Antimicrobial Cyclic Peptides for Plant Disease Control
Article information
Abstract
Antimicrobial cyclic peptides derived from microbes bind stably with target sites, have a tolerance to hydrolysis by proteases, and a favorable degradability under field conditions, which make them an attractive proposition for use as agricultural fungicides. Antimicrobial cyclic peptides are classified according to the types of bonds within the ring structure; homodetic, heterodetic, and complex cyclic peptides, which in turn reflect diverse physicochemical features. Most antimicrobial cyclic peptides affect the integrity of the cell envelope. This is achieved through direct interaction with the cell membrane or disturbance of the cell wall and membrane component biosynthesis such as chitin, glucan, and sphingolipid. These are specific and selective targets providing reliable activity and safety for non-target organisms. Synthetic cyclic peptides produced through combinatorial chemistry offer an alternative approach to develop antimicrobials for agricultural uses. Those synthesized so far have been studied for antibacterial activity, however, the recent advancements in powerful technologies now promise to provide novel antimicrobial cyclic peptides that are yet to be discovered from natural resources.
Fungal diseases of plants are responsible for major yield losses during agricultural production, and they also affect the quality and safety of fresh and processed food. Until recently the control of fungal diseases relied heavily on chemical fungicides, however, public concerns about the potential side effects on human health and the environment, has stimulated research to develop new antimicrobial agents that meet current health and safety standards (Xu and Choi, 2014; Yoon and Kim, 2013). Microbially-produced antimicrobial cyclic peptides provide much appeal for the development of fungicides (Montesinos, 2007). This is partly because the breakdown of their structure to its amino acid derivatives upon exposure to agricultural environments prevents the accumulation of compounds to potentially harmful levels. Cyclization of a linear peptide endows a considerable rigidity over its linear form. This structural change provides enhanced and stable binding with target sites, and tolerance against hydrolysis by proteases due to the absence of amino and carboxyl termini. These characteristics ensure that the cyclized molecules have an improved and more reliable activity, and that they have a suitable residual period (Edman, 1959; Horton et al., 2002; Rezai et al., 2006). Antimicrobial cyclic peptides work by targeting fundamental features of the fungal cell envelope, and such modes of action are thought to reduce the risk of resistance developing in microbial populations (Perron et al., 2006).
Antimicrobial cyclic peptides are produced as secondary metabolites composed of up to 50 amino acid residues, among which complex molecules have covalently linked fatty acid chains (lipopeptides) or other substitutions. Antimicrobial cyclic peptides of microbial origin are non-ribosomally synthesized by non-ribosomal peptide synthetases (NRPSs), utilizing both coded and non-proteinogenic amino acids. NRPSs can also incorporate specific traits into the peptide chains such as epimerization, N-methylation, and heterocyclization, increasing the functional diversity of these molecules (Hur et al., 2012).
So far, numbers of cyclic peptides with a variety of structures have been reported to be active against pathogenic fungi and bacteria. These peptides mainly targeted components in microbial cell envelope such as chitin, glucan, and sphingolipids. In addition, a diverse range of cyclic peptides with improved properties in terms of activity, specificity, biodegradability, and toxicity have recently been synthesized using combinatorial chemistry. The synthesis of new molecules coupled with high-throughput screening systems and data processing using design of experiments (DOE) methodology has generated promising results for quantitative structure-activity relationship (QSAR) models and optimized compounds (Montesinos and Bardaji, 2008).
Since several comprehensive review articles have previously discussed recent progresses in the antimicrobial peptide research (Bockus et al., 2013; De Lucca and Walsh, 1999; Montesinos, 2007; Singh et al., 2013), this mini-review now focusses on potential use of cyclic peptides as agricultural fungicides.
Structural diversity of cyclic peptides
Cyclic peptides are polypeptide chains where the amino acid residues are covalently linked to generate the ring. The ring structure can be formed by linking one end of the peptide with the other using an amide bond, or other chemically stable bonds such as lactone, ether, thioether or disulfide. Most biologically active cyclic peptides are formed by N-to-C (or head-to-tail) cyclization, side chain to C-terminus cyclization, side chains to side chain cyclization, side chain to N-terminus cyclization, or backbone to backbone cyclization (Diederich et al., 2008; Joo, 2012).
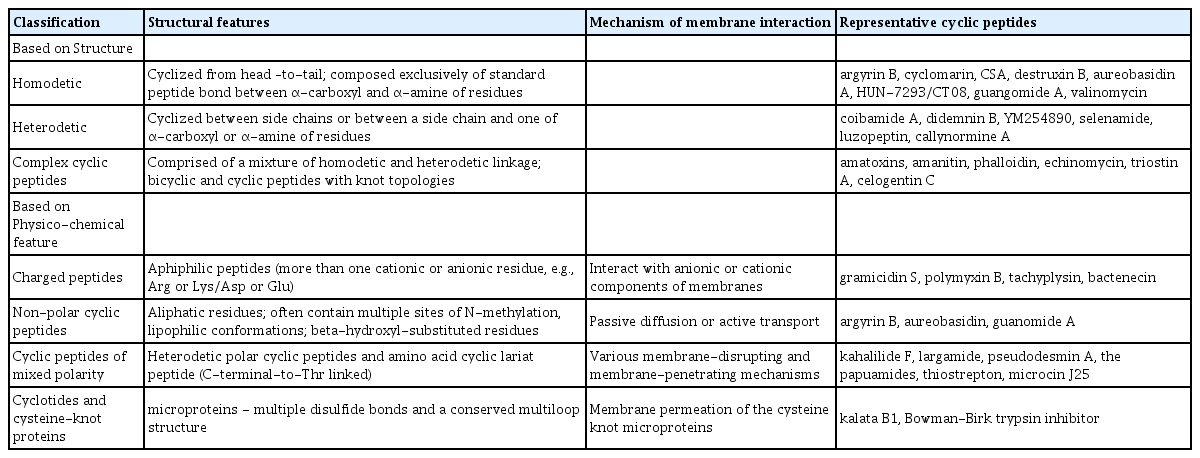
Cyclic peptides are classified according to the types of bonds within the ring. Homodetic cyclic peptides (cyclized from head-to-tail, e.g., cyclosporine A) are composed exclusively of standard peptide bonds (i.e., between the alpha carboxyl of one residue and the alpha amine of another). Heterodetic cyclic peptides are cyclized between side chains or between a side chain and one of the termini. A lactone ring is generated by cyclization of the C-terminal carboxylic acid with the side chain of serine or threonine, for example, kahalalide F, theonellapeptolide, and didemnin B (Boger et al., 1999; SirDeshpande and Toogood, 1995). Cyclization between the C-terminal carboxylic acid and the side chain of lysine or ornithine forms a lactam ring structure (e.g., microcystin and bacitracin). These are known as cyclic isopeptides because a non-alpha amide linkage is also called an isopeptide bond. The heterodetic peptides can also be capped on their amino termini with a lipid of varying length and composition. Complex cyclic peptides are comprised of a mixture of homodetic and heterodetic linkages, which include bicyclic and cyclic peptides with knot topologies. Bicyclic peptides such as the amatoxins, amanitin and phalloidin contain a bridging group, usually between two of the side chains. In the amatoxins, the bridge is formed as a thioether between the tryptophan and cysteine residues. Other bicyclic peptides include echinomycin, triostin A, and celogentin C.
Variations in amino acid residues and structural conformations of cyclic peptides produce differences in physico-chemical properties that appear to be closely related to biological function. In particular: 1) highly charged polycationic and polyanionic molecules mostly function as antimicrobial agents by disrupting bacterial membranes; 2) non-polar cyclic peptides containing mostly lipophilic side chains and modifications to the amide backbone (e.g., N-methylation) can penetrate eukaryotic cells by passive diffusion; 3) cyclic peptides with mixed polarity, most of which are amphiphilic and not limited to microbial targets (e.g., intracellular targets in mammalian cells); 4) cyclotides (cyclic cysteine-knot peptides) are 2–8 kD cyclic peptides with unusual interlocking arrangement of multiple disulfide bonds within the ring, which show good absorption in animal cells and high affinity to pharmacological targets (Table 1).
Natural cyclic peptides as a disruptor of structural integrity of microbial cell
Many antimicrobial cyclic peptides are potent disruptors of structural integrity, targeting components of the cell envelope through lysis of the membrane or inhibition of the cell wall and membrane biosynthesis (Shai, 1995). Cyclic peptides causing membrane lysis are amphipathic molecules with a positive or neutral charge, and hydrophobic moieties. Some molecules bind only to the membrane surface and disrupt its structure without traversing the membrane. Other cyclic peptides traverse membranes and interact specifically with particular membrane-bound structures (e.g., ion channels, transporters, and receptors). At the post-binding stage, cyclic peptide molecules aggregate in a selective manner, forming aqueous pores of variable sizes that allow the uncontrolled passage of ions and other solutes through the channels, ultimately leading to cell death. Several cyclic peptides can impair biosynthesis of macromolecular components of the microbial cell wall such as glucan, chitin, and mannoproteins (Debono and Gordee, 1994). The microbial cell wall is a selective target for antimicrobial agents, excluding target-related deleterious effects on host cells (Georgopapadakou and Tkacz, 1995; Gooday, 1977; Gozalbo et al., 1993).
Cyclic peptides lysing cell membrane
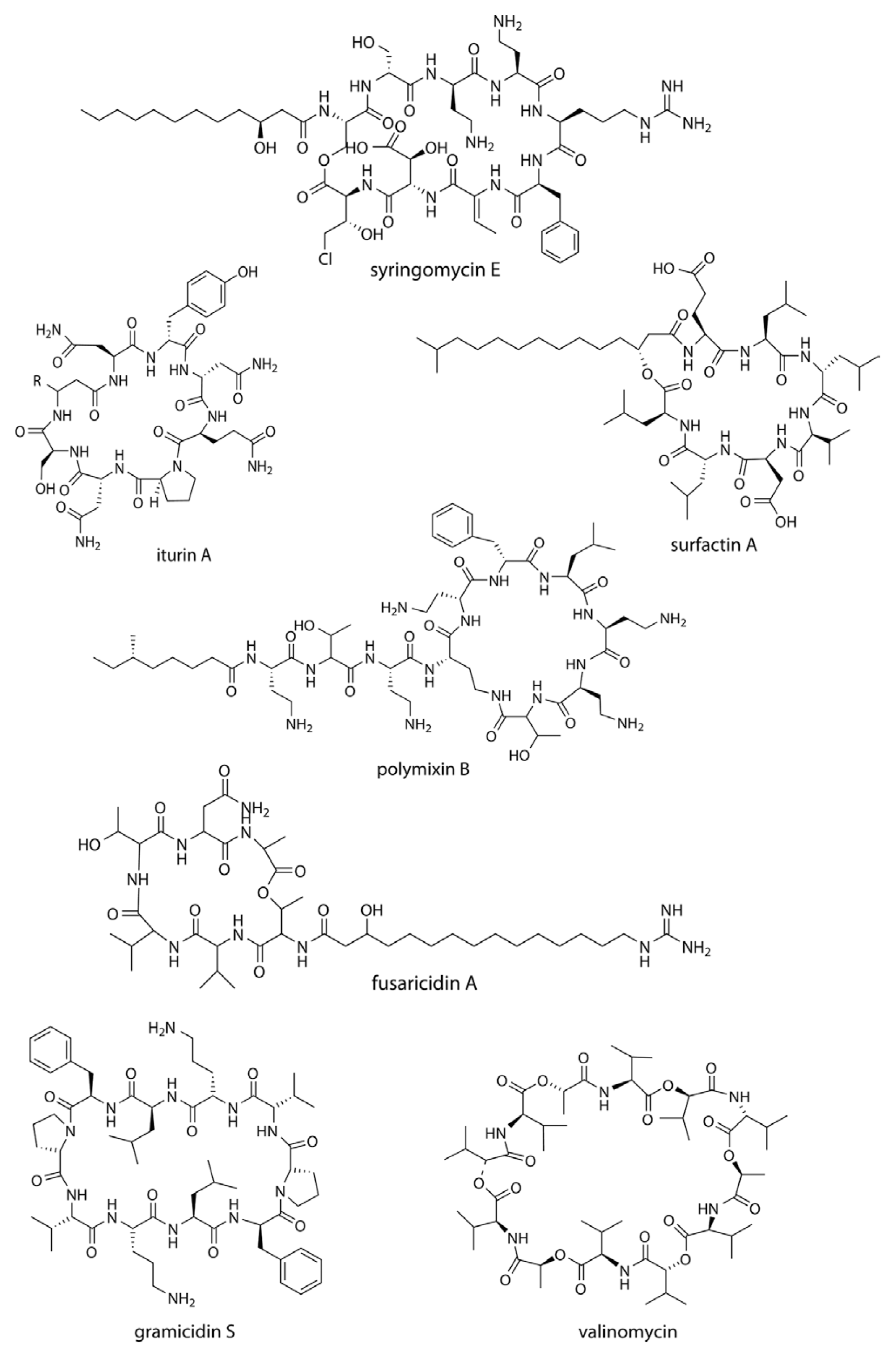
Cyclic lipopeptides (CLPs) are composed of a fatty acid tail linked to a cyclized oligopeptide, and possess antifungal, antibacterial, cytotoxic or surfactant properties. One of the modes of action of the CLPs is through membrane integration and the formation of pores, causing leakage and an imbalance of the ionic potential across the membrane, followed by cell lysis. This is typically seen in the syringomycins secreted by Pseudomonas syringae pv. syringae.
Syringomycins (Fig. 1) and syringopeptins act as virulence factors in Pseudomonas syringae, and also possess strong inhibitory activities against the growth of Gram-positive bacteria at a concentration of 18.75 μM (Grgurina et al., 2005) and mycelial growth of Botrytis cinerea at 1.56–12.5 μM (Lavermicocca et al., 1997). The syringopeptin-producing strain P. syringae 508 has antagonistic property against Venturia inaequalis, the causal agent of apple scab (Burr et al., 1996). Tolaasin D inhibits the growth of Rhizoctonia solani and the Gram-positive bacterium Rhodococcus fascians at a minimal inhibitory quantity (MIQ) of 0.16 μg (Bassarello et al., 2004). Pseudophomins A and B produced by Pseudomonas fluorescens BRG100 significantly inhibit the growth of Phoma lingam, Alternaria brassicae and Sclerotinia sclerotiorum (Pedras et al., 2003). Massetolide A is produced by P. fluorescens SS101, and possesses a biosurfactant property that affects plant pathogenic oomycetes. Massetolide A is also zoosporicidal towards Pythium intermedium (de Souza et al., 2003), where zoospores initially lose their motility before rupturing into fragments. Culture extracts of P. fluorescens SS101 can reduce the surface tension of water to 30 mN/m and attain a critical micelle concentration at 25 μg/ml. Amphisin, lokisin, tensin, and viscosinamide are produced by P. fluorescens spp., and possess in vitro antagonistic activities against the oomycete Pythium ultimum and the basidiomycete R. solani (Nielsen et al., 2002; Nielsen and Sorensen, 2003).
In addition to the pseudomonads, Bacillus spp. are major producers of CLPs. Fusaricidins, iturins, fengycins, polymixins, and agrastatins have been identified in culture extracts of several species of Bacillus (Stein, 2005). These CLPs are composed of seven (surfactins, iturins) or ten α-amino acids (fengycins) linked to one unique β-amino (iturins) or β-hydroxy (surfactins, fengycins) fatty acids. The carbon numbers of β-amino fatty acid chain of surfactins, iturins, and fengycins are C13–C16, C14–C17, and C14–C18, respectively. Multiple derivatives of each lipopeptide family are usually coproduced by a strain (Akpa et al., 2001; Leenders et al., 1999). Iturins have a potent antifungal activity against plant pathogens, affecting the surface tension of membranes, resulting in the formation of pores that allow leakage of potassium and other vital ions, and ultimately cell death (Thimon et al., 1992). Iturin production of antagonistic bacteria was related to their control efficacy as biocontrol agents. The iturin-producing strain Bacillus subtilis B-3 was able to control peach brown rot caused by Monilinia fructicola (Gueldner et al., 1988). Fengycin-type cyclic peptides produced by B. subtilis M4 inhibit the mycelial growths of Fusarium oxysporum, R. solani and B. cinerea at an MIQ of 1.85 μg, and also reduce the incidence of grey mold disease of apple fruits caused by B. cinerea during post-harvest storage (Ongena et al., 2005). B. subtilis CMB32 produces iturin A, fengycin, and surfactin A, that are each able to suppress the growth of Colletotrichum gloeosporioides, the causative agent of anthracnose in a variety of crops (Kim et al., 2010). Surfactin (Fig. 1) produced by B. subtilis RB14 suppresses damping-off by R. solani in tomato seedlings (Asaka and Shoda, 1996). Surfactin is the most powerful biosurfactant known to date, and it has strong antimicrobial activities on Candida albicans, Lactococcus lacti subsp. Lactis, Mycobacterium smegmatis, Staphylococcus aureus, and Streptococcus mutant (Peypoux et al., 1999; Singh and Cameotra, 2004). The derivatives of iturin such as bacillomycins have seven α-amino acids and a β-amino fatty acids with 14–16 C atoms, which can control a broad range of plant diseases. Podosphaera fusca, the principal cause of powdery mildew in melons is controlled by different strains of B. subtilis producing bacillomycin, fengycin or iturin (Romero et al., 2007). Derivatives of bacillomycin D produced by Bacillus amyloliquefaciens SD-32 inhibits infections of B. cinerea in cucumber leaves at concentrations of 30–80 μM (Tanaka et al., 2014), and bacillomycin F prevents the growth of phytopathogenic fungi such as B. cinerea (MIC 20 μg/ml), Mycosphaerella pinode (MIC 10 μg/ml), and Pleospora herbarum (MIC 10 μg/ml) (Mhammedi et al., 1982). Other antibiotics of the iturin group, mycosubtilin, consists of seven α-amino acids in an L-Asn-D-Tyr-D-Asn-L-Gln-L-Pro-D-Ser-L-Asn sequence closed by a β-amino acid linkage (Peypoux et al., 1986). Overproducing mycosubtilin mutants of B. subtilis ATCC6633 are more effective at controlling Pythium damping-off on tomato plants than the parent strain (Leclère et al., 2005). Paenibacillus sp. strain B2 isolated from the sorghum rhizosphere and was found to produce polymixin B and had antagonistic characteristics towards Erwinia carotovora. Polymixin B is also effective against the root pathogenic fungus Fusarium solani (2.6 μg/ml; isolated from Lascaux caves, Lot, France) and Fusarium acuminatum (8.0 μg/ml) (Selim et al., 2005). The fusaricidins are a group of cyclic depsipeptides with an unusual 15-guanidino-3-hydroxypentadecanoic acid moiety bound to a free amino group (Kajimura and Kaneda, 1997) (Fig. 1). The compound is produced by Paenibacillus polymyxa E681 and controls Phytophthora blight infections in red pepper caused by Phytophthora capsici (Lee et al., 2013). The antibiotic agrastatin A is produced by B. subtilis AQ713 and has a broad fungicidal spectrum in vitro. Agrastatin A is a potent control agent on grey mold disease of bean and geranium leaves and, early blight of tomato seedlings caused by Alternaria solani. Grape downy mildew caused by Plasmopara viticolar is also effectively controlled by agrastatin A and the efficacy is comparable with the synthetic fungicide metalaxyl (Heins et al., 2000). Gramicidin S (Fig. 1) is considered unusual because it has both antifungal activity against Fusarium nivale and sporicidal qualities towards the spores of Bacillus sp. and Dictyostelium discoideum (Murray et al., 1986). Brevibacillus brevis (formerly Bacillus brevis) synthesizes gramicidin S, and acts as a biocontrol agent against B. cinerea, the causative agent of grey mold of Chinese cabbage (Edwards and Seddon, 2001).
Cyclic peptides are also widely produced by Streptomyces spp. Valinomycin (Fig. 1) was first identified from culture extract of Streptomyces griseus. Valinomycin has insecticidal and acaricidal activities (Heisey et al., 1988) and acts as an ionophore that specifically modulates the transport of potassium ions across biological membranes. Based on these properties, valinomycin has been used as an insecticide and nematocide, and also as a key component in the instrumental measurement of potassium ions in biomedicine (Perkins et al., 1990). Antifungal property of valinomycin was subsequently identified from Streptomyces sp. M10 against B. cinerea, and its disease control efficacy was confirmed against Botrytis blight (Park et al., 2008). Antioomycete activity has also been shown against P. capsici at an IC50 value of 15.9 μg/ml (Lim et al., 2007).
Chitin synthase inhibitors
Chitin is a linear homopolymer of β-(1,4)-linked N-acetylglucosamine (GlcNAc) residues, and is a major component of fungal cell walls. It is synthesized on the cytoplasmic surface of the plasma membrane, extruded perpendicularly to the cell surface as microfibrils, and then crystallized outside the cell through extensive hydrogen bonding (the poly-GlcNAc chains run antiparallel) (Bulawa, 1993; Georgopapadakou, 1992). Polyoxins and nikkomycins are nucleoside-peptide antibiotics that are competitive inhibitors of chitin synthase, acting as analogs of the substrate UDP-GlcNAc (Isono and Suzuki, 1979). Their potency and excellent selectivity ensure they have much commercial appeal as fungicides.
Arthrichitin (Fig. 2) is produced by Arthrinium phaeospermum and is non-nucleoside type inhibitor of chitin biosynthesis. Arthrichitin has a cyclic depsipeptide structure and causes morphological abnormalities in B. cinerea in vitro. It also showed 75 and 85% disease control efficacies against Pyricularia oryzae infection in rice and B. cineria infection in cucumber at 5 mg/ml, respectively (Vijayakumar et al., 1996).
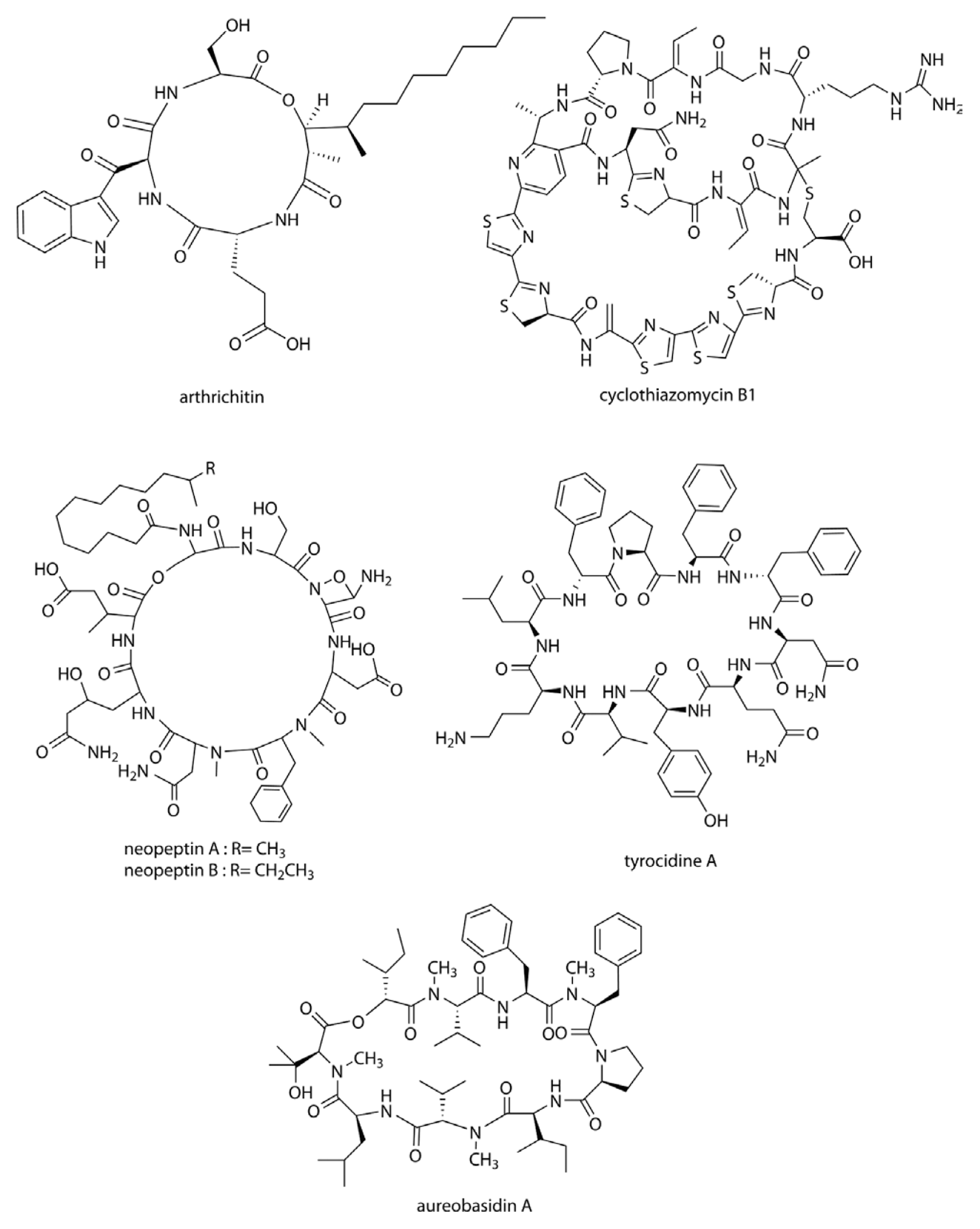
Molecular structures of antimicrobial cyclic peptides that modulate the biosynthesis of chitin, glucan, and inositol phosphorylceramide (IPC).
Thiopeptide antibiotics are an important class of natural products resulting from post-translational modifications of ribosomally synthesized peptides. Cyclothiazomycin is a typical thiopeptide antibiotic that has a unique bridged macrocyclic structure derived from an 18 amino acid structural peptide (Wang et al., 2010). Cyclothiazomycin B1 (Fig. 2) is an antifungal cyclic thiopeptide isolated from the culture broth of Streptomyces sp. HA 125-40 (Mizuhara et al., 2011). Cyclothiazomycin B1 binds to chitin in the fungal cell wall and disrupts the crystallization of the chitin chain. This mechanism inhibits the growth of filamentous fungi such as plant pathogens, and causes swelling of the hyphae and spores. Growths of Mucor and Fusarium spp. can be inhibited at low concentrations (0.020–0.33 μM) of cyclothiazomycin B1.
Cyclic peptides affecting glucan synthesis
Glucans are homopolymers of glucose arranged as long (≥60 units) coiling strings of β-(1,3)-linked residues with occasional side chains involving β-(1,6)-linkages. The enzyme β-(1,3)-glucan synthase catalyzes polymerization and consists of two functional components: a catalytic part that acts on the UDP-glucose substrate and a regulatory component that binds GTP (Arellano et al., 1996; Hartland et al., 1991). The β-1,3-glucan polymers form a network of fibrils that surround the fungal cell, helping to maintain mechanical strength and osmotic resistance of the fungal cell wall (Smits et al., 1999). Glucan synthases hold much appeal as targets for antifungal drug development.
Neopeptins (Fig. 2) are produced by Streptomyces spp. and are cyclic lipopeptides composed of nine unusual amino acids and one acyl chain. Neopeptins have potent antifungal properties, inhibiting mannoprotein, proteoheteroglycan, and β-1,3-glucan synthetases found in Saccharomyces cerevisiae (Satomi et al., 1982; Ubukata et al., 1984; Ubukata et al., 1986), and causing mycelial swelling in several plant pathogens. The growth of Glomerella cingulata, P. oryzae, Cochliobolus miyabeanus, and Colletotrichum lagenaium can be completely inhibited by neopeptins at a concentration of 4 μg/ml. In one study, the development of powdery mildew on cucumber plants was effectively controlled by a mixture of neopeptins isolated from a culture of Streptomyces neopeptinius (Han et al., 2008; Kim et al., 2007). The tyrocidines (Fig. 2) are a group of antimicrobial cyclic decapeptides identified from the soil bacterium Bacillus aneurinolyticus. They have a potent antifungal activity against a range of phytopathogens including F. solani and B. cinerea, where they induce morphological abnormalities of the mycelia. The potency of tyrocidines against agronomically important phytopathogens (significantly higher activity than that of the commercial fungicide bifonazole), combined with their relative salt stability, highlights the potential for their development as antifungal agents in the agricultural sector (Troskie, 2014).
Cyclic peptides affecting inositol phosphorylceramide synthase
Aureobasidins (Fig. 2) are cyclic depsipeptide antifungal antibiotics produced by Aureobasidium pullulans (Takesako et al., 1991). Their molecular structures consist of eight lipophilic amino acid residues and one α-hydroxy acid (Ikai et al., 1991a; Ikai et al., 1991b). Most of the aureobasidin derivatives are effective against a wide range of fungi and protozoa. Aureobasidin A inhibits inositol phosphorylceramide (IPC) synthase, a key enzyme catalyzing sphingolipid synthesis in fungi (Nagiec et al., 1997). The inhibition of IPC synthase affects spore germination, germ tube elongation and hyphal growth of the pathogenic fungi Penicillium digitatum, Penicillium italicum, Penicillium expansum, B. cinerea and M. fructicola, which are major pathogens causing postharvest diseases of a variety of fruits (Liu et al., 2007). Aureobasidin A is effective (50 μg/ml) at controlling the citrus green mold (P. digitatum) and also reducing the incidence and severity of strawberry gray mold (B. cinerea).
Other cyclic peptides with unknown antimicrobial mechanism
The modes of action of several antifungal cyclic peptides isolated from bacteria, fungi, blue-green alga and cyanobacterium remain unclear, but their potent activities against plant pathogens are well established.
Cepacidines A1 and A2 (Fig. 3) are glycopeptides produced by Pseudomonas cepacia (Lee et al., 1994; Lim et al., 1994). The MICs for cepacidine A range from 0.098 to 0.391 mg/ml against Aspergillus niger, F. oxysporum, and Rhizopus stolonifer. Fungicin M4 is a hydrophilic antifungal peptide produced by Bacillus licheniformis M-4 (Lebbadi et al., 1994). Fungicin M4 is resistant to proteolytic enzymes and lipase, and inhibits the growth of Microsporum canis, Mucor species, Bacillus megaterium, Corynebacterium glutamicum, and Sporothrix schenckii. Peptide antibiotic PKB1 (Fig. 3) is produced by P. polymyxa PKB1 and has antifungal activity against the causative agent of blackleg disease in canola (Leptosphaeria maculans) as well as other economically destructive pathogens including S. sclerotiorum, Marasmius oreades, Pythium pythioides, R. solani, Fusarium avenaceum, and Alternaria brassicae (Kharbanda et al., 2003). P. polymyxa PKB1 can also act as a biocontrol agent against blackleg and other fungal diseases of canola. Success in using P. polymyxa PKB1 as a biocontrol agent was achieved by coating canola seeds with freeze-dried or living cells of the bacterium to protect germinating shoots from L. maculans infection in stubble.
Cryptocandin (Fig. 3) is a potent lipopeptide antimycotic produced by the endophytic fungus Cryptosporiopsis cf. quercina (Strobel et al., 1999), and is active against several plant pathogenic fungi including S. sclerotiorum (MIC 0.78 μg/ml) and B. cinerea (MIC 0.62 μg/ml). Verlamelin (Fig. 3) is a peptide antibiotic produced by Acremonium strictum (Kim et al., 2002) and the entomopathogenic fungus Lecanicillum sp. (Ishidoh et al., 2014). It has in vitro antifungal activity against phytopathogenic fungi such as Margnaporthe grisea, Bipolaris maydis, and B. cinerea. In vivo analysis has shown strong protective and curative activities, particularly against barley powdery mildew caused by Blumeria graminis f. sp. hordei. The development of barley powdery mildew can be controlled by an application of 100 μg/ml verlamelin in 7-day protective treatments and 2-day curative applications.
Calophycin is a broad-spectrum antifungal cyclic decapeptide that has been isolated from Calothrix fusca EU-10-1, a terrestrial blue-green alga belonging to the Nostocaceae family. In experiments using disc-diffusion assays in soft agar, calophycin showed zones of inhibition of 13 mm (1.2 μg/disc) against Aspergillus oryza (Moon et al., 1992).
The cyanobacterium Schizotrix (TAU strain IL-89-2) produces schizotrin A (Fig. 3), a cyclic undecapeptide (Pergament and Carmeli, 1994), that inhibited the radial growth of Sclerotium rolfsii (13.4 nM/disc), R. solani (13.4 nM/disc), F. oxysporum (33.5 nM/disc) and C. gloeosporioides (13.4 nM/disc) in zone-of-inhibition assays.
Xanthostatin (Fig. 3) produced by Streptomyces spiroverticillatus is a depsipeptide antibiotic with an N-acetylglycine side chain and selective antimicrobial activity against Xanthomonas spp. (Kim et al., 2003). Bacterial canker affecting citrus plants by causing necrotic spots on citrus fruits, leaves, and stems, is induced by Xanthomonas campestris pv. citri. Control measures include windbreaks of trees or netting, pruning of diseased shoots, and chemical sprays, but the canker on susceptible or highly susceptible trees has not yet been properly controlled (Osada and Isono, 1986; Stall and Seymour, 1983).
Synthetic cyclic peptides
Most studies using synthetic cyclic peptides have focused on smaller chains of 4–10 residues, which were prepared using solid-phase methods (Andreu et al., 1983) and procedures that facilitate the performance of combinatorial synthesis (Monroc et al., 2006b; Reed et al., 1997). The development of high-throughput systems have eased the burden of large scale analyses and allowed massive numbers of synthetic peptides to be screened for antibacterial activity.
Monroc et al. (2006b) synthesized a combinatorial library of head-to-tail cyclic peptides (4–10 residues) consisting of alternating hydrophilic (Lys) and hydrophobic (Leu and Phe) amino acids and tested them against the economically important plant pathogenic bacteria Erwinia amylovora, Xanthomonas vesicatoria and P. syringae. The cyclic cationic peptide BPC10L (KLKLKFKLKQ) showed activity towards X. vesicatoria (MIC, 6.25 mM) and P. syringae (MIC, 12.5 mM). An investigation of tetra- to decapeptides based on the structure c(X1X2X3X4KFKKLQ), where X is Lys or Leu (Monroc et al., 2006a), produced two cyclic peptides (BPC194 and BPC198). The two peptides showed improved activities against E. amylovora, and this was coupled with a low eukaryotic cytotoxicity at concentrations 30–120 times higher than the MIC values. Activities of the two peptides are comparable to streptomycin, a commercially available antibiotic used in agriculture. For bacterial disease control, streptomycin (MIC values 2–9 mM) was applied to agricultural fields at about 100 mM. Field doses of the peptides were similar to those of streptomycin, however, they are not expected to present any cytotoxic issues. These findings indicate that synthetic cyclic peptides have much appeal for consideration as antimicrobial agents for plant protection. Synthetic cyclic peptides with antifungal properties have not been created, however the success in developing antibacterials provides a glimpse of the potential this approach holds.
Concluding remarks
Cyclization of a linear peptide gives a considerable rigidity over the linear form, providing an enhanced and stable binding with target sites, and a tolerance to hydrolysis by proteases. These features are ideal properties for considering the merits of using cyclic peptides as fungicides in the control of plant diseases. Antimicrobial cyclic peptides that are effective against plant pathogens have a broad spectrum of activity (e.g., Alternaria, Botrytis, Cochliobolus, Geotrichum, Penicillium, Sclerotinia, Fusarium species, and M. grisea), which may be due to similarities in the fungal cell envelopes, where antimicrobial cyclic peptides are active. Despite the apparent merits, there are other issues associated with the practical field-based applications of antimicrobial cyclic peptides that need to be resolved. For example, the effectiveness of the peptides under certain physiological conditions, the selective toxicity for pathogenic fungi over their hosts, and the synergistic effects with other fungicides all need to be considered. Approaches that enhance molecular diversity of cyclic peptides should also be investigated. Combinatorial chemistry has the potential to synthesize novel cyclic peptides, optimize structures for improved potency, reduce cytotoxicity, and increase molecule stability towards proteases. Initial studies on naturally occurring antimicrobial cyclic peptides focused on bacteria and yeasts. However, promising results for antimicrobial cyclic peptides active against plant pathogens are increasingly reported as described here. The use and synthesis of cyclic peptides has provided an insight to a potentially rich source of antimicrobial agents suitable for the control of plant diseases. Continued efforts to diversify cyclic peptide structures, validate structure-activity relationships, and optimize formulation and treatment methods may provide a new efficient tool to improve and diversify the current management strategies of plant diseases that heavily rely on conventional fungicides.
Acknowledgements
This work was partly supported by the National Research Foundation of Korea (NRF) grant (NRF-2013K2A2A2000535) funded by the Ministry of Science, ICT & Future Planning.