Differential Control Efficacies of Vitamin Treatments against Bacterial Wilt and Grey Mould Diseases in Tomato Plants
Article information
Abstract
Bacterial wilt and grey mould in tomato plants are economically destructive bacterial and fungal diseases caused by Ralstonia solanacearum and Botrytis cinerea, respectively. Various approaches including chemical and biological controls have been attempted to arrest the tomato diseases so far. In this study, in vitro growths of bacterial R. solanacearum and fungal B. cinerea were evaluated using four different vitamins including thiamine (vitamin B1), niacin (vitamin B3), pyridoxine (vitamin B6), and menadione (vitamin K3). In planta efficacies of the four vitamin treatments on tomato protection against both diseases were also demonstrated. All four vitamins showed different in vitro antibacterial activities against R. solanacearum in dose-dependent manners. However, treatment with 2 mM thiamine was only effective in reducing bacterial wilt of detached tomato leaves without phytotoxicity under lower disease pressure (106 colony-forming unit [cfu]/ml). Treatment with the vitamins also differentially reduced in vitro conidial germination and mycelial growth of B. cinerea. The four vitamins slightly reduced the conidial germination, and thiamine, pyridoxine and menadione inhibited the mycelial growth of B. cinerea. Menadione began to drastically suppress the conidial germination and mycelial growth by 5 and 0.5 mM, respectively. Grey mould symptoms on the inoculated tomato leaves were significantly reduced by pyridoxine and menadione pretreatments one day prior to the fungal challenge inoculation. These findings suggest that disease-specific vitamin treatment will be integrated for eco-friendly management of tomato bacterial wilt and grey mould.
Introduction
Bacterial wilt and grey mould have occurred prevalently and devastated tomato plant cultures in open fields and greenhouses. Hot and humid environments are favourable for tomato bacterial wilt occurrence, but prolonged cool wetness conditions increase grey mould incidence. Grey mould on tomato fruits has been also nuisance during postharvest periods like distribution and export. Planting resistant cultivars and grafting resistant rootstocks were conventionally chosen for reducing the bacterial and fungal tomato diseases (Cristescu et al., 2002; Jyothi et al., 2012; Molan and El-Komy, 2010; Rivard et al., 2012; Yamazaki and Hoshina, 1995). Bacterial wilt- or grey mould-resistant tomato cultivars or wild accessions were screened to identify novel genetic resources of the disease resistance (Carmeille et al., 2006; Egashira et al., 2000; Han et al., 2009). However, it was found to be difficult to excavate resistance germplasm and to introduce the genetic sources into commercial susceptible cultivars so far. Crop cultural managements including altered transplantation timing, soil amendment and fertilizers have been considered as environmental friendly approaches to reduce the bacterial wilt during tomato growing seasons as well (Liu et al., 2015; Wei et al., 2015). Calcium supplement increased resistance of tomato plants against Ralstonia solanacearum infection in concentration-, application timing- and cultivar-dependent manners (Yamazaki and Hoshina, 1995; Yamazaki et al., 1999). Grey mould occurred in the tomato foliage and fruits were also distinctly suppressed by calcium fertilizers applied in the soils (Elad and Volpin, 1993). Non-pathogenic bacteria and fungi have been evaluated for developing biological control agents to manage bacterial wilt and grey mould during the tomato cultures. For controlling tomato bacterial wilt, antagonistic bacteria and plant growth-promoting rhizobacteria were suggested in the disease management program (Anith et al., 2004; Nguyen and Ranamukhaarachichi, 2010). Foliar spraying antagonistic bacteria and soil-drenching plant growth-promoting fungi effectively reduced grey mould of tomato plants (Harel et al., 2014; Lee et al., 2006). However, it is still difficult to commercialise the biocontrol agents and apply them successfully in the tomato fields.
In recent decades, through growing concerns about adverse effects of frequently applied synthetic chemical pesticides and antibiotics, a variety of eco-friendly alternatives have been suggested for sustainable crop production against different pathogens (McFeeters and McFeeters, 2012; Yuliar et al., 2015). Antimicrobial plant essential oils were suggested as eco-friendly agents to decrease the tomato bacterial wilt and grey mould. Treatments with clove oil and thymol efficiently delayed bacterial wilt of tomato plants (Hong et al., 2011; Huang and Lakshman, 2010; Ji et al., 2005; Lee et al., 2012). Essential oils extracted from wild thyme and origanum plants showed high protection efficacies against grey mould in the tomato leaves (Ben-Jabeur et al., 2015; Soylu et al., 2010). Antimicrobial and/or defence-activating activities of hydrogen peroxide and nitric oxide led to reduced bacterial wilt and grey mould of tomato plants (Hong et al., 2013; Lai et al., 2011). More recently, novel materials suppressing bacterial wilt and grey mould have been investigated to establish more efficient and sustainable disease control strategies for tomato production. Silicon and cold plasma were suggested for tomato bacterial wilt control, and selenium treatment and 405-nm light irradiation effectively decreased grey mould of tomato plants (Imada et al., 2014; Jiang et al., 2014; Wang et al., 2013; Wu et al., 2016).
Some vitamins have been discovered to be antibacterial agents. Although direct antibacterial activity of thiamine (vitamin B1) itself was not demonstrated, thiamine addition synergistically enhanced antibacterial activities of commercial sanitizers and disinfectants for the purpose of food preservation (Lee and Ha, 2008). Menadione (vitamin K3) superoxide anion generator has strong antimicrobial properties against broad ranges of fungal species via eliciting oxidative stresses (Abbá et al., 2009; Igbaria et al., 2008; Nikolaou et al., 2009; Yan et al., 2011). Nikolaou et al. (2009) demonstrated that phytopathogenic fungi was generally more sensitive to oxidative stresses caused by menadione and H2O2 treatments than human pathogenic fungi. However, menadione-sensitive bacterial species were rarely described so far. Direct bactericidal activity of menadione against Escherichia coli and the bacterial cellular responses to menadione were demonstrated (Tamarit et al., 1998). Menadione induced antioxidant responses such as glutathione peroxidase gene expression (Arenas et al., 2010), decreased intracellular total glutathione and increased oxidized glutathione in E. coli cells (Smirnova et al., 2000). No direct bactericidal activity of menadione against phytopathogenic bacteria was described, and only inducible expression of catalase gene in Xanthomonas oryzae pv. oryzae causing rice blight disease, detected by non-lethal low concentrations of menadione, indicated an antioxidant response of phytopathogenic bacteria in response to menadione (Mongkolsuk et al., 1996).
Vitamins can reinforce plant disease resistance and environmental stress tolerance, as well as act directly as exert antimicrobial activities. Several vitamins including vitamin B1 (thiamine), vitamin B6 (pyridoxine) and vitamin K3 (menadione) mediated disease defences in plants like Arabidopsis, rice, cucumber, grapevine and tobacco plants (Ahn et al., 2005; Bahuguna et al., 2012; Borges et al., 2009; Boubakri et al., 2012; Denslow et al., 2005). Seed treatments with menadione, riboflavin (vitamin B2), folic acid (vitamin B9), niacin (vitamin B3), pyridoxine and biotin (vitamin B9) protected pearl millet plants against downy mildew pathogen, Sclerospora graminicola, by inducing disease resistance (Pushpalatha et al., 2007). Abiotic stress tolerance of plants could be enhanced by exogenous vitamin applications. Different abiotic stresses like cold, high salinity and herbicide paraquat led to elevated thiamine accumulation in Arabidopsis. Arabidopsis plants supplemented with thiamine increased oxidative stress tolerance by excess superoxide anion production, suggesting antioxidant property of thiamine (Tunc-Ozdemir et al., 2009). Maize seedlings under drought, high salinity and oxidative stresses have increased thiamine content compared to the plants under normal growing condition, although it has not been demonstrated that exogenous thiamine provided enhanced abiotic stress tolerance in the maize plants under adverse environments (Rapala-Kozik et al., 2008). However, limited information is available in vitamin-induced disease resistance and abiotic stress tolerance in tomato plants so far.
The present study was carried out to evaluate in vitro antibacterial and antifungal efficacies of four different vitamins thiamine, niacin, pyridoxine and menadione on R. solanacearum and Botrytis cinerea. Differential tomato protection against bacterial wilt and grey mould mediated by these vitamin treatments was also investigated to show possibility of the vitamin treatments in the eco-friendly tomato disease management.
Materials and Methods
Plant and pathogen growths
Tomato plants (cv. Cupirang) were grown in commercial soil mixture named Toshil (Shinan Growth Co., Ltd., Jinju, Korea) in pots (8 cm in diameter, 7.5 cm in height) in a walk-in growth chamber in which temperatures ranged from 19–21°C at night and 26–28°C during the day with 60% of relative humidity. Third true leaves were detached from 5-leaf stages of tomato plants and used for bacterial and fungal inoculation.
R. solanacearum strain GMI1000 were cultured in 4 ml of CPG broth (1 g of casamino acid, 10 g of peptone, and 5 g of glucose per litre) by shaking overnight at 30°C (Schaad et al., 2001). After centrifugation of the bacterial culture, bacterial suspension was adjusted at optical density at 600 nm wavelength (OD600) = 0.15 to set 108 colony-forming unit [cfu]/ml in sterile water according to our previous studies (Hong et al., 2013; Lee et al., 2012). B. cinerea KACC40573 (from the Korean Agricultural Culture Collection) was cultured on 1/2 potato dextrose agar (PDA) media at 20°C for 3 days and 14 days, for mycelial growth and conidial germination assays, respectively. Conidial suspension was prepared in 1/4 potato dextrose broth.
Vitamin treatments
Thiamine hydrochloride (Daejung Chemicals & Metals Co., Ltd., Siheung, Korea), nicotinic acid sodium salt (Tokyo Chemical Industry Co., Ltd., Tokyo, Japan), pyridoxine hydrochloride (Tokyo Chemical Industry Co., Ltd.) and menadione sodium bisulfite (Sigma-Aldrich Co., St. Louis, MO, USA) prepared in distilled water were applied for treatment with thiamine, niacin, pyridoxine and menadione, respectively. Different concentrations of the four vitamins (0, 0.5, 1, 2, 5, 10, and 20 mM) were supplemented in 4 ml of CPG broth for R. solanacearum in vitro cultures and in 10 ml of dipping solution for protection assay of the detached tomato leaves. Same concentrations of the four vitamins were added in 1/4 potato dextrose broth and 1/2 PDA for in vitro conidial germination and mycelial growth of B. cinerea, respectively. Different vitamin solutions prepared in distilled water foliar-sprayed onto the tomato seedlings one day before B. cinerea inoculation.
In vitro growth of R. solanacearum during liquid culture
In vitro growth of R. solanacearum was investigated indirectly using a spectrophotometer after liquid culture. Forty microliter aliquot of the bacterial suspension (108 cfu/ml) was inoculated to 4 ml of CPG broth in 15-ml conical tubes to make bacterial inoculum 106 cfu/ml prior to overnight culture at 30°C. Different concentrations of thiamine, niacin, pyridoxine and menadione were also added to the initial bacterial culture. When OD600 value of the untreated bacterial culture arrived at 0.25 ± 0.02, all the bacterial cultures were stopped, and then OD600 value of each bacterial culture was measured. Relative OD600 value of the vitamin-treated bacterial cultures was calculated comparable to those of the untreated cultures to demonstrate bacterial growth affected by the different concentrations of the four vitamins. Independent experiments were conducted repeatedly four times, and four replicates were prepared for each experiment.
Bacterial inoculation and disease evaluation
The detached tomato leaves were inoculated using a petiole-dipping method described in our previous study (Lee et al., 2012). All leaflets except for terminal three leaflets were removed, and fresh weight (FW) of the leaves was measured just before bacterial inoculation. Petioles of the detached tomato leaves were dipped into 10 ml of the solution containing R. solanacearum (106 or 107 cfu/ml) supplemented with different concentrations of the vitamins in 50-ml conical tubes. FW of the inoculated leaves were measured daily. Relative FW from the vitamin-treated leaves were compared to those from untreated ones, and expressed as percentage (%).
In vitro germination and mycelial growth of B. cinerea
In vitro antifungal activity of the vitamins against B. cinerea was evaluated according to the modified methods of our previous studies (Hong et al., 2015; Kim et al., 2013).
Conidial suspension (5 × 105 conidia/ml) of B. cinerea was treated with different concentrations of the four vitamins in 1.5 ml-microtubes. Four drops (20 μl/drop) of the vitamin-untreated and -treated conidial suspensions were incubated on sterilized glass slides without covering cover glasses in moist chambers at 20°C for 6 h, at which circa (ca.) 90% of the conidia germinated. Trypan blue-stained conidia were counted under a light microscope. Relative conidal germination was calculated compared to those of the untreated conidia, and expressed as percentage (%). Mycelial agar plugs (5 mm in diameter) was inoculated on the center of PDA media supplemented with different concentrations of the four vitamins. The fungal colony diameter was measured 3 days after incubation at 20°C. Relative mycelial growth was calculated comparable to those of the untreated mycelia, and expressed as percentage (%).
Fungal inoculation and disease evaluation
Ten microliter of the conidial suspension (105 conidia/ml) was dropped at the center of adaxial surface of the detached tomato leaves. After fungal inoculation, the leaves were placed on water-saturated cheesecloth in a plastic box (13 × 9 × 5 cm) to maintain humid conditions and to let lesions develop at 20°C. Diameters of brown necrotic lesions on the inoculated leaves were measured at 4 days after inoculation.
Statistical analyses
Analysis of variance (ANOVA) was used to determine the effects of treatments. Means were compared using least significant difference tests. Statistical analysis was performed with the SAS version 8.1 (SAS Institute Inc., Cary, NC, USA).
Results
In vitro antibacterial activities of vitamins on R. solanacearum
Direct antibacterial activities of the four vitamins (thiamine, niacin, pyridoxine and menadione) against R. solanacearum were evaluated (Fig. 1). The four vitamins have different in vitro bactericidal effects against R. solanacearum in a dose-dependent manner. The bacterial growth was not affected by 0.5–5 mM of thiamine. Ten mM of thiamine drastically arrested the bacterial growth to ca. 34.5% compared to that in the untreated cultures, and no bacteria were grown in the liquid culture supplemented with 20 mM of thiamine. Growth of R. solanacearum was relatively slightly retarded to ca. 91.4% by 1 mM of niacin, and increasing niacin concentrations led to gradual limit bacterial growth to ca. 46.4% by 20 mM of niacin. Lower concentration of pyridoxine (0.5–5 mM) did not change the bacterial growth, whereas higher concentration of pyridoxine (10 mM) was also effective in inhibiting the bacterial growth to ca. 61.7%. Twenty mM of pyridoxine significantly suppressed the bacterial growth to ca. 14.2%. Treatment with menadione dramatically suppressed the bacterial growth by 0.5 mM concentration, and then bacterial growth gradually decreased to ca. 0.8% by 2 mM of menadione. More than 5 mM of menadione completely restrained the bacterial growth.

Effect of different vitamins on in vitro growth of Ralstonia solanacearum. In vitro bacterial growths in response to different concentrations of four vitamins (thiamine, niacin, pyridoxine, and menadione) during liquid cultures were evaluated. Bacterial numbers were initially inoculated with 106 cfu/ml and indirectly measured using a spectrophotometer with optical density at 600 nm. Relative bacterial growth in the vitamin-treated cultures was expressed as percentage (%) compared to that in non-treated cultures. Error bars represent the standard errors of the means of the four independent experimental replications. Means followed by the same letter are not significantly different at 5% level by least significant difference test. The same letter above bars represented no significant difference between treatments.
Protection efficacies of vitamins against tomato bacterial wilt
Vitamin-mediated protection against tomato bacterial wilt was evaluated in planta using the detached leaves by the petiole-dipping inoculation (Fig. 2A). Increasing concentrations (0–20 mM) of thiamine, niacin, pyridoxine and menadione were applied to the detached leaves simultaneously inoculated with the bacterial suspension of R. solanacearum, and the bacterial wilting symptom was assessed at 3 days post-inoculation (dpi). Treatment with the four vitamins led to different disease suppression against tomato bacterial wilt. Lower concentration of thiamine (0.5 and 1 mM) could not reduce the bacterial wilt, whereas relatively higher doses of 2 to 20 mM were effective in delaying tomato bacterial wilting. But more than 5 mM of thiamine had phytotoxic effects on the detached tomato leaves indicated by arrow in Fig. 2. Disease suppression by 2 mM thiamine without phytotoxicity was only limited at 3 dpi, and no disease control was found at 5 dpi (data now shown). Lower concentration of niacin (0.5–2 mM) had no effect on the FW change, but treatment with relatively higher niacin doses was followed by decreased FW of the leaves. More than 5 mM of niacin had phytotoxic effects on the detached tomato leaves indicated by arrow. Pyridoxine treatment did not show any FW change by 5 mM concentration, and 10 mM of pyridoxine increased FW with phytotoxic effect. Twenty mM of pyridoxine was rather phytotoxic but decreased FW loss. Menadione was also effective in decreasing the FW. Narrow range of menadione doses (5–10 mM) conferred the protection efficacy. Twenty mM of menadione slightly decreased FW, and no FW difference was found compared to that in the untreated bacterial culture. More than 5 mM of menadione also led to phytotoxic effect indicated by arrow.
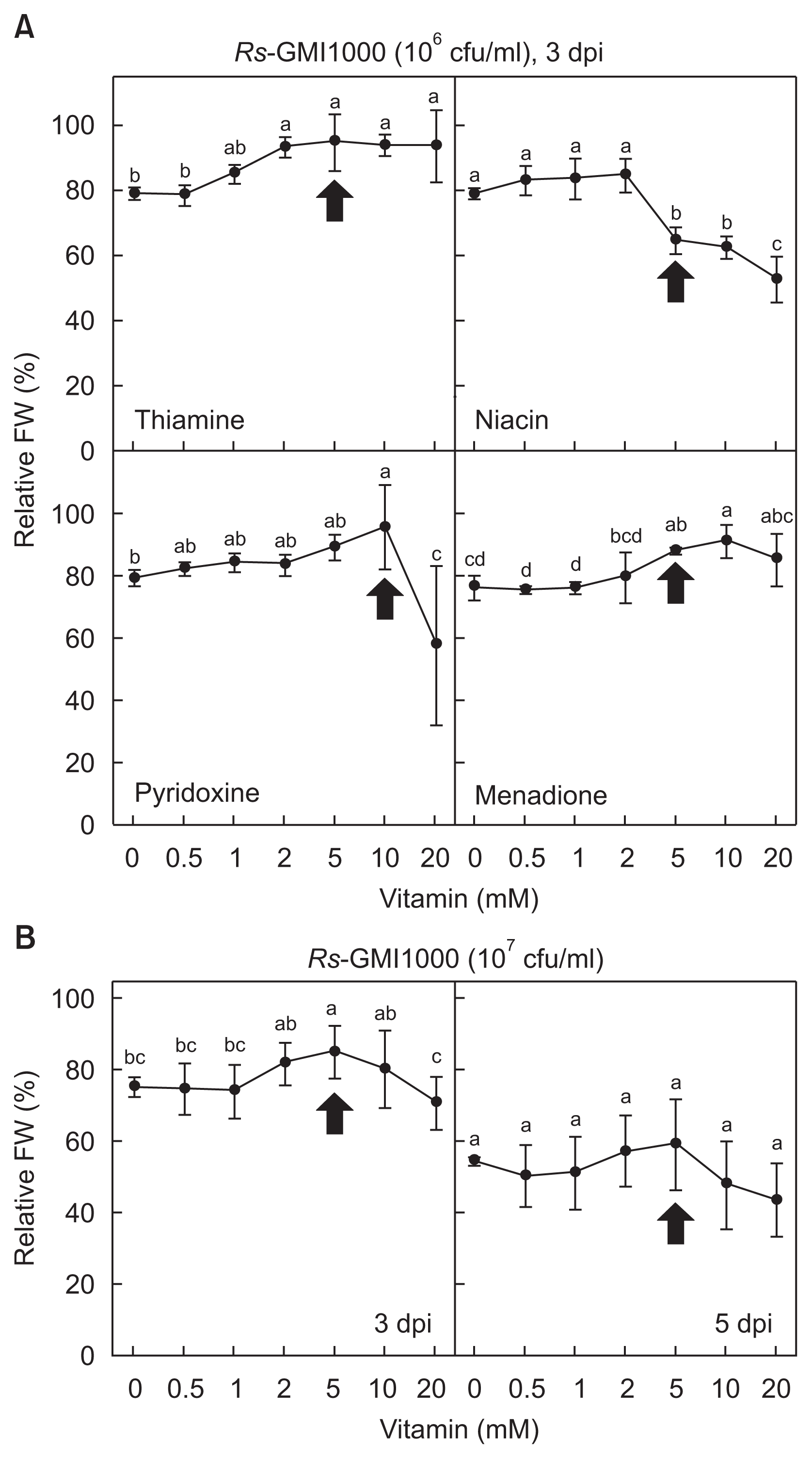
Disease suppression by vitamin treatments against bacterial wilt of detached tomato leaves. (A) Different concentrations of four vitamins (thiamine, niacin, pyridoxine, and menadione) were evaluated for disease development of the tomato bacterial wilt at 3 days post-inoculation (dpi). Relative fresh weight (FW) of the detached tomato leaves by petiole-dipping inoculation with R. solanacearum (106 cfu/ml) was shown. Protection by vitamin treatment against tomato bacterial wilt was presented as relative FW (%) of the vitamin-treated leaves compared with that of the inoculated leaves without any vitamin. Arrows indicate the minimum visible phytotoxic doses of each vitamin to the detached tomato leaves. (B) Relative reduced wilting symptom against tomato bacterial wilt by R. solanacearum (107 cfu/ml) with different doses of thiamine treatment at 3 and 5 dpi. Error bars represent the standard errors of the means of the four independent experimental replications. Means followed by the same letter are not significantly different at 5% level by least significant difference test. The same letter above bars represented no significant difference between treatments.
We investigated whether thiamine-mediated disease protection was still effective against higher bacterial inoculum (Fig. 2B). Relatively higher numbers of bacteria (107 cfu/ml) were inoculated to the detached tomato leaves in the presence of different dose of thiamine, and disease progression was evaluated. Only 5 mM of thiamine treatment effectively reduced the bacterial wilt at 3 dpi, and any dose of thiamine did not suppress the symptom development at 5 dpi.
Mixture of two vitamins for reducing in vitro growth of R. solanacearum and tomato bacterial wilt
Two different vitamins were applied together to investigate whether synergistic effects on in vitro bacterial growths and tomato protection efficacies were demonstrated or not (Fig. 3).
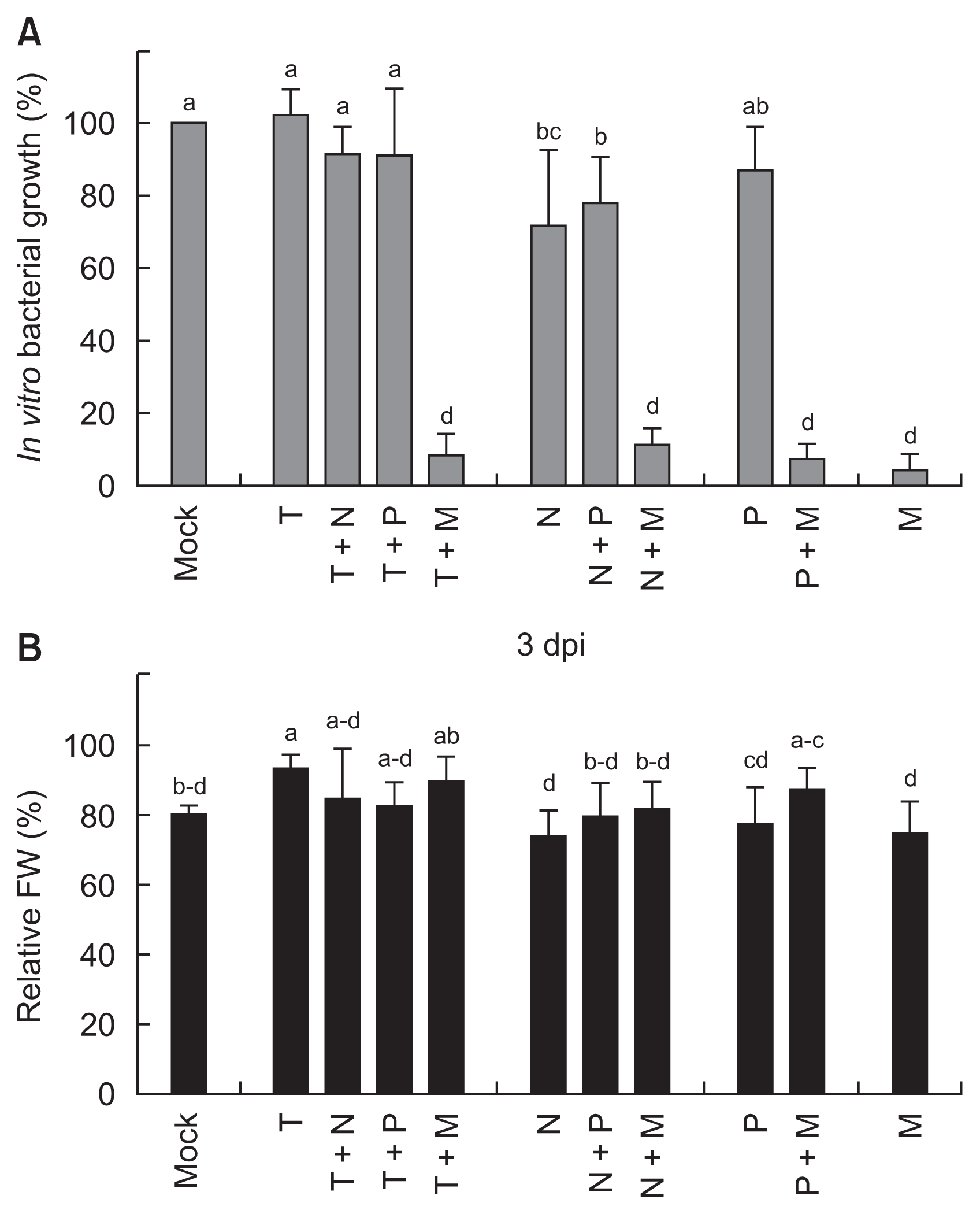
Combined treatment effects of two different vitamins on in vitro antibacterial activities to Ralstonia solanacearum and in planta protection against tomato bacterial wilt. (A) Relative in vitro growths of R. solanacearum during liquid cultures supplemented with combination of two different vitamins were investigated. Bacterial numbers were initially inoculated with 106 cfu/ml and indirectly measured using a spectrophotometer with optical density at 600 nm. Relative bacterial growth in vitamin-treated cultures was expressed as percentage (%) compared to that in non-treated cultures. (B) Relative protection against tomato bacterial wilt by R. solanacearum (106 cfu/ml) with different combinations of vitamins 3 days post-inoculation (dpi). Error bars represent the standard errors of the means of four independent experimental replications. Means followed by the same letter are not significantly different at 5% level by least significant difference test. The same letter above bars represented no significant difference between treatments. T, 2 mM thiamine; N, 2 mM niacin; P, 2 mM pyridoxine; M, 0.5 mM menadione; FW, fresh weight.
Synergistic antibacterial activity of vitamin mixtures on the bacterial growth was not found (Fig. 3A). Two mM of thiamine had no direct antibacterial activity shown in Fig. 2A, and additional treatments with niacin or pyridoxine did not alter the bacterial growth. Slightly suppressed bacterial growth by niacin alone was not changed by additional treatment with pyridoxine. Significant reductions of the bacterial growth by thiamine + menadione mixture and niacin + menadione mixture were same with the bacterial growth treated with menadione alone.
In planta protection efficacy was investigated with different vitamin mixtures in the detached tomato at 3 dpi (Fig. 3B). Only treatment with 2 mM of thiamine delayed loss of FW by the bacterial infection (106 cfu/ml). Supplemental vitamin into thiamine-treated bacterial cultures could not synergistically increase tomato protection. Other combinations of two vitamin mixtures also were not efficient in minimizing FW loss by the bacterial infection.
Different in vitro antifungal activities of the four vitamins during conidial germination and mycelial growth of B. cinerea
The four vitamins were evaluated in terms of their in vitro activities suppressing conidial germination of B. cinerea (Fig. 4A). Relatively higher concentrations of thiamine (10 and 20 mM), niacin (20 mM) and pyridoxine (5–20 mM) slightly reduced the conidial germination. However, 5 and 10 mM of menadione treatment drastically inhibited the conidial germination to ca. 26% and 1.25%, respectively. Treatment with 20 mM of menadione completely curbed the germination of B. cinerea at 6 h after incubation of B. cinerea.
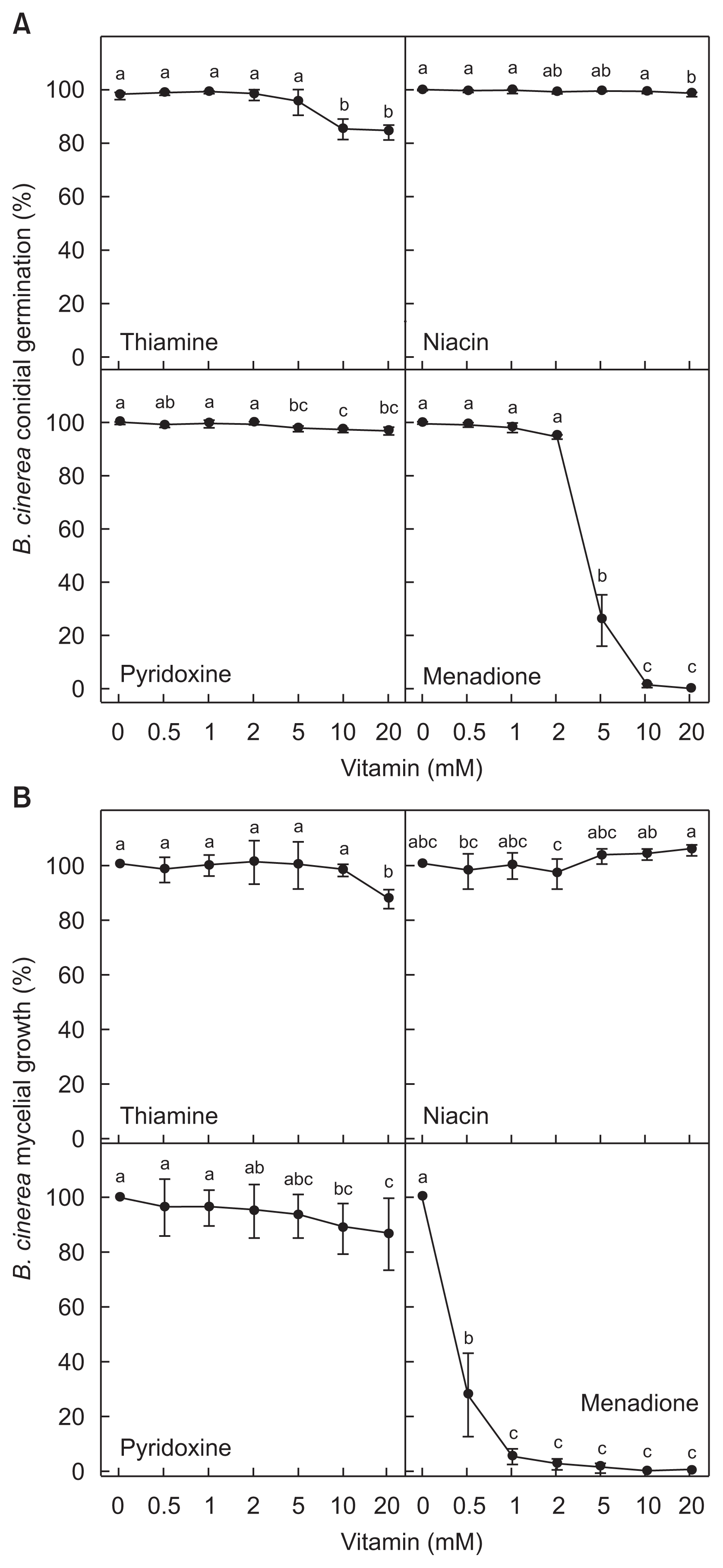
Effect of different vitamins on in vitro conidial germination and mycelial growth of Botrytis cinerea. (A) Relative conidial germinations in the vitamin-treated cultures on glass slides expressed as percentage (%) compared to that in non-treated conidia. (B) Relative mycelial growths in the vitamintreated cultures on potato dextrose agar media expressed as percentage (%). Means followed by the same letter are not significantly different at 5% level by least significant difference test. The same letter above bars represented no significant difference between treatments.
Efficient antifungal activity of the menadione was also shown during mycelial growth (Fig. 4B). Relatively higher concentrations of thiamine (20 mM) and pyridoxine (10–20 mM) slightly reduced the mycelial growth, and niacin did not show any mycelial growth inhibiting activities. At least 0.5 mM of menadione distinctly suppressed the mycelial growth to ca. 28%, and more than 1 mM of menadione considerably inhibited the mycelial growth to lower than ca. 5.5%.
Synergistic effects of two vitamins on in vitro growth of B. cinerea and grey mould lesion development on the tomato leaves
Each vitamin was mixed with one other vitamin to investigate synergistic effects on in vitro fungal growths and tomato protection efficacies against grey mould disease (Fig. 5).
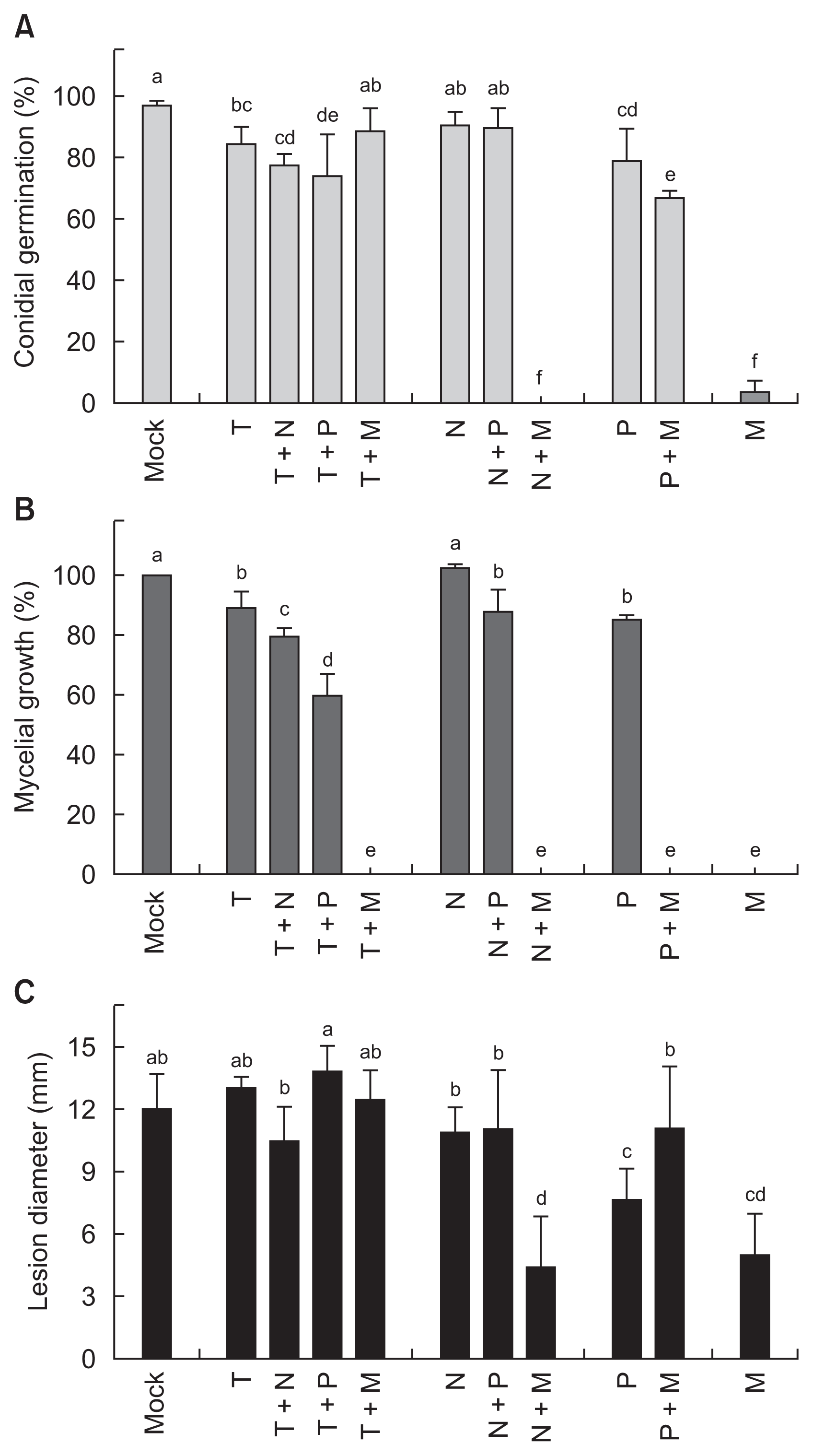
Combined treatment effects of different vitamins on in vitro antifungal activities to Botrytis cinerea and in planta protection against grey mould infection in tomato leaves. (A) In vitro conidial germination and (B) in vitro mycelial growth of B. cinerea treated with different combinations of vitamins. (C) Lesion development on the B. cinerea-inoculated tomato leaves pretreated with different combinations of vitamins. Means followed by the same letter are not significantly different at 5% level by least significant difference test. The same letter above bars represented no significant difference between treatments. T, 20 mM thiamine; N, 20 mM niacin; P, 20 mM pyridoxine; M, 20 mM menadione.
Mixture of two vitamins did not significantly enhance the conidial germination inhibitory activities of single vitamin treatments (Fig. 5A). Slightly reduced conidial germination by 20 mM of thiamine was suppressed more by additional treatment with niacin or pyridoxine. Niacin treatment with pyridoxine did not change the conidial germination. Pyridoxine-inhibited conidial germination was suppressed more by menadione addition that completely abolished the germination alone.
Mixture of two vitamins enhanced the mycelial growth inhibitory activities of single vitamin treatments (Fig 5B). Treatment with 20 mM of thiamine showed slight inhibitory activity against B. cinerea mycelial growth. Additional treatment with niacin or pyridoxine relatively increased the antifungal activities against the mycelial growth. Menadione completely suppressed the mycelial growth of B. cinerea. Niacin treatment alone did not alter mycelial growth, but pyridoxine single treatment suppressed it. Mycelial growth by niacin and pyridoxine mixture treatment was similar to that reduced by pyridoxine treatment alone.
Grey mould suppressing activities of two vitamin mixtures were evaluated in the detached tomato leaves (Fig. 5C). Thiamine treatment alone and thiamine mixture with other vitamin did not show any disease suppression effect. Neither niacin treatment alone nor niacin-pyridoxine mixture treatment was effective in reducing grey mould symptom. Most effective disease reducing activity of niacin-menadione mixture was not different from that of menadione treatment alone. Pyridoxine or menadione treatment alone significantly reduced grey mould lesion size.
Dose-dependent reduction in tomato grey mould by menadione pretreatment
Pretreatment with increasing menadione doses could gradually reduce the grey mould on the detached tomato leaves (Fig. 6).
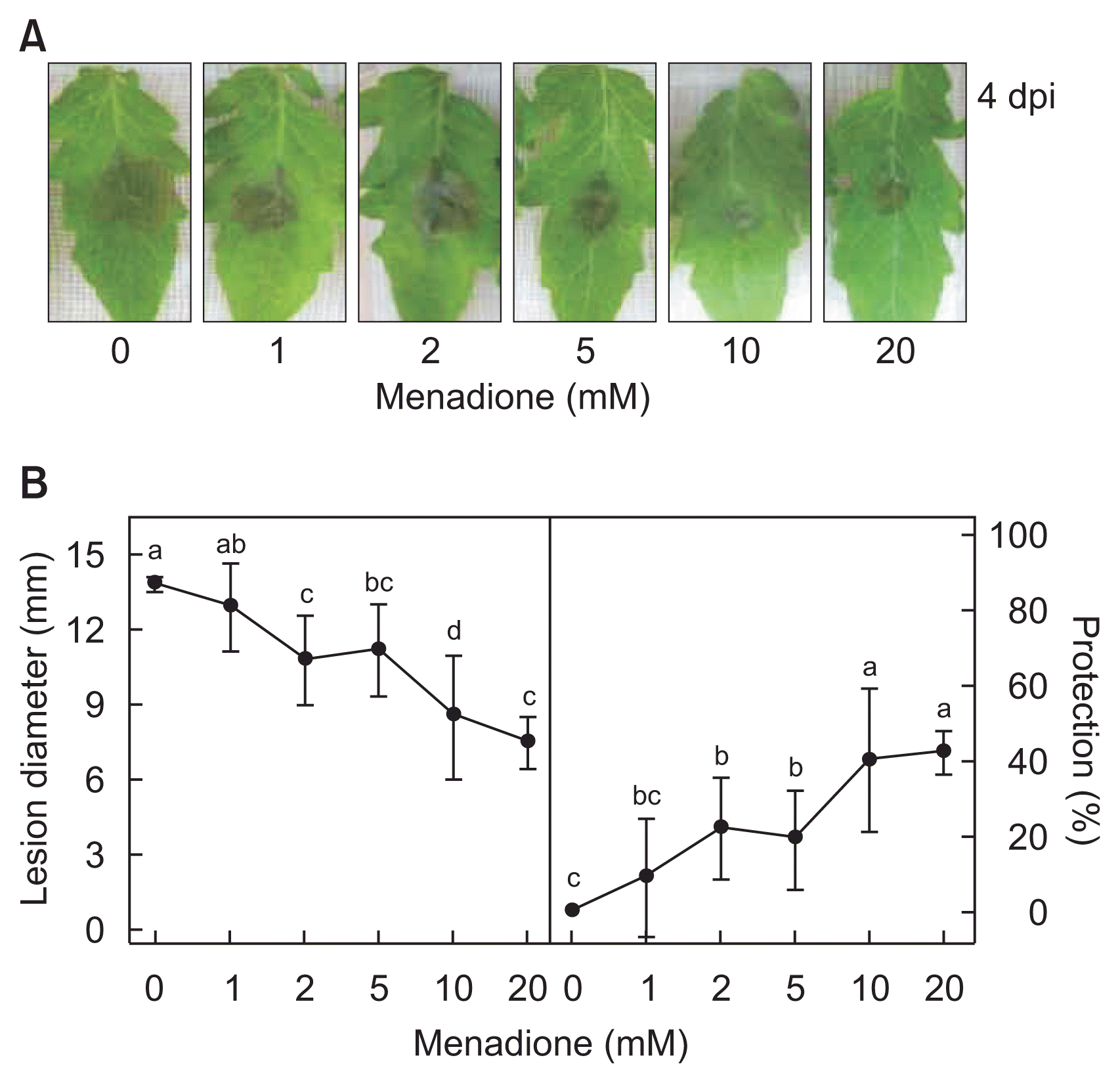
Suppression of grey mould in tomato leaves by menadione pretreatment. (A) Reduced symptom development on the Botrytis cinerea-inoculated tomato leaves pretreated with different concentrations of menadione. Photos were taken 4 days post-inoculation (dpi). (B) Decreased lesion diameters on the grey mould infected tomato leaves and relative protection efficacies mediated by menadione pretreatment. Means followed by the same letter are not significantly different at 5% level by least significant difference test.
Increasing menadione pretreatments one day prior to the fungal inoculation efficiently inhibited lesion enlargement on the B. cinerea-inoculated tomato leaves (Fig. 6A). Lesion diameters of the inoculated tomato leaves with or without menadione pretreatment were measured, and protection efficacies were calculated (Fig. 6B). Without menadione, brown necrotic lesions were spreading through the inoculated leaves with ca. 13.8 mm in diameter at 4 dpi. Pretreatment with 2 mM of menadione began to reduce the lesion to ca. 10.8 mm in diameter. Higher menadione concentrations of 10 and 20 mM led to significant decrease of the lesion diameter to ca. 8.5 and ca. 7.4 mm, respectively. Increasing tomato protection efficacies were positively correlated with accretive menadione-reduced lesion formation.
Discussion
Eco-friendly disease controls have been recommended during tomato productions owing to increasing concerns about environment and human health. Recently, treatments with clove oil and H2O2-nitric oxide mixture were suggested to reduce tomato bacterial wilt in our previous studies (Hong et al., 2013; Lee et al., 2012). In the current study, we also evaluated effects of the four vitamins thiamine, niacin, pyridoxine and menadione to manage bacterial wilt and grey mould diseases in tomato plants as alternatives for chemical pesticides.
Some vitamins have shown antimicrobial activities. Thiamine dilauryl sulfate has antimicrobial and preservative effects on human pathogenic bacteria E. coli and Staphylococcus aureus (Kim et al., 2005). Menadione showed in vitro antibacterial activities to phytopathogenic bacteria such as Xanthomonas campestris pv. phaseoli and X. oryzae pv. oryzae causing leaf blight of bean and rice plants, respectively (Mongkolsuk et al., 1996; Vattanaviboon et al., 2003). However, antibacterial activities of niacin and pyridoxine were hardly detected so far. The four vitamins we investigated, thiamine, pyridoxine, niacin and menadione, showed different levels of in vitro antibacterial activity against R. solanacearum in dose-dependent manners, which suggested that these vitamins can be applied to the tomato plants to reduce bacterial wilt unless phytotoxicity is not found.
Treatments with thiamine, pyridoxine and menadione reduced the tomato bacterial wilt, whereas niacin did not show any protective effect. Notably, higher doses of thiamine, pyridoxine and menadione showing bacterial wilt reducing activity led to phytotoxic effect on the tomato leaves. Only 2 mM of thiamine treatment resulted in decreased bacterial wilt caused by the lower inoculum (106 cfu/ml) without any adverse effect on the tomato leaves. But the same concentration of thiamine was not effective to control the tomato bacterial wilt caused by the higher inoculum (107 cfu/ml). It is noteworthy that the 2 mM of thiamine did not show direct antibacterial activity during in vitro culture, indicating the thiamine-induced tomato protection against the bacterial wilt may be mediated by enhanced defence responses of tomato plants partially, not by bactericidal effect only. Thiamine is related to biotic and abiotic stress tolerance in many plant species, but its involvement in tomato protection against bacterial wilt has never been demonstrated so far (Ahn et al., 2005; Boubakri et al., 2012; Rapala-Kozik et al., 2012; Tunc-Ozdemir et al., 2009). Application of acibenzolar-S-methyl triggered tomato disease resistance to the bacterial wilt, suggesting that tomato bacterial wilt can be controlled by inducing resistance using various chemical elicitors (Pradhanang et al., 2005). Thiamine-mediated disease resistance has been investigated in many plants against broad range of plant pathogens. Direct antimicrobial activities of thiamine against rice blast fungus (Magnaporthe grisea), rice leaf blight bacterium (X. oryzae pv. oryzae), cucumber anthracnose fungus (Colletotrichum lagenarium), and Arabidopsis leaf speck bacterium (Pseudomonas syringae pv. tomato) have not been determined, although thiamine application conferred enhanced disease resistance to these plants to the pathogen challenges (Ahn et al., 2005). Recently, thiamine showed direct anti-oomycete activity during sporangia germination of Plasmopara viticola causing downy mildew in grapevine, as well as indirect triggering activity of the grape defence grapevine via H2O2 generation, defense-related gene expression, callose deposition, phenolics accumulation and onset of hypersensitive cell death (Boubakri et al., 2012). Elevated contents in H2O2 and phenolics, and activation of phenylalanine ammonia lyase and superoxide dismutase were involved in the thiamine-induced resistance of rice plants against sheath blight by Rhizoctonia solani fungus (Bahuguna et al., 2012). In this context, pretreatment with 2 mM of thiamine can drive induced resistance of tomato plants against R. solanacearum. Application timing and frequency may be reconsidered to improve thiamine-induced resistance of tomato plants against R. solanacearum. Molecular and biochemical defences of the thiamine-mediated protection of tomato plants need to be investigated as well.
Pyridoxine and menadione were unveiled as potent agents for controlling grey mould of tomato plants. Direct antifungal activity of pyridoxine was found during the conidial germination and mycelial growth of B. cinerea, although its activity has not been demonstrated to many other phytopathogenic fungi yet. Pyridoxine biosynthesis was important to mediate defence activation against B. cinerea in tomato plants. Pyridoxine biosynthetic gene expressions were induced in the tomato leaves inoculated by B. cinerea, whilst tomato plants silencing these gene expressions exhibited lowered pyridoxine content followed by highly accumulated reactive oxygen species (ROS) in response to B. cinerea infection (Zhang et al., 2014). The increased ROS may facilitate the necrotrophic fungal invasion into the host tissues much easier (Lehmann et al., 2015). It was found that pyridoxine pretreatment (20 mM) at least 1 day prior to B. cinerea inoculation efficiently arrested lesion enlargement on the inoculated tomato leaves in this study. These findings indicate that pyridoxine played critical roles to reduce tomato grey mould via direct antifungal activity and enhancement of mitigating the fungal infection-triggered oxidative stress in plant tissues. Pyridoxine-mediated disease resistance may not be limited in tomato plants inoculated by B. cinerea. Arabidopsis containing lowered pyridoxine content also showed enhanced susceptibility to B. cinerea and P. syringae pv. tomato infections (Zhang et al., 2015). By contrast, pyridoxine treatment rather accelerated chlorotic and necrotic symptom development of tobacco plants inoculated by P. syringae pv. tabaci, implying that different role of pyridoxine for controlling plant diseases dependent on plant-pathogen relationship (Denslow et al., 2005).
More than 2 mM of menadione led to reduction in grey mould on the detached tomato leaves in a dose-dependent manner. Foliar-spraying 20 mM menadione efficiently protected the tomato leaves more than 40% compared to untreated control plants against B. cinerea. Tomato protection by menadione could be due to direct antifungal activity against B. cinerea found in this study. Menadione, superoxide generator, has been suggested to trigger oxidative stresses directly in fungal cells and effectively arrest the growth of phytopathogenic fungi B. cinerea, Fusarium graminearum, M. grisea, and Ustilago maydis (Nikolaou et al., 2009; Yan et al., 2011). Nevertheless, we cannot exclude possibility of menadione-enhanced tomato immunity against B. cinerea infection. It was evident that highly activated transcriptional reprogramming and phytoalexin accumulation were involved in the menadione-induced disease resistant plants such as Arabidopsis, banana and oilseed rape (Borges et al., 2003a, 2003b, 2009). Interestingly, a recent report found that menadione regulated DNA cytosine methylation in the Arabidopsis plants to increase osmolyte proline accumulation to cope with high salt stress (Jiménez-Arias et al., 2015). Genetic and epigenetic control for tomato disease resistance induced by menadione remains elucidated.
Taken together, different vitamin treatments were evaluated to control bacterial wilt and grey mould in tomato plants. Thiamine delayed the tomato bacterial wilt, and pyridoxine and menadione were effective in reducing the grey mould disease. Practical applications of the disease-suppressing vitamins during tomato cultures in open fields and glasshouses are being anticipated for eco-friendly and integrated tomato disease management.
Acknowledgments
This research was financially supported by Gyeongnam National University of Science and Technology (GNTech) Grant 2015 to Jeum Kyu Hong.
Notes
Articles can be freely viewed online at www.ppjonline.org.