Antifungal Activity of Green and Chemically Synthesized ZnO Nanoparticles against Alternaria citri, the Causal Agent Citrus Black Rot
Article information
Abstract
Citrus black rot is a serious disease of citrus plants caused by Alternaria citri. The current study aimed to synthesize zinc oxide nanoparticles (ZnO-NPs) by chemically or green method and investigate their antifungal activity against A. citri. The sizes of synthesized as measured by transmission electron microscope of ZnO-NPs were 88 and 65 nm for chemical and green methods, respectively. The studied prepared ZnO-NPs were applied, in vitro and in situ, at different concentrations (500, 1,000, and 2,000 μg/ml) in post-harvest treatment on navel orange fruits to verify the possible control effect against A. citri. Results of in vitro assay demonstrated that, at concentration 2,000 μg/ml, the green ZnO-NPs was able to inhibit about 61% of the fungal growth followed by 52% of chemical ZnO-NPs. In addition, scanning electron microscopy of A. citri treated in vitro with green ZnO-NPs showed swelling and deformation of conidia. Results showed also that, using a chemically and green ZnO-NPs at 2,000 μg/ml in situ in post-harvest treatment of orange, artificially-infected with A. citri, has reduced the disease severity to 6.92% and 9.23%, respectively, compared to 23.84% of positive control (non-treated fruits) after 20 days of storage. The out findings of this study may contribute to the development of a natural, effective, and eco-friendly strategy for eradicating harmful phytopathogenic fungi.
Citrus fruits are produced worldwide in tropical and subtropical areas and are the largest fruit commodities in international trade (FAOSTAT, 2018). After harvest, citrus fruits are susceptible to physiological disorders and microbial diseases that result in post-harvest losses. Furthermore, post-harvest citrus decay is the most serious cause of wastage and quality deterioration because it renders fresh fruit unfit for consumption, resulting in significant economic losses (Cheng et al., 2020; Garganese et al., 2019; Khorram and Ramezanian, 2021). In less developed countries, losses can reach 30% of total production and even 50% (Strano et al., 2022).
Some fruit post-harvest decays can be caused by latent infections in farms, which typically include infections caused by Alternaria alternata pv. citri Whiteside, and Phytophthora citrophthora, the causal agents of black and brown rots (Strano et al., 2022). The causal agent of citrus black rot is Alternaria citri, which is the first citrus-associated Alternaria species (Peever et al., 2004). Black rot of fruit, caused by A. citri, is a significant fungal disease of citrus that causes severe losses in citrus orchards of the world. It affects most of the members of the citrus family and often causes severe losses. The disease primarily affects plant above-ground parts, particularly leaves and fruits. The first symptoms, however, usually appear on the leaves as black necrotic lesions. Later, black necrotic lesions form on the fruits, and as the plants’ age, the fruits become soft and black-rotted (Umer et al., 2021). In a 10-year survey of citrus-associated diseases from several countries in the Mediterranean region, high disease pressure has been detected in all areas, forcing citrus growers to adapt fungicidal spray programs.
In fact, chemical control management poses serious risks to human health and the environment; additionally, continuous fungicide applications may result in the emergence of resistance to pathogenic species (Sánchez-Torres and Tuset, 2011; Vitale et al., 2021). Thus, there is a great interest to search for natural antifungal agents to control plant disease (Elshafie et al., 2017a, 2017b; Yang et al., 2019). Although these techniques are costly and time-consuming, it is critical to extend the technical life of fungicides after a certain period (Piccirillo et al., 2018). Furthermore, consumers are worried about the utilization of chemical fungicides since their active ingredients and co-formulants have been related to health problems and ecological contamination (Nicolopoulou-Stamati et al., 2016). Moringa oleifera Lam. (moringa) is a multi-purpose herbal plant used as human food and as an alternative for medicinal purposes worldwide. It has been identified by researchers as a plant with numerous health benefits, including nutritional and medicinal advantages (Abdull Razis et al., 2014).
Nanomaterials have been proposed and accepted due to their properties and critical applications in food and agriculture (Khan et al., 2019). In the field of post-harvest technology of fruits and vegetables, the nanotechnology approach is helpful for controlling post-harvest diseases, introducing an invention for packaging films, preventing the impact of gases and unsafe rays, improving packaging appearance, and helping to label fresh products using multiple chips (nano biosensors). Nanotechnology was used to produce nanomaterials for use as antifungal agents in several commodities, including fruits and vegetables (Ruffo Roberto et al., 2019). Due to their antibacterial properties, zinc oxide nanoparticles (ZnO-NPs) have received substantial attention (Meruvu et al., 2011). Even at low concentrations, the ZnO-NPs exhibited antibacterial and antifungal activities, making them acceptable for their coating applications (Sharma et al., 2010).
The present study used green-synthesized ZnO-NPs of M. oleifera as an alternative to toxic fungicides, in order to investigate ZnO-NPs’ antifungal effects against the A. citri Ellis & N. Pierce in vitro and in situ compared to the chemically-synthesized one. The study’s findings may help develop a new, efficient approach for controlling phytopathogenic fungi.
Materials and Methods
Chemicals
Zinc nitrate hexahydrate, sodium borohydride, and sodium hydroxide were obtained from Merck (KGaA, Darmstadt, Germany). Switch 62.5WG Fungicide was obtained from Syngenta USA (Greensboro, NC, USA).
Chemically synthesis of ZnO-NPs
The ZnO-NPs were prepared following the Wet-chemical method as reported by Arora et al. (2014). In particular, zinc nitrate and sodium hydroxide have been used as precursors, whereas sodium borohydride has been used as a stabilizing agent. Following the reaction and the formation of zinc hydroxide, calcinations should be performed to obtain the required size. Briefly, 2.79 g of Zn(NO3)2·6H2O was weighed and dissolved in 100 ml of distilled, deionized water, which was then agitated vigorously for 1 h. Then 0.48 g NaOH dissolved in 60 ml distilled deionized water was prepared, and then 1 ml NaBH4 (1%) was added dropwise after zinc nitrate dissolution, and stirred overnight for 12 h to obtain a low nano size. The precipitate was then allowed to settle before being filtered under suction and washed several times with distilled, deionized water (pump). The precipitate was then dried in an oven at 80°C. Zn(OH)2 was formed and then converted to ZnO. The muffle calcination had been performed at 500°C to obtain the smaller size.
Green synthesis of ZnO-NPs
The green synthesis of ZnO-NPs has been carried out using M. oleifera as follows. Ten grams of M. oleifera were obtained from the greenhouse of the Desert Research Center (Cairo, Egypt), washed with distilled water, and bot leaves surfaces were sterilized using alcohol by gentle rubbing. These leaves were heated for 40 min in 100 ml of distilled water at 50°C. The extract was filtered with Whatman 41 (Merck, KGaA). The filtrate was stored in a cool and dry place.
Zinc oxide nanoparticles were prepared by mixing 10 ml of M. oleifera leaf extract with 90 ml of a 0.05 mM zinc nitrate hexahydrate [Zn(NO3)2·6H2O] solution. The mixture was heated on a hot plate at 80°C for 30 min with continuous stirring. The presence of white-colored particles indicated the formation of nanoparticles. The extract was centrifuged and the pellets were collected, dried, and calcinated for 2 h at 300°C. White-colored nanoparticles were obtained as per the protocol as follows (El-Saber, 2021; Pal et al., 2018; Zambri et al., 2019).
Characterization of green and chemically-synthesized ZnO-NPs
The shape and size of ZnO-NPs were practically obtained using high-resolution transmission electron microscopy (TEM; HRTEM, JEOL JEM-2100, JEOL, Tokyo, Japan). Specimens for TEM measurements were prepared by placing a drop of colloidal solution on a 400-mesh carbon-coated copper grid and evaporating the solvent in the air at room temperature. The programme (ImageJ, U.S. National Institutes of Health, Bethesda, MD, USA) was used to calculate the average size of nanoparticles. The crystalline structure of the ZnO-NPs was confirmed via X-ray diffractometer (XRD; X‘Pert Pro, PanAlytical, Almelo, The Netherlands). Malvern zeta sizer nano was used to measure particle size and zeta potential. The sample in solution form was placed in a cuvette and mitigated to become transparent until the error in the reading was reduced, then was placed inside the device, and the device start required the diameter of the particles and determination of the extent of homogeneity through the polydispersity index (PDI; dispersed index), which ranges from 0 to 1, and when approached zero increased homogeneity.
Isolation and identification of tested pathogenic fungi
Samples with visible disease symptoms were removed by a sterilized knife in such a manner as to contain the lesion edges too. These were then immersed in HgCl2 (1:10,000) for 2–3 min for surface sterilization and washed thoroughly in sterile distilled water until all the HgCl2 was removed. The surface-sterilized tissues were further cut into smaller pieces using a sterile blade and placed on potato dextrose agar (PDA) medium using disinfected forceps. Plates were incubated at 28°C for 7 days. The isolated fungi were identified by molecular methods based on polymerase chain reaction (PCR) (Camele et al., 2005) at Laboratory of Mycology, School of Agricultural, Forestry, Food and Environmental Sciences (SAFE), University of Basilicata (Potenza, Italy). The genomic DNA (gDNA) were extracted from each pure fungal isolate using DNeasy Plant Mini Kit (Qiagen, Heidelberg, Germany). The gDNAs were amplified using the universal primer pair ITS4/ITS5 of rDNA (White et al., 1990). The obtained amplicons were directly sequenced and the obtained sequences were compared with those available in the GenBank nucleotide archive using Basic Local Alignment Search Tool software BLAST (Bethesda, MD, USA) (Altschul et al., 1997). The purified isolates were maintained on PDA slants for further studies (Talibi et al., 2014).
In vitro antifungal assay
The poisoned food in vitro technique described by Das et al. (2010) was employed to evaluate the antifungal activity of the tested materials (moringa leaves aqueous extract, ZnO-NPs, and green zinc nanoparticles) against A. citri at different concentrations (0, 500, 1,000, and 2,000 μg/ml) by incorporating appropriate quantities of the treatments with an autoclaved PDA at 45°C, mixing very well, then plating in sterile Petri dishes and allowing to solidify (Elshafie et al., 2020a, 2020b, 2021). With the assistance of a sterile cork borer, a 5 mm mycelial disc of the old culture of A. citri was taken and aseptically placed in the center of the treated plates. The inoculated plates were incubated at 28°C for 7 days, or until the control plate grew to 9 cm. The inhibition percentage was measured with the help of the mean colony diameter and computed according to the following formula (O’Rourke et al., 2009).
Where, C = Average mycelial growth in control petri plates, and T = Average mycelial growth in treated Petri plates.
Scanning electron microscopy of tested fungi
Scanning electron microscopy (SEM) of A. citri treated with studied antifungal agents at 2,000 μg/ml compared to control (without treatment) for 4 h at room temperature was estimated according to Sitohy et al. (2013) as described by Abbas et al. (2020).
Pathogenicity assay
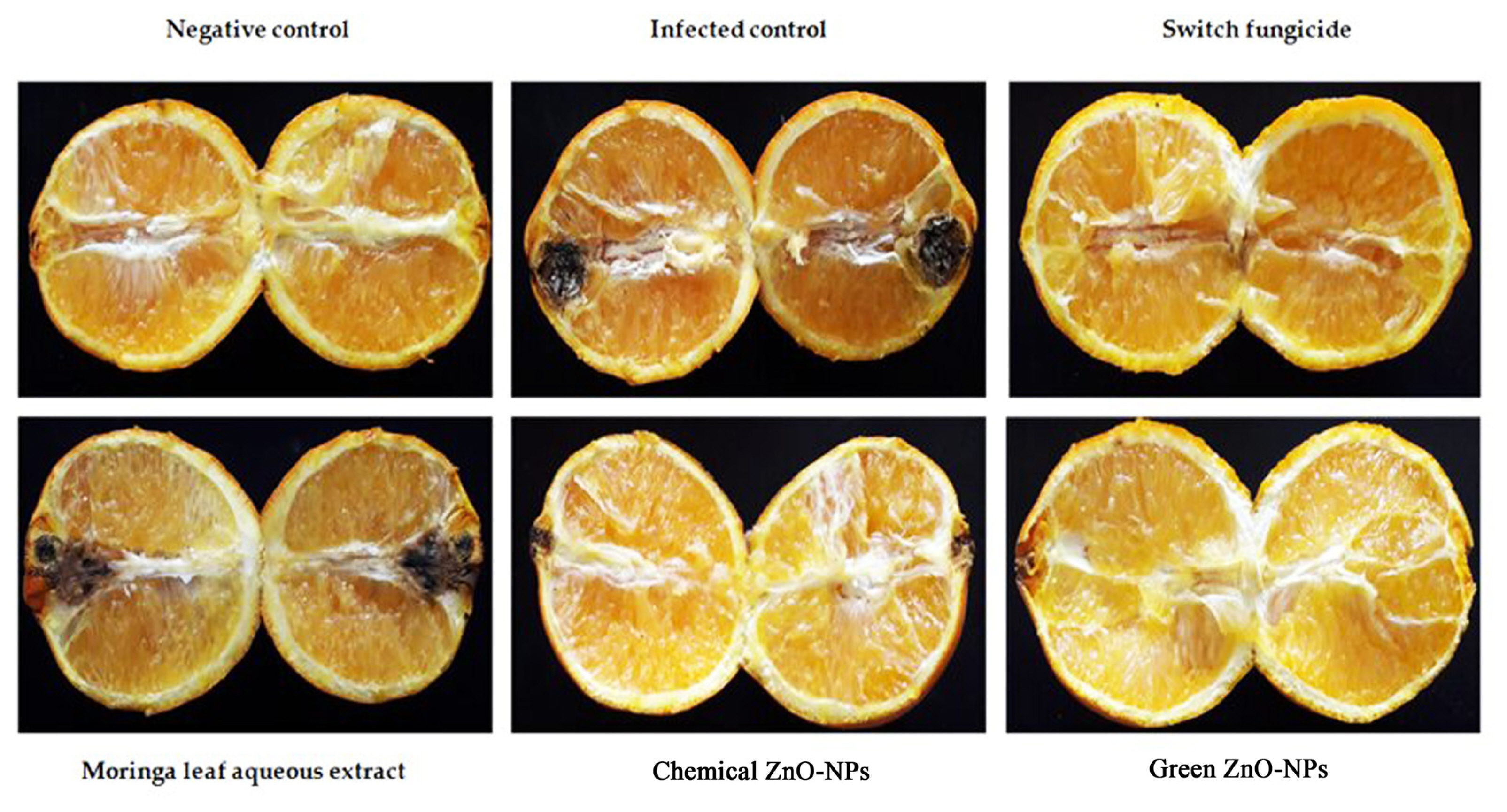
An in situ experiment has been carried out using navel orange fruit obtained from the local market. Harvested orange fruits were immediately transported to the Plant Pathology Department, Faculty of Agriculture, Zagazig University, Zagazig, Egypt some fruits were selected based on uniformity of size and color, then were disinfected using 70% ethanol for 1 min, 1% sodium hypochlorite for 1 min, and washed three times with sterilized distilled water, then drained, rinsed with fresh air, and air dried at room temperature (25°C) before being treated on the same day. A fresh PDA culture of A. citri, was prepared and used for artificial infection of navel orange fruits (six groups of six fruits each, in three replicates). Twenty microliters (2,000 μg/ml) of each tested material (moringa leaves aqueous extract, ZnO-NPs, and green zinc nanoparticles) were injected into the central axis of a sterilized fruit using a micropipette. Switch fungicide was similarly applied using the recommended dose as positive control. The negative control was treated likewise, except that the fruits were neither infected with the fungus nor treated with nanoparticles. After that, 100 μl of spore suspension containing 106 spores/ml was injected directly into the central axis of a sterilized fruit following the method described by Katoh et al. (2006). The inoculated fruits were then incubated in a sealed, sterilized plastic box at 24°C for 3 weeks, and the fruits were sliced in half for observation of symptom development. Disease severity was determined according to the area of the lesion on each fruit using the following formula:
Results
Fungal identification
The PCR amplification of the isolated fungi produced amplicons with molecular weights of approximately 600 bp. No amplification was observed in the case of the negative control. The obtained amplicons were directly sequenced (BMR Genomics, Padova, Italy) and the obtained sequences were compared with those available in the GenBank nucleotide archive using the Basic Local Alignment Search Tool software (BLAST) (Altschul et al., 1997). The sequences analysis for the isolated fungi showed high similarity with the sequences already presented in the GenBank database >99% for A. citri.
Characterization of chemically-synthesized ZnO-NPs
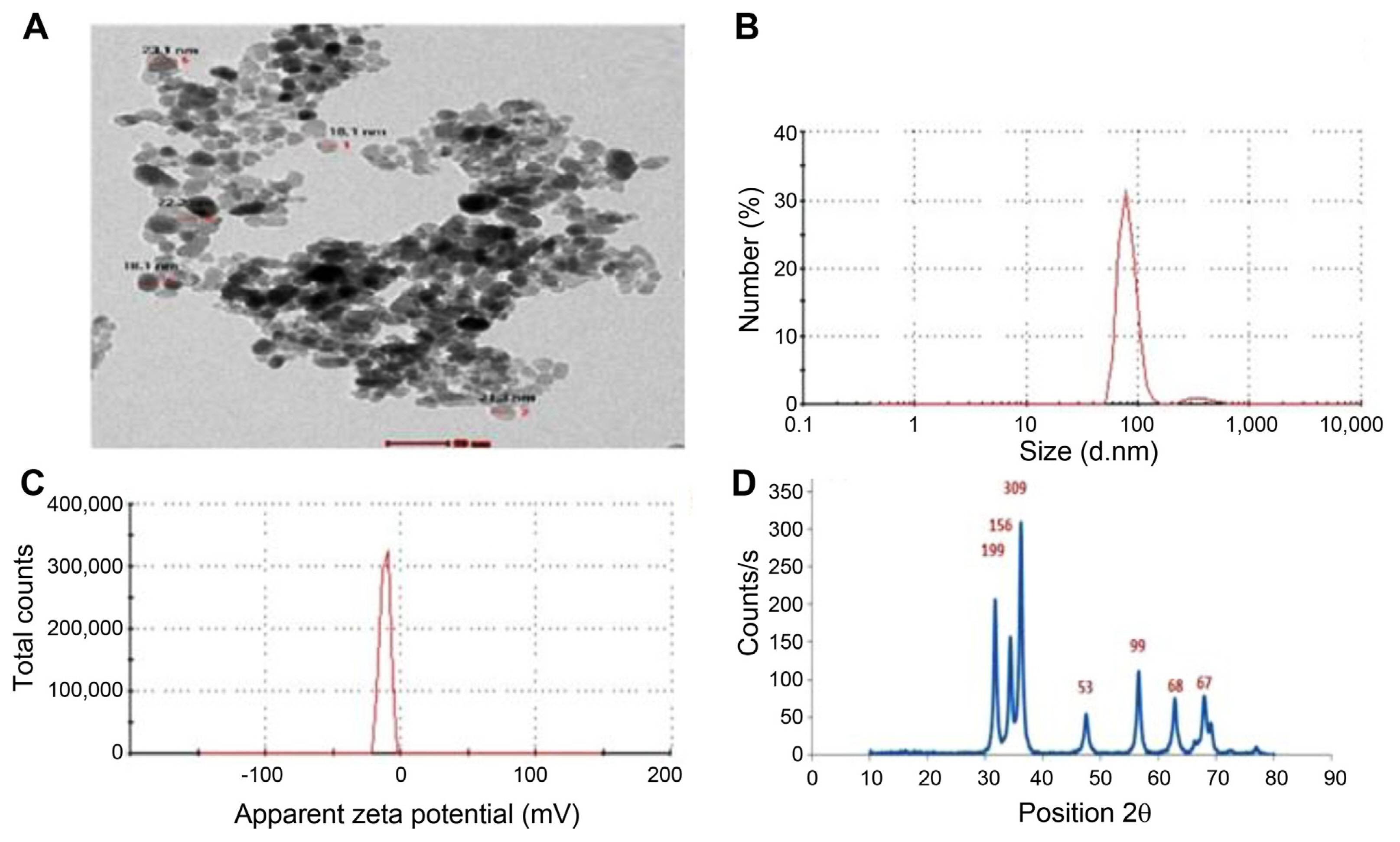
The chemical characterization of ZnO-NPs showed a well-dispersed, nearly spherical shape with a particle size of approximately 80 ± 5 nm as shown in the TEM image (Fig. 1A) and for extra-confirmatory identification by dynamic light scattering, which was performed to evaluate the nano-dispersity and particle size determination, an average particle size of 80 ± 5 nm with 0.552 PDI indicated a narrow size range (Fig. 1B). Stability and surface charge were estimated by the zeta potential exhibited by the −11.5 mV moderately stable NPs (Fig. 1C). For phase analysis and chemical structural identification, the XRD technique was conducted, where the XRD pattern of ZnO-NPs showed peaks at 2 = 31.69, 34.39, 36.21, 47.51, 56.47, 62.97, and 68.13, card number 01-078-2585 hexagonal (Fig. 1D).
Characterization of green-synthesized ZnO-NPs
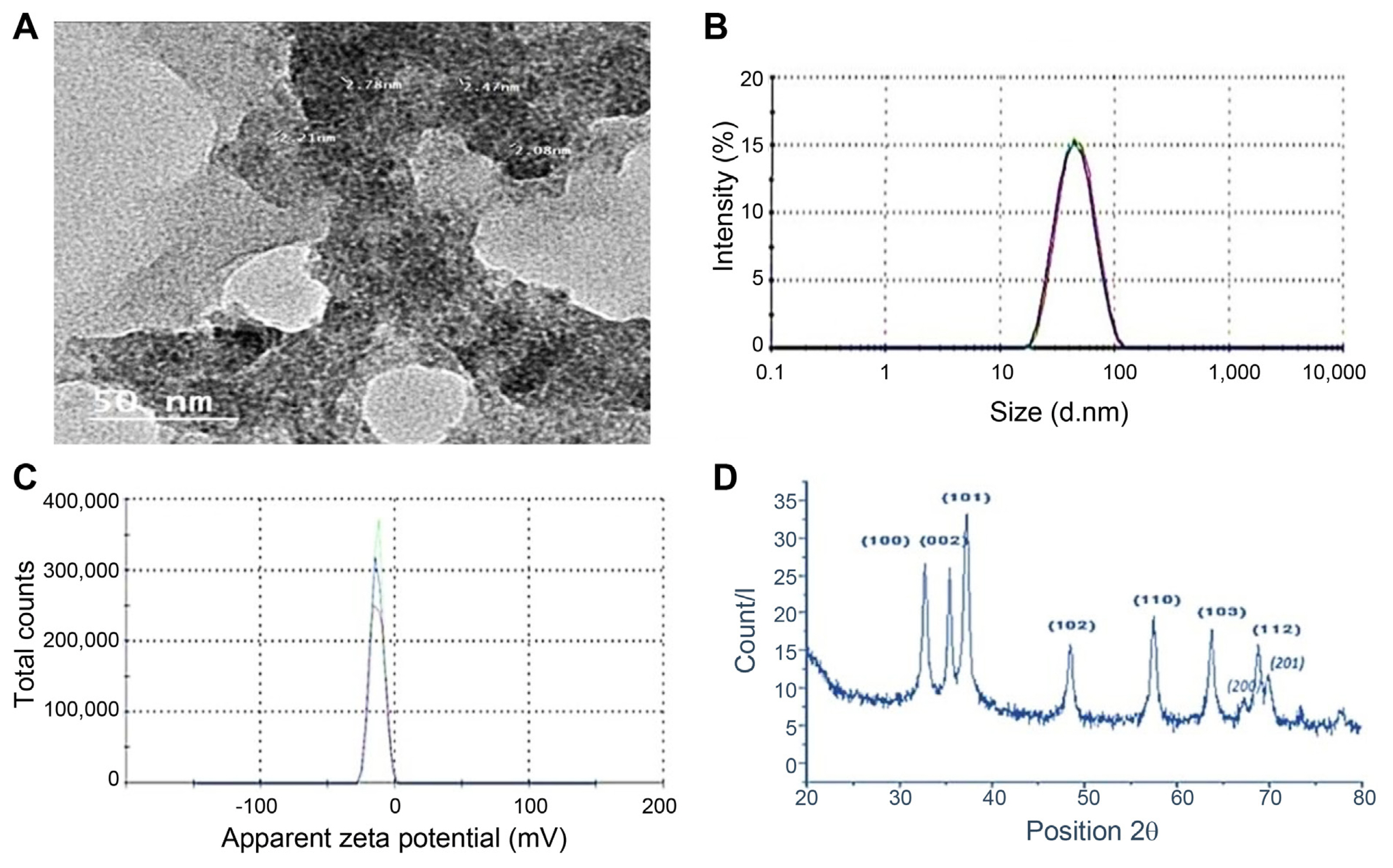
Different characterization techniques including TEM, XRD, zeta potential, and zeta size were used to ascertain the formation of ZnO-NPs green synthesized by moringa leaf. The shape and the size of the biosynthesized ZnO-NPs were studied and investigated by using TEM (Fig. 2A). The average size and the stability of the nanoparticles were confirmed by the zeta size (Fig. 2B) and the zeta potential analysis (Fig. 2C). The obtained results showed that the mean diameter of ZnO-NPs was about 65 nm, and the zeta potential was −13.7 mv. Finally, The XRD pattern of the ZnO-NPs powder showed various diffractions peaks at 2 theta degree 31.24, 34.11, 35.71, 47.77, 56.22, 62.72, 66.34, 67.78, 72.84, and 76.79. As matched with the crystallographic database JCPDS 36–1451, these peaks were assigned to (100), (002), (101), (102), (110), (103), (200), (112), (004), and (202) planes of zinc, respectively (Fig. 2D).
In vitro antifungal activity of M. oleifera extract and ZnO-NPs
Figs. 3 and 4 illustrate the in vitro antifungal activity of M. oleifera leaf aqueous extract and ZnO-NPs prepared either chemically or green-synthesis against A. citri mycelium growth. All treatments showed a promising effect on the linear growth of A. citri in a dose-dependent manner compared to control. A linear growth percentage reduction of A. citri at a concentration was 2,000 μg/ml, which was the most effective, being 3.5, 4.36, and 5.3 cm for greenly synthesized ZnO-NPs, chemically-synthesized ZnO-NPs, and moringa aqueous extract, respectively. Furthermore, data showed that the green-synthesized ZnO-NPs at 2,000 μg/ml was the most effective dose decreasing the linear growth of A. citri, where it showed 61.1%, followed by chemical NPs (51.55%), and finally moringa aqueous leaf extract (41.11%).
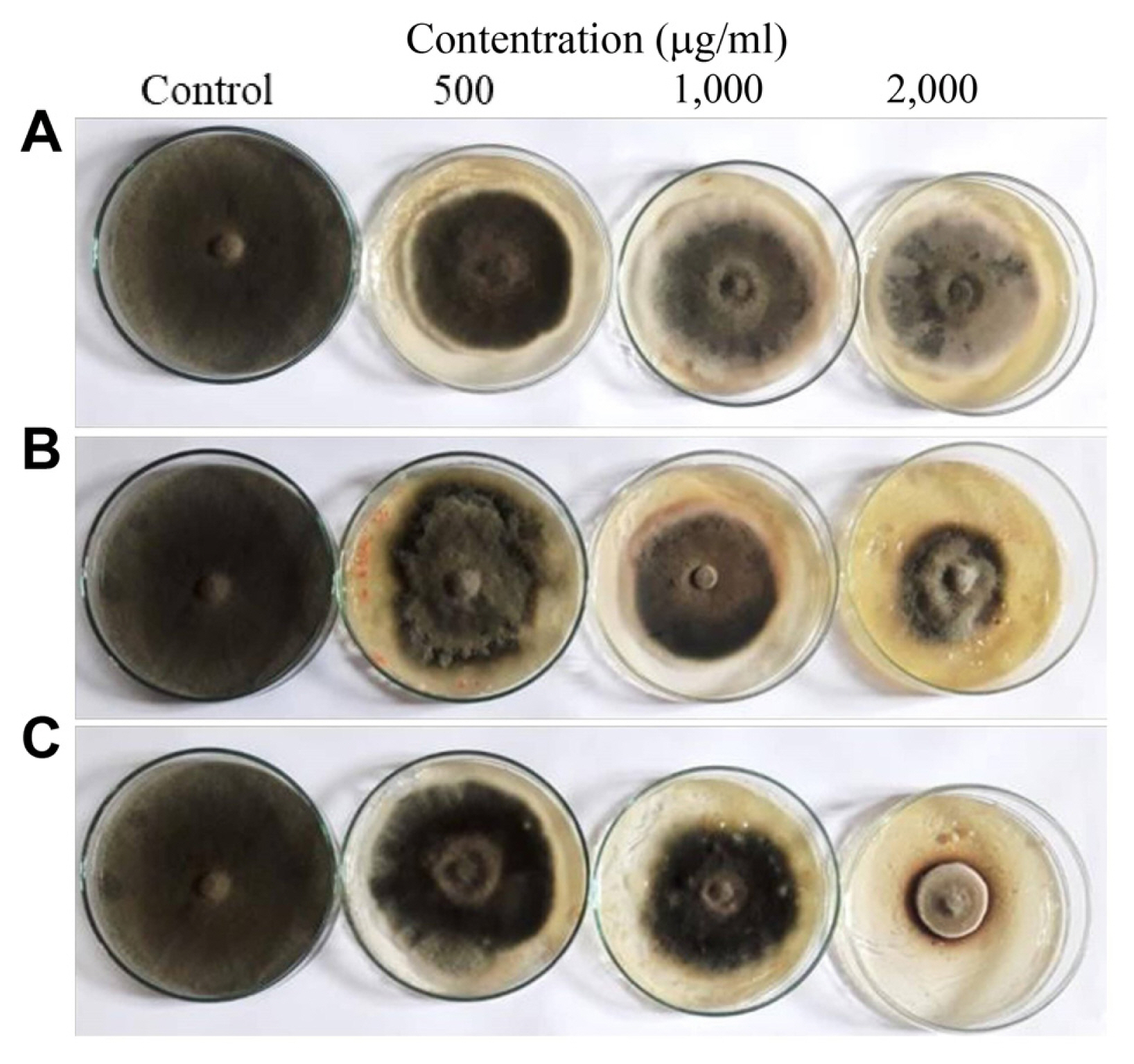
Fungal growth of Alternaria citri on potato dextrose agar medium after incubation at 28°C for 7 days with Moringa oleifera leaf aqueous extract (A), chemical zinc oxide nanoparticles (ZnO-NPs) (B), and green ZnO-NPs (C) at different concentrations (500, 1,000, and 2,000 μg/ml) compared to control (non-treated plates).

Linear growth (cm) (A) and growth reduction percent (B) of Alternaria citri on potato dextrose agar medium after 7 days of incubation at 28°C with Moringa oleifera leaf aqueous extract, chemical zinc oxide nanoparticles (ZnO-NPs), green ZnO-NPs at different concentrations (500, 1,000, and 2,000 μg/ml) compared to control (non-treated plates).
Scanning electron microscopy
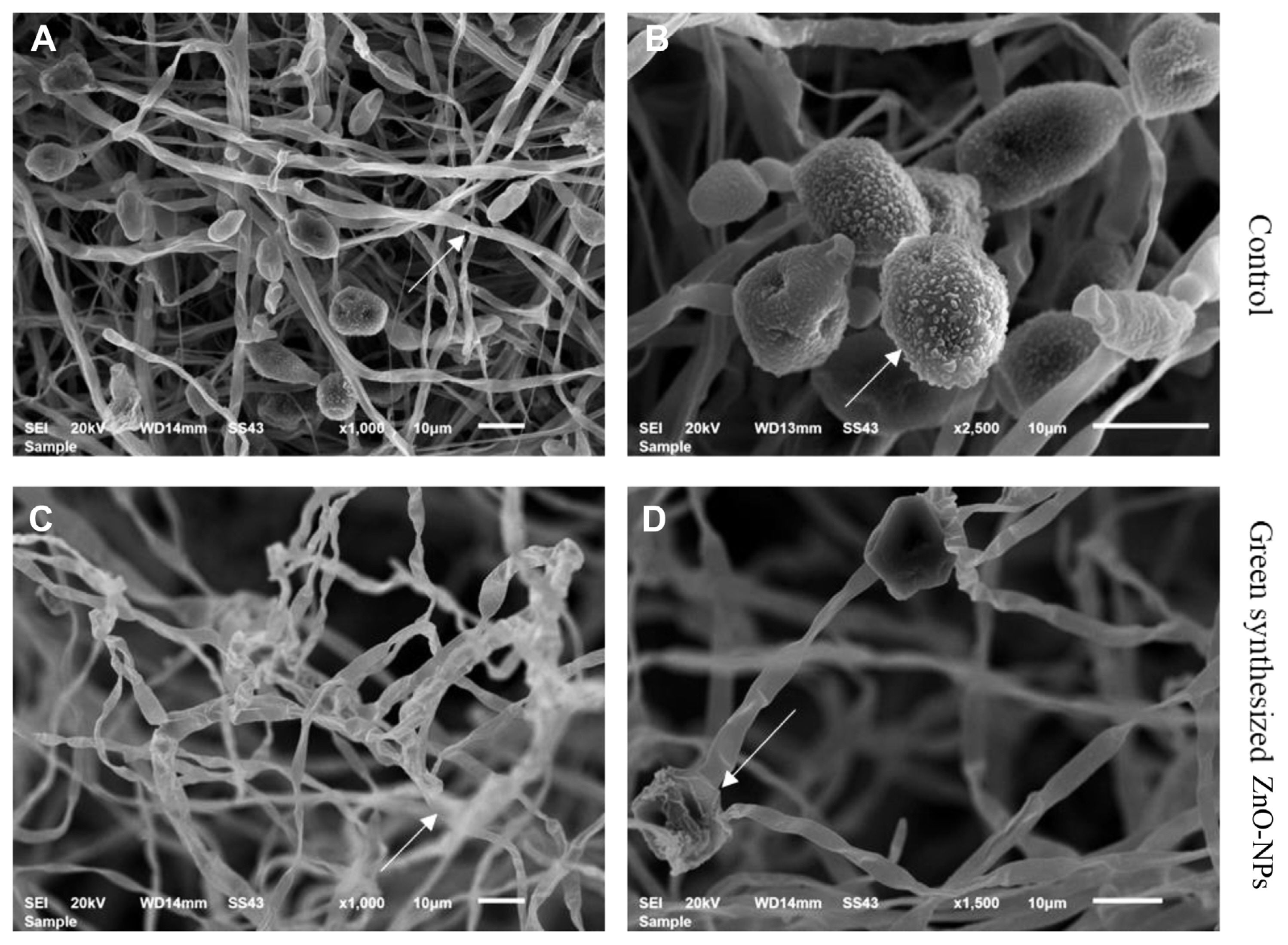
SEM was used to examine the morphological features of A. citri mycelia and cells treated with green-synthesized ZnO-NPs at 2,000 μg/ml to investigate the possible antifungal mechanism of these NPs against A. citri. The observation explicated that the mycelia and conidia of A. citri grown on PDA were smooth, homogeneous mycelial morphology. In contrast, the mycelial and conidial morphology was conspicuously changed when exposed to green-synthesized ZnO-NPs at 2,000 μg/ml (Fig. 5).
Pathogenicity assay
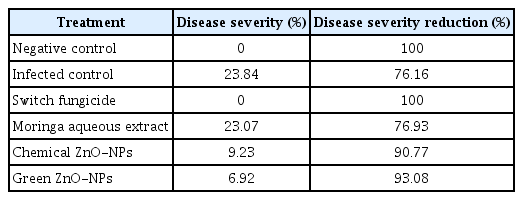
Table 1 and Fig. 6 showed the effect of moringa leaf aqueous extract and new green and chemically-synthesized ZnO-NPs on the severity of black rot disease on navel orange. Data presented in Table 1 indicates that switch fungicide completely inhibited black rot disease severity. Furthermore, data showed that green ZnO-NPs at 2,000 μg/ml was the most effective in reducing black rot disease severity (93.08%), followed by chemical ZnO-NPs (90.77%), whereas moringa aqueous extract exhibited only 76.93% effectiveness.
Discussion
Citrus fruit rot disease is incited by A. citri is one of the serious diseases that attack citrus cultivars, causing severe damage and causing many diseases, i.e., black fruit rot, stem end rot, brown spot, and leaf spot (Isshiki et al., 2001; Sadowsky et al., 2002). Citrus black rot is a major fungal disease of sweet oranges (Citrus sinensis L.) that affects the fruit even after post-harvest, leading to a devastating impact on the overall production of oranges. A. citri, the prime etiological agent of the disease, has been reported to develop fungicide-resistance during the last decade (Sardar et al., 2022; Zelmat et al., 2021). Hence, there is a need to use alternative techniques to control fungal infections. Nanoparticles can be used as possible alternative to control phytopathogens in comparison to synthetic fungicides. ZnO-NPs have wider applications and are generally recognized as safe materials by the US Food and Drug Administration (Isshiki et al., 2001). The application of ZnO-NPs has been recently increased including cosmetics ointments, food packaging, etc. (Bobo et al., 2016; Espitia et al., 2012; Marcous et al., 2017; Pillai et al., 2020). Pillai et al. (2020) reported that the antifungal activity of ZnO-NPs was prepared by the green method, and found that ZnO-NPs prepared using Beta vulgaris were active against Aspergillus niger, while those prepared from Cinnamomum tamala were active against Candida albicans. Additionally, ZnO-NPs were prepared from the extract of Brassica oleracea var. italica has shown activity against both fungal strains.
Antifungal activity of ZnO-NPs (35–45 nm), silver (20–80 nm), and titanium dioxide (85–100 nm) has been tested against Macrophomina phaseolina, a major soil-borne pathogen of pulse and oilseed crops. Silver nanoparticles (AgNO3 NPs) had a greater antifungal effect at lower concentration than ZnO-NPs and TiO2 NPs. Maize treated with nanosilica (20–40 nm) has been screened for resistance against Fusarium oxysporum and A. niger compared to that of bulk silica (Shyla et al., 2014).
The normal mycelial and conidial morphology changed noticeably when exposed to green ZnO-NPs at 2,000 μg/ml in the current study. The mode of action of prepared NPs may be explained through the electrostatic interaction between metal ions and the microbial cell membrane, resulting in damage to the cell membrane and intracellular organelles (Nisar et al., 2019). These continued steps enhance the inhibitory activity of NPs (Gold et al., 2018). The penetration of NPs into microbial cells increases the microbial inhibition interacting with the electron transport chain, disrupting DNA by breaking phosphate and hydrogen bonds, denaturation proteins by altering tertiary structure, and killing mitochondria via oxidative stress (Stanić and Tanasković, 2020). Due to the interactions between inorganic metal and metal oxide NPs, reactive oxygen species are produced causing cell damage (Abd-Ellatif et al., 2022; Canaparo et al., 2020). Greenly manufactured inorganic metal and metal oxide nanoparticles have a wide range of applications in agriculture, particularly in the management of fungal plant diseases (Abd-Ellatif et al., 2022).
The usage of green NPs as potential bio-pesticides should replace the detrimental effects of conventional pesticides for a sustainable environment. One of the most prevalent post-harvest diseases affecting navel oranges is citrus black rot, which has a significant negative influence on citrus fruit worldwide. The diseases resistance which frequently occurred in case of conventional fungicides draws the researchers’ attention to find out novel, effective natural alternatives. Nanoparticles have been reported to have a good long-term antifungal effect. Green-synthesized ZnO-NPs showed a greater antifungal efficacy than the chemically synthesized one against A. citri, the causal agent of citrus black rot disease. In conclusion, the use of eco-friendly green-synthesized metal oxide NPs may reduce the fungal infection of navel orange and improve the farmers’ socio-economic standings.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Acknowledgments
This research was funded by the project entitled “Fighting plant fungi post-harvest using environmentally friendly bio products’, financially supported by the Science and Technology Development Fund (STDF), Egypt. Grant type/name: Research Support. Project ID: 43673.