang et al., 2018), did transformation and quantified their β-galactosidase activities in the wild-type strain G05 and its derivative mutants. As shown in Fig. 4, β-galactosidase activities expressed by pME15N (prnA′-′lacZ) in the mutant G05Δvfr were much less than those in the wild-type strain G05. However, β-galactosidase activities expressed by pME15Z (phzA′-′lacZ) in the mutant G05Δvfr were almost same to those in the wild-type strain G05. As shown in Fig. 5, β-galactosidase activities expressed by pME22N (prnA-lacZ) in the mutant G05Δvfr were almost same to those in the wild-type strain G05. Similarly, β-galactosidase activities expressed by pME22Z (phzA-lacZ) in the mutant G05Δvfr were also same to those in the wild-type strain G05. To verify these results with direct evidences, we also carried out RT-qPCRs to check the transcription of the prnA. As shown in Fig. 6, the copies of mRNA transcribed from the prnA in the mutant G05Δvfr were almost same to those in the wild-type strain G05, confirming that there were no remarkable differences in transcriptional levels of the prn operon in the vfr-deletion mutant G05Δvfr and its parental strain G05. Taken together, no matter whether the vfr gene was mutated with the random transposon insertion or the site-directed deletion in P. chlororaphis G05, deficiency of Vfr dramatically down-regulated the prn operon expression at the posttranscriptional level, but not at the transcriptional level. Meanwhile, Vfr did not exert any regulatory effects on the phz expression.
As an important global regulator, Vfr first was identified and designated in P. aeruginosa duo to its regulatory effects on the biosynthesis of virulence factors (West et al., 1994). In fact, it is a homologue of a transcriptional regulator cyclic AMP receptor protein (Crp) in E. coli, which mediates the expression of more than 100 genes, as well as the biosynthesis of at least 60 proteins (Suh et al., 2002; Wolfgang et al., 2003). Today, a few of homologues of the Crp regulator have been identified in different bacterial genera and their many regulatory effects on virulence-associated phenotypes have been elucidated, such as iron uptake ability and virulence-host relationships (Taguchi and Ichinose, 2013). In general, Vfr is not only related tightly to the pathogenicity of some bacteria, but also plays a critical role in their infection. In one other previous study, it was reported Vfr in P. fluorescens had a negative regulation on the biosynthesis of secondary antifungal metabolites, such as pyrrolnitrin, PLT, and DAPG (Zhang et al., 2016). Knockout of the vfr gene brought increased production of antifungal compounds. Surprisingly, we happened to find that transposon insertion mutagenesis in the vfr gene in the fusion mutant G05ΔphzΔprn::lacZ led to much less β-galactosidase activities, suggesting that mutation of the vfr could inhibit the biosynthesis of pyrrolnitrin in P. chlororaphis G05. To confirm this hypothesis, we made a site-directed knockout of the vfr gene in the wild-type strain G05 and the fusion mutant G05ΔphzΔprn::lacZ. Their pyrrolnitrin production and β-galactosidase activities verified that deletion of the vfr actually suppressed the expression of the prn operon and biosynthesis of pyrrolnitrin in P. chlororaphis G05. Meanwhile, we also found that Vfr did not exert any regulatory effects on the expression of the phz operon and phenazine-1-carboxylic acid biosynthesis. This is the first report about Vfr-mediated regulation on phenazine production although phenazine biosynthesis is regulated by many well-known regulators (Bilal et al., 2017; Mavrodi et al., 2006). The fact that Vfr differentially regulates two antifungal compounds production in a strain suggests each of two secondary metabolites, pyrrlnitrin and phenazine-1-carboxylic acid, has respectively been synthesized under the control of their own specific regulatory cascade. Obviously, this differential regulation mechanism helps to keep stability of total production of antifungal compounds in the strain G05 and also is helpful in maintaining its biological control function.
Although it has been reported that Vfr could regulate a quite few of metabolites production, the regulation mechanism of Vfr has not been elucidated in detail. Using the translational and transcriptional fusions and RT-qPCR, in this study, we tried to understand whether the Vfr-mediated regulation of the prn operon occurs at the transcriptional level or the posttranscriptional level. β-Galactosidase activities and qPCR indicated that the expression of the prn operon is regulated by Vfr at the posttranscriptional level, not the transcriptional level. Based on these data, we deduced that there might be an intermediate (s) at the downstream of the Vfr-mediated regulatory cascade. This intermediate should be controlled by the Vfr, and in turn, it might directly or indirectly regulate the prn operon expression in P. chlororaphis G05. For the detailed Vfr regulation pathway, therefore, further study should be conducted later.
Acknowledgments
We thank Stephan Heeb (University of Nottingham, Nottingham, the United Kingdom) for providing plasmid pME6015 and pME6522 friendly, Stephen Perle (University of Bridgeport, the Unite States) for his language revision of this manuscript, and Yuquan Xu (Shanghai Jiaotong University, China) for sending us phenazine-1-carboxylic acid. This study was financially supported by the Natural Science Foundation of China (Grant No. 31260080 and 31571997).
Fig. 1
Characterizations of the conjugant G05W02 and its derivatives. (A) Color of colonies shown in the LB medium supplemented with X-gal. Arabic numbers from 1 to 4 stand for the wild-type strain G05, the fusion mutant G05ΔphzΔprn::lacZ, the transposon mutant G05W02, and the transformant G05W02/pME10V, respectively. (B) β-Galactosidase activities were quantified when they were grown in GA medium at 30°C for 72 h. The values from three independent experiments were presented as the average ± standard deviation. Superscript of asterisk followed the strains indicated no significant differences (P > 0.05).
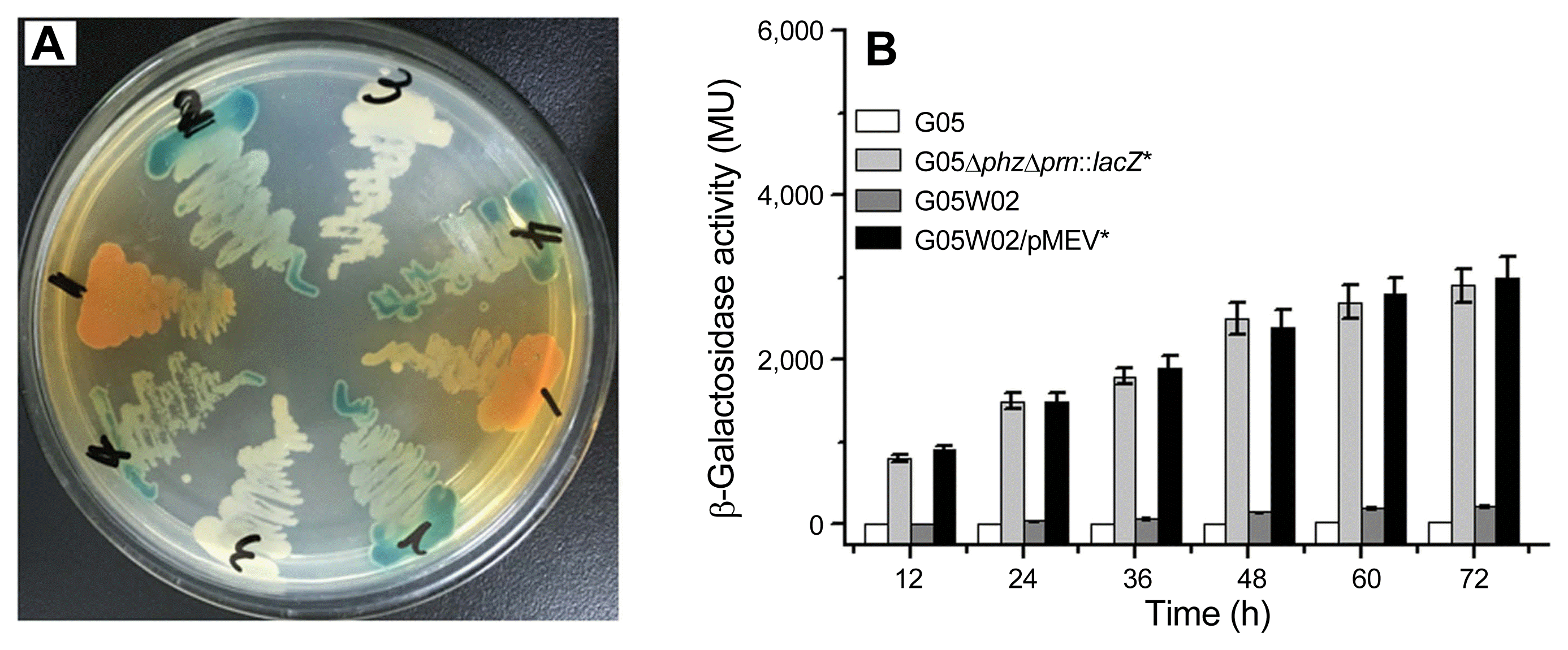
Fig. 2
Characterizations of the site-directed knockout mutant G05ΔphzΔprn::lacZΔvfr and its derivatives. (A) Color of colonies shown in the LB medium plate supplemented with X-gal. Arabic numbers from 2 to 7 stand for the fusion mutant G05ΔphzΔprn::lacZ, the vfr-knockout mutant G05ΔphzΔprn::lacZΔvfr, the transformant G05ΔphzΔprn::lacZΔvfr/pME10V, and the transformant G05ΔphzΔprn::lacZΔvfr/pME6010, respectively. (B) β-Galactosidase activities were quantified when they grown in GA medium at 30°C for 72 h. The values from three independent experiments were presented as the average ± standard deviation. Different superscript lowercase letters followed strains indicate significant difference (P < 0.05) according to duncan’s multiple range test, and different superscript uppercase letters indicate extremely significant difference (P < 0.01).
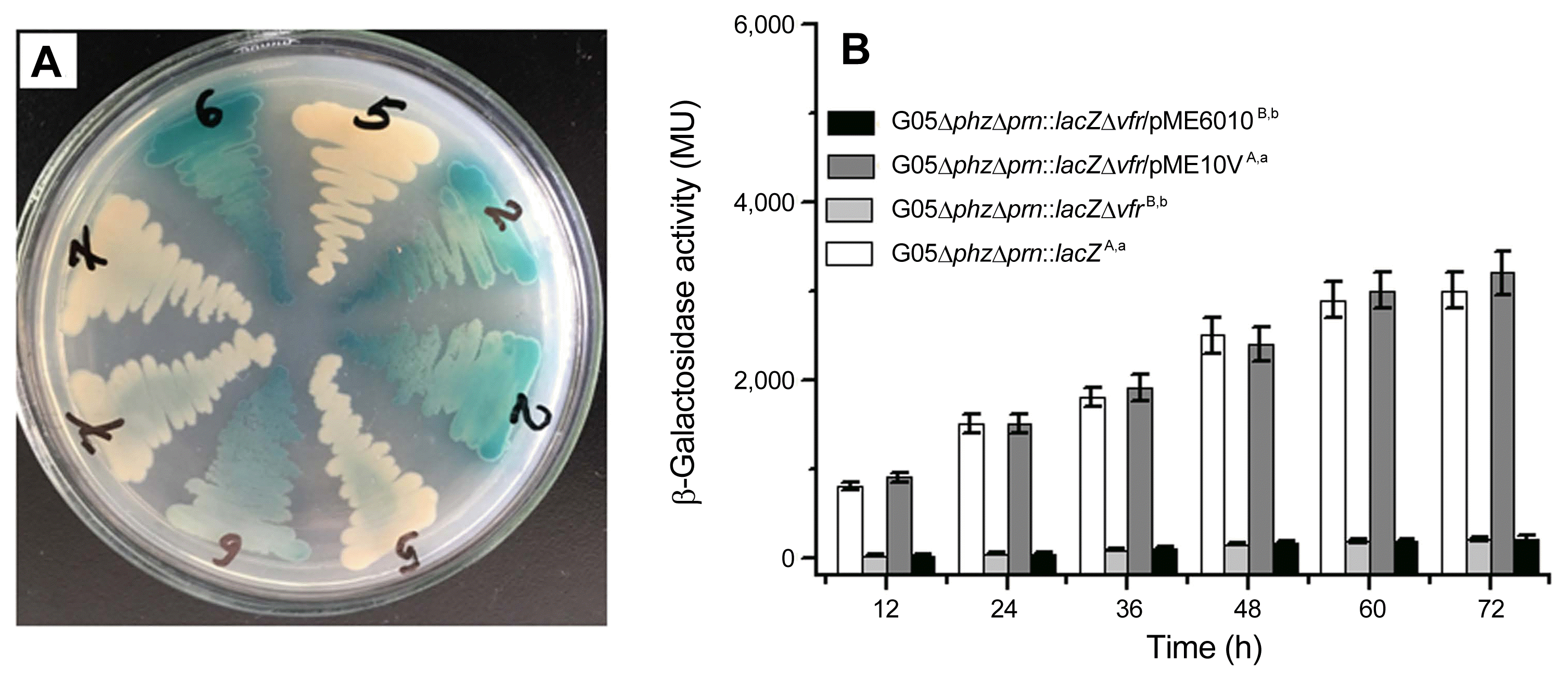
Fig. 3
Regulatory effects of deletion of the vfr on fungal metabolites production in P. chlororaphis G05. All experiments were performed in triplicate, and each value was presented as the means ± standard deviation. (A) Pyrrolnitrin produced by the wild-type strain G05 and its derivatives in GA broth. According to duncan’s multiple range test, different superscript lowercase letters followed the strains indicated significant difference (P < 0.05), and different superscript uppercase letters followed the strains indicated extremely significant difference (P < 0.01). (B) Phenazine-1-carboxylic acid produced by the wild-type strain G05 and its derivatives in GA broth. Asterisks at top of columns mean no significant difference (P > 0.05).
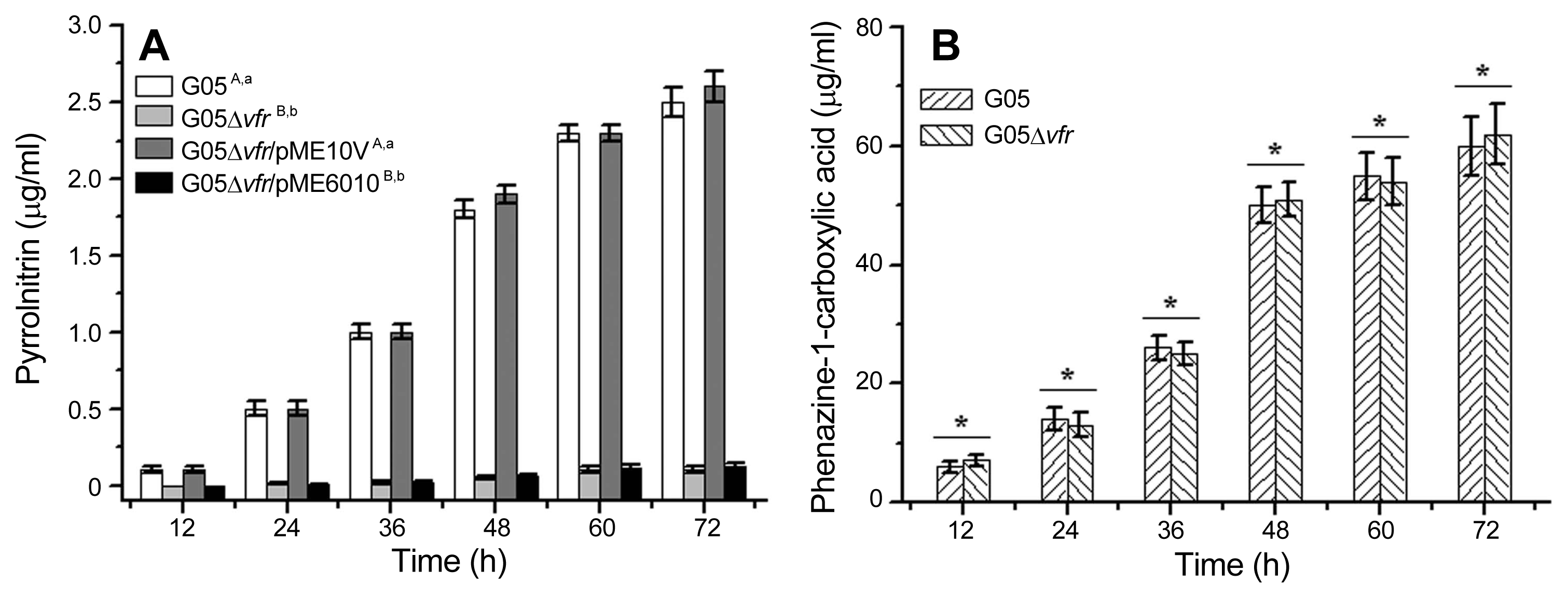
Fig. 4
Translational lacZ fusion vectors pME15Z and pME15N were employed to examine Vfr regulation in P. chlororaphis G05. (A) β-Galactosidase activities produced by pME15N in the wild-type strain G05 and the mutant G05Δvfr were quantified. The transformants G05/pME6015 and G05Δvfr/pME6015 were used as negative controls. (B) β-Galactosidase activities produced by pME15Z in the wild-type strain G05 and the mutant G05Δvfr were quantified. The transformants G05/pME6015 and G05Δvfr/pME6015 were used as negative controls. All experiments were performed in triplicate, and each value was presented as the means ± standard deviation. Asterisks at top of columns mean no significant difference (P > 0.05).

Fig. 5
Translational lacZ fusion vectors pME22Z and pME22N were employed to examine Vfr regulation in P. chlororaphis G05. (A) β-Galactosidase activities produced by pME22Z in the wild-type strain G05 and the mutant G05Δvfr were quantified. The transformant G05/pME6522 and G05Δvfr/pME6522 were used as negative controls. (B) β-Galactosidase activities produced by pME22N in the wild-type strain G05 and the mutant G05Δvfr were quantified. The transformant G05/pME6522 and G05Δvfr/pME6522 were used as negative controls. All experiments were performed in triplicate, and each value was presented as the means ± standard deviation. Asterisks at top of columns mean no significant difference (P > 0.05).
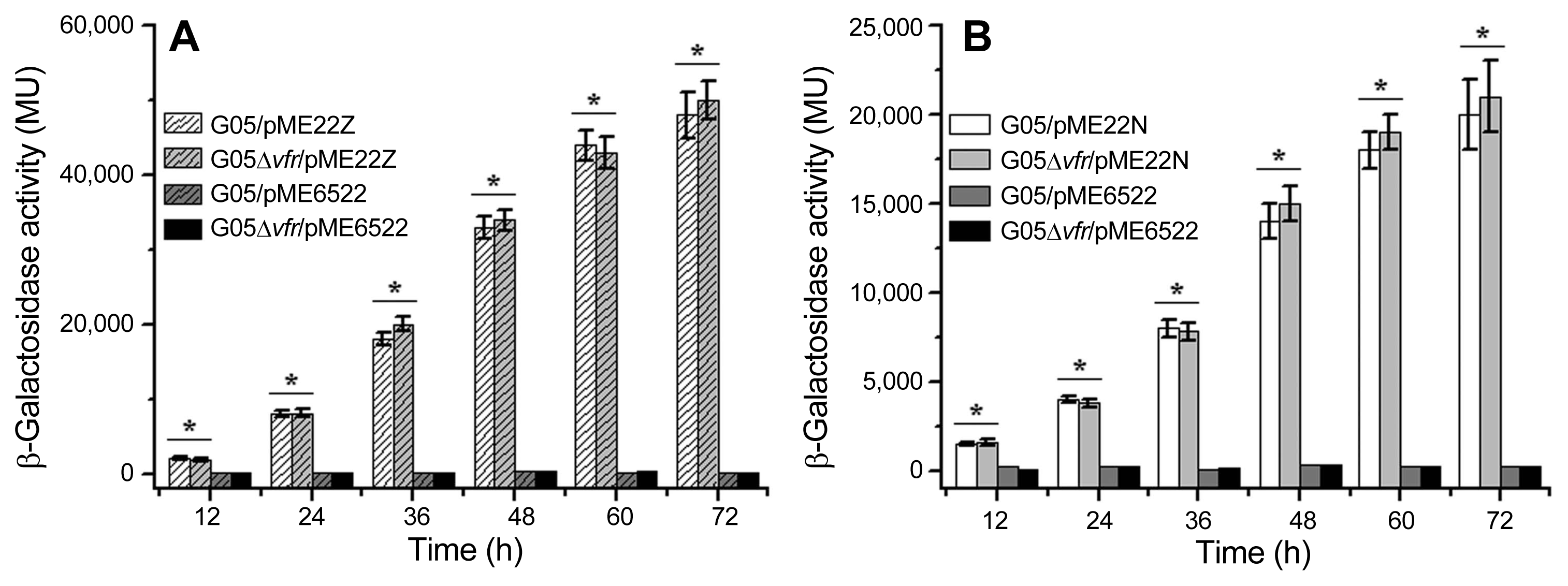
Fig. 6
Gene expression of prnA by RT-qPCR assay in P. chlororaphis G05 and its derivative mutant G05Δvfr. Expression level of the tested prnA in the wild-type strain G05 was considered 1. Relative expressions of prnA in the mutant G05Δvfr compared to the wild-type strain G05 grown in GA medium for 24 h, 48 h, and 72 h were determined by the 2−ΔΔCT method. Asterisks at top of columns mean no significant difference (P > 0.05).
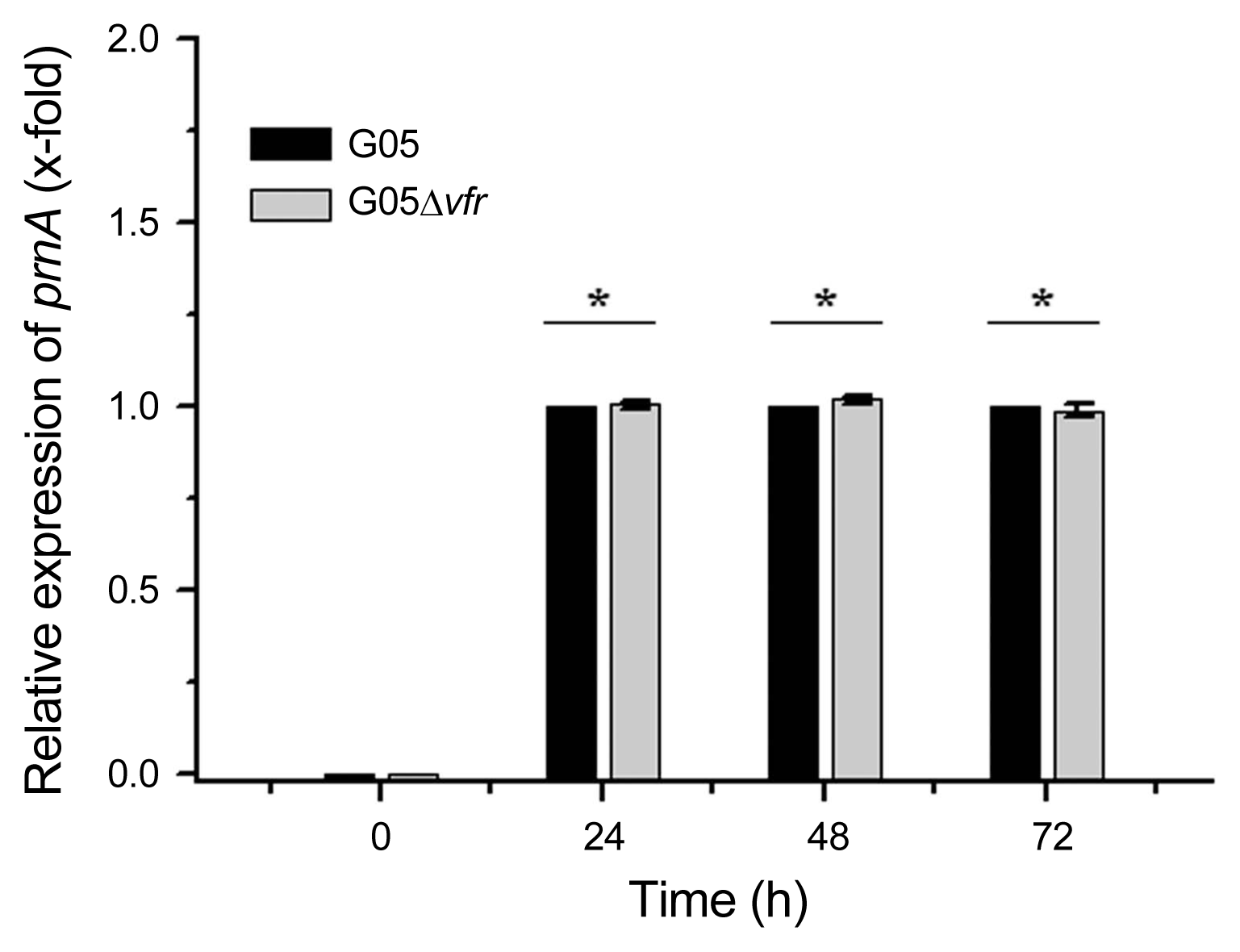
Table 1
Bacterial strains and plasmids used in this study
| Strain and plasmid | Relevant characteristics | Source/reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5α | Φ80 lacZΔM15 Δ (lacZYA-argF) U169 hsdR17 recA1endA1 thi−1 | Lab collection |
| SM10 (λvfr) | F− thi−1 thr−1 leuB6 recA tonA21 lacY1 supE44 (MuC+) λpir KanR | Lab collection |
| P. chlororaphis | ||
| G05 | The wild-type strain, phenazine-1-carboxylic acid and pyrrolnitrin producer, PCA+, PRN+, SpeR | Lab collection |
| G05ΔphzΔprn::lacZ | The phzABCDEFG and prnABCD operons deleted and the prnA' fused with the truncated lacZ gene in frame in the wild-type strain G05, SpeRGenR | Luo et al., 2018 |
| G05Δvfr | The vfr deleted and inserted with gentamicin resistance cassette in the wild-type strain G05, SpeRGenR | This study |
| G05W02 | A white conjugant isolated on LB plates by transposon random insertion on the chromosome of the fusion mutant G05ΔphzΔprn::lacZ, SpeRKanR | This study |
| G05ΔphzΔprn::lacZΔvfr | The vfr deleted in the fusion mutant G05ΔphzΔprn::lacZ, SpeRGenR | This study |
| Plasmids | ||
| pUCm-T | T-vector, ColE, AmpR | Sangon |
| pUCTW02 | Transposon-flanking DNA fragment amplified by inverse PCR cloned into pUCm-T, AmpR | This study |
| pEX18Tc | Gene replacement vector with MCS from pUC18, oriT+ sacB+, TetR | Hoang et al., 1998 |
| pEXV | pEX18Tc containing a 2.0 kb vfr-flanking PCR fragment, TetR | This study |
| pEXVG | A 0.8 kb XbaI-digested aacC1 fragment (gentamicin resistance cassette) inserted in XbaI site in pEXV, TetRGenR | This study |
| pME6010 | Low-copy shuttle vector between E. coli and Pseudomonas spp., TetR | Heeb et al., 2000 |
| pME10V | A 1.2 kb vfr amplified by PCR cloned in pME6010, TetR | This study |
| pME6015 | Pvs1-p15A shuttle vector for translational lacZ fusion, TetR | Heeb et al., 2000 |
| pME15N | A 0.9 kb DNA DNA fragment containing the promoter region and the first 10 condon of prnA cloned in pME6015, TetR | Zhang et al., 2018 |
| pME15Z | A 0.9 kb DNA DNA fragment containing the promoter region and the first 8 condon of phzA cloned in pME6015, TetR | Zhang et al., 2018 |
| pME6522 | pVS1-p15A shuttle vector for transcriptional lacZ fusion and promoter probing, TetR | Blumer et al., 1999 |
| pME22N | pME6522 carrying a 0.8 kb upstream region of prn (promoter region) and transcriptional fusion prnA-lacZ, TetR | Zhang et al., 2018 |
| pME22Z | pME6522 carrying a 0.8 kb upstream region of phz (promoter region) and transcriptional fusion phzA1-lacZ, TetR | Zhang et al., 2018 |
| pUCGm | Gentamicin resistance gene cassette (aacC1) resource, cloning vector, AmpRGenR | Schweizer, 1993 |
Table 2
Oligonucleotide primers used in this study
References
Baehler, E, Bottiglieri, M, Péchy-Tarr, M, Maurhofer, M and Keel, C 2005. Use of green fluorescent protein-based reporters to monitor balanced production of antifungal compounds in the biocontrol agent Pseudomonas fluorescens CHA0. J Appl Microbiol. 99:24-38.


Bilal, M, Guo, S, Iqbal, HMN, Hu, H, Wang, W and Zhang, X 2017. Engineering Pseudomonas for phenazine biosynthesis, regulation, and biotechnological applications: a review. World J Microbiol Biotechnol. 33:191



Blumer, C, Heeb, S, Pessi, G and Haas, D 1999. Global GacA-steered control of cyanide and exoprotease production in Pseudomonas fluorescens involves specific ribosome binding sites. Proc Natl Acad Sci USA. 96:14073-14078.



Chen, WP and Kuo, TT 1993. A simple and rapid method for the preparation of Gram-negative bacterial genomic DNA. Nucleic Acids Res. 21:2260



Chen, Y, Wang, J, Yang, N, Wen, Z, Sun, X, Chai, Y and Ma, Z 2018. Wheat microbiome bacteria can reduce virulence of a plant pathogenic fungus by altering histone acetylation. Nat Commun. 9:3429



Chi, X, Wang, Y, Miao, J, Feng, Z, Zhang, H, Zhai, J, Zhang, H, Tian, L, Xue, W, Yang, T, Huang, R, Hu, X and Ge, Y 2017. Development and characterization of a fusion mutant with the truncated lacZ to screen regulatory genes for phenazine biosynthesis in Pseudomonas chlororaphis G05. Biol Control. 108:70-76.

Chieda, Y, Iiyama, K, Yasunaga-Aoki, C, Lee, JM, Kusakabe, T and Shimizu, S 2005. Pathogenicity of gacA mutant of Pseudomonas aeruginosa PAO1 in the silkworm, Bombyx mori. FEMS Microbiol Lett. 244:181-186.


Cui, Q, Lv, H, Qi, Z, Jiang, B, Xiao, B, Liu, L, Ge, Y and Hu, X 2016. Cross-regulation between the phz1 and phz2 operons maintains a balanced level of phenazine biosynthesis in Pseudomonas aeruginosa PAO1. PLoS One. 11:e0144447



de Lorenzo, V, Herrero, M, Jakubzik, U and Timmis, KN 1990. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J Bacteriol. 172:6568-6572.



D’Mello, JPF, Macdonald, AMC, Postel, D, Dijksma, WTP, Dujardin, A and Placinta, CM 1998. Pesticide use and mycotoxin production in Fusarium and Aspergillus phytopathogens. Eur J Plant Pathol. 104:741-751.
Fenton, AM, Stephens, PM, Crowley, J, O’Callaghan, M and O’Gara, F 1992. Exploitation of gene(s) involved in 2,4-diacetylphloroglucinol biosynthesis to confer a new biocontrol capability to a Pseudomonas strain. Appl Environ Microbiol. 58:3873-3878.



Ge, Y, Huang, X, Wang, S, Zhang, X and Xu, Y 2004. Phenazine-1-carboxylic acid is negatively regulated and pyoluteorin positively regulated by gacA in Pseudomonas sp. M18. FEMS Microbiol Lett. 237:41-47.


Ge, Y, Yang, S, Fang, Y, Yang, R, Mou, D, Cui, J and Wen, L 2007. RpoS as an intermediate in RsmA-dependent regulation of secondary antifungal metabolites biosynthesis in Pseudomonas sp M18. FEMS Microbiol Lett. 268:81-87.


Ge, Y, Chen, L, Wang, L, Su, H, Zhou, J and Cheng, X 2008 Effects of insertional inactivation of gacS gene on two secondary metabolites in Pseudomonas chlororaphis G05. Acta Microbiol Sinica. 48:1595-1601 (in Chinese)..
Haas, D and Défago, G 2005. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat Rev Microbiol. 3:307-319.


Haas, D and Keel, C 2003. Regulation of antibiotic production in root-colonizing Pseudomonas spp. and relevance for biological control of plant disease. Annu Rev Phytopathol. 41:117-153.


Heeb, S, Itoh, Y, Nishijyo, T, Schnider, U, Keel, C, Wade, J, Walsh, U, O’Gara, F and Haas, D 2000. Small, stable shuttle vectors based on the minimal pVS1 replicon for use in gram-negative, plant-associated bacteria. Mol Plant-Microbe Interact. 13:232-237.


Hoang, TT, Karkhoff-Schweizer, RR, Kutchma, AJ and Schweizer, HP 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene. 212:77-86.


Huang, R, Feng, Z, Chi, X, Sun, X, Lu, Y, Zhang, B, Lu, R, Luo, W, Wang, Y, Miao, J and Ge, Y 2018. Pyrrolnitrin is more essential than phenazines for Pseudomonas chlororaphis G05 in its suppression of Fusarium graminearum. Microbiol Res. 215:55-64.


Kim, KJ 2000. Phenazine 1-carboxylic acid resistance in phenazine 1-carboxylic acid producing Bacillus sp. B-6. J Biochem Mol Biol. 33:332-336.
Laville, J, Voisard, C, Keel, C, Maurhofer, M, Défago, G and Haas, D 1992. Global control in Pseudomonas fluorescens mediating antibiotic synthesis and suppression of black root rot of tobacco. Proc Natl Acad Sci USA. 89:1562-1566.



Liu, Y, Wang, Z, Bilal, M, Hu, H, Wang, W, Huang, X, Peng, H and Zhang, X 2018. Enhanced fluorescent siderophore biosynthesis and loss of phenazine-1-carboxamide in phenotypic variant of Pseudomonas chlororaphis HT66. Front Microbiol. 9:759



Luo, W, Miao, J, Feng, Z, Lu, R, Sun, X, Zhang, B, Ding, W, Lu, Y, Wang, Y, Chi, X and Ge, Y 2019. Construction of a β-galactosidase-gene-based fusion is convenient for screening candidate genes involved in regulation of pyrrolnitrin biosynthesis in Pseudomonas chlororaphis G05. J Gen Appl Microbiol. 64:259-268.


Mavrodi, DV, Ksenzenko, VN, Bonsall, RF, Cook, RJ, Boronin, AM and Thomashow, LS 1998. A seven-gene locus for synthesis of phenazine-1-carboxylic acid by Pseudomonas fluorescens 2-79. J Bacteriol. 180:2541-2548.



Mavrodi, DV, Blankenfeldt, W and Thomashow, LS 2006. Phenazine compounds in fluorescent Pseudomonas spp. biosynthesis and regulation. Annu Rev Phytopathol. 44:417-445.


Miller, JH 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, USA. 466.
Minton, NP 1984. Improved plasmid vectors for the isolation of translational lac gene fusions. Gene. 31:269-273.


Mulet, M, Bennasar, A, Lalucat, J and García-Valdés, E 2009. An rpoD-based PCR procedure for the identification of Pseudomonas species and for their detection in environmental samples. Mol Cell Probes. 23:140-147.


Livak, KJ and Schmittgen, TD 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-ΔΔC(T)) method. Methods. 25:402-408.


Nandi, M, Selin, C, Brawerman, G, Fernando, WG and de Kievit, TR 2016. The global regulator ANR is essential for Pseudomonas chlororaphis strain PA23 biocontrol. Microbiology. 162:2159-2169.


Oh, SA, Kim, JS, Park, JY, Han, SH, Dimkpa, C, Anderson, AJ and Kim, YC 2013. The RpoS sigma factor negatively regulates production of IAA and siderophore in a biocontrol Rhizobacterium, Pseudomonas chlororaphis O6. Plant Pathol J. 29:323-329.



Ovadis, M, Liu, X, Gavriel, S, Ismailov, Z, Chet, I and Chernin, L 2004. The global regulator genes from biocontrol strain Serratia plymuthica IC1270: cloning, sequencing, and functional studies. J Bacteriol. 186:4986-4993.



Park, JY, Kang, BR, Ryu, CM, Anderson, AJ and Kim, YC 2018. Polyamine is a critical determinant of Pseudomonas chlororaphis O6 for GacS-dependent bacterial cell growth and biocontrol capacity. Mol Plant Pathol. 19:1257-1266.


Sambrook, J and Russell, DW 2001. Molecular cloning: A laboratory manual. 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, USA. 2100.
Schweizer, HD 1993. Small broad-host-range gentamycin resistance cassettes for site-specific insertion and deletion mutagenesis. BioTechniques. 15:831-834.

Shah, N, Klaponski, N, Selin, C, Rudney, R, Fernando, WG, Belmonte, MF and de Kievit, TR 2016. PtrA is functionally intertwined with GacS in regulating the biocontrol activity of Pseudomonas chlororaphis PA23. Front Microbiol. 7:1512



Smith, AW and Iglewski, BH 1989. Transformation of Pseudomonas aeruginosa by electroporation. Nucleic Acids Res. 17:10509



Suh, SJ, Runyen-Janecky, LJ, Maleniak, TC, Hager, P, MacGregor, CH, Zielinski-Mozny, NA, Phibbs, PV and West, SE 2002. Effect of vfr mutation on global gene expression and catabolite repression control of Pseudomonas aeruginosa. Microbiology. 148:1561-1569.


Taguchi, F and Ichinose, Y 2013. Virulence factor regulator (Vfr) controls virulence-associated phenotypes in Pseudomonas syringae pv tabaci 6605 by a quorum sensing-independent mechanism. Mol Plant Pathol. 14:279-292.


Thomashow, LS and Weller, DM 1988. Role of a phenazine antibiotic from Pseudomonas fluorescens in biological control of Gaeumannomyces graminis var tritici. J Bacteriol. 170:3499-3508.



Trippe, K, McPhail, K, Armstrong, D, Azevedo, M and Banowetz, G 2013. Pseudomonas fluorescens SBW25 produces furanomycin, a non-proteinogenic amino acid with selective antimicrobial properties. BMC Microbiol. 13:111



Voisard, C, Keel, C, Haas, D and Dèfago, G 1989. Cyanide production by Pseudomonas fluorescens helps suppress black root rot of tobacco under gnotobiotic conditions. EMBO J. 8:351-358.



West, SE, Sample, AK and Runyen-Janecky, LJ 1994. The vfr gene product, required for Pseudomonas aeruginosa exotoxin A and protease production, belongs to the cyclic AMP receptor protein family. J Bacteriol. 176:7532-7542.



Wolfgang, MC, Lee, VT, Gilmore, ME and Lory, S 2003. Coordinate regulation of bacterial virulence genes by a novel adenylate cyclase-dependent signaling pathway. Dev Cell. 4:253-263.


Zhang, Q, Xiao, Q, Xu, J, Tong, Y, Wen, J, Chen, X and Wei, L 2015. Effect of retS gene on antibiotics production in Pseudomonas fluorescens FD6. Microbiol Res. 180:23-29.





 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




