Reaction of Global Collection of Rye (Secale cereale L.) to Tan Spot and Pyrenophora tritici-repentis Races in South Dakota
Article information
Abstract
Rye (Secale cereale L.) serves as an alternative host of Pyrenophora tritici-repentis (PTR) the cause of tan spot on wheat. Rye is cultivated as a forage or cover crop and overlaps with a significant portion of wheat acreage in the U.S. northern Great Plains; however, it is not known whether the rye crop influences the evolution of PTR races. We evaluated a global collection of 211 rye accessions against tan spot and assessed the diversity in PTR population on rye in South Dakota. All the rye genotypes were inoculated with PTR races 1 and 5, and infiltrated with Ptr ToxA and Ptr ToxB, at seedling stage. We observed 21% of the genotypes exhibited susceptibility to race 1, whereas, 39% were susceptible to race 5. All 211 accessions were insensitive to both the Ptr toxins. It indicates that though rye exhibits diversity in reaction to tan spot, it lacks Ptr ToxA and ToxB sensitivity genes. This suggests that unknown toxins or other factors can lead to PTR establishment in rye. We characterized the race structure of 103 PTR isolates recovered from rye in South Dakota. Only 22% of the isolates amplified Ptr ToxA gene and were identified as race 1 based on their phenotypic reaction on the differential set. The remaining 80 isolates were noted to be race 4. Our results show that races 1 and 4 are prevalent on rye in South Dakota with a higher frequency of race 4, suggesting a minimal role of rye in the disease epidemiology.
Introduction
The fungus Pyrenophora tritici-repentis (Died.) Drechs. (anamorph = Drechslera tritici-repentis [Died.] Shoem.) is an important foliar pathogen of wheat (Triticum aestivum L.), and causes tan spot throughout major wheat-growing countries (De Wolf et al., 1998). The fungus has a broad host-range and can infect barley, oat, rye and many non-cereal grasses (Ali and Francl, 2003; Hosford, 1971; Krupinsky, 1992; Sprague, 1950). The fungus produces oval-shaped tan necrotic spots on the leaf surrounded by a chlorotic halo with a pinhead size black spot in the center on susceptible wheat and alternative hosts genotypes (Hosford, 1971; Krupinsky, 1992). Eight races have been identified in the fungal population based on an isolate’s ability to produce necrosis and/or chlorosis symptoms on appropriate tan spot differential genotypes (Lamari et al., 2003). The fungus is also known to produce three host-selective toxins, Ptr ToxA, Ptr ToxB, and Ptr ToxC, which are associated with development necrosis and chlorosis symptoms (Faris et al., 2013; Lamari et al., 2003). Of these 8 races, 1 through 5 have been reported in North America (Ali and Francl, 2003; Lamari et al., 2003). The most prevalent race on wheat worldwide is race 1, whereas race 4 is the least observed (Aboukhaddour et al., 2013; Ali and Francl, 2002, 2003; Lamari et al., 2003). In contrast, race 4 was the most prevalent race observed on its alternative hosts such as non-cereal grasses (Ali and Francl, 2003).
Knowledge of genetic variation in the pathogen virulence and the host plant is crucial in the development of effective and durable disease management strategies especially breeding for cultivars with durable disease resistance. Variability of virulence and aggressiveness has been reported in the P. tritici-repentis (PTR) isolates recovered from various alternative hosts such as Altai wild rye, basin wild rye, and Russian wild rye (Ali and Francl, 2003; Krupinsky, 1992). However, the prevalence of PTR population on rye has not been studied. Further, diversity to tan spot reaction in rye has not been explored. Alternative host plants can play a significant role in pathogen diversity and disease epidemic, especially when they are cultivated in the vicinity of the economically important host crops (Burdon, 1993). In contrast, alternative hosts can also be a part of the solution by contributing resistance genes to pests and diseases (Dinoor, 1974). Rye (Secale cereale subsp. cereale) is one of the most important cereal crops worldwide that is primarily grown as forage for livestock, cover crop for green manure, or as a food product for use in bread (Bushuk, 2001). Moreover, rye has played a significant role in modern wheat improvement through its contribution of resistance genes for combating leaf rust, stem rust, stripe rust, powdery mildew, Barley yellow dwarf virus (BYDV) and insect resistance to Hessian fly, Russian wheat aphids, and green bug (Rabinovich, 1998; Saulescu et al., 2011; Zhang et al., 2001) as examples.
In the Northern Great Plains (NGP), rye is most often grown as a green manure and a rotational crop that is often planted adjacent to wheat fields. This can potentially play a role in PTR virulence variation and evolution; harbor completely different pathogen population; and/or serve as an additional source of inoculum for tan spot development on wheat. The objectives of this study were (i) to evaluate a global collection of rye genotypes for their reaction to tan spot to determine if rye could be exploited as a source of resistance to PTR; (ii) to determine if rye could be playing any role in the PTR diversity in South Dakota. The study will contribute to our understanding of the genetic diversity for tan spot resistance in rye that could be exploited by both wheat and rye breeders. Further, our study underpins the virulence pattern of PTR on rye that could guide in tan spot management strategies.
Materials and Methods
Evaluation of rye genotypes for tan spot reaction
Two hundred and eleven rye genotypes from 54 countries representing Asia, Africa, Europe, North America and South America, (Supplementary Fig. 1, Supplementary Table 1) were evaluated for their reaction to tan spot using PTR races 1 and 5 and their host selective toxins, Ptr ToxA and Ptr ToxB, respectively. Due to lack of seed, only 171 out of 211 genotypes were evaluated against race 5. The seed of all genotypes were obtained from United State Department of Agriculture-Agriculture Research Service (USDA-ARS) National Small Grains Collection, Aberdeen, Idaho. Additionally, eight rye cultivars, Rymin, Dreb 15, ND 5, Fredrick, ND Dylan, Muskter, Dacold, and Spooner grown in the region were included in this study. All 211 genotypes, except four (Secale varilovii, n = 1; S. cereale subsp. ancestrale, n = 1; S. strictum, n = 2), were of S. cereale subsp. cereale.
Race 1 being most prevalent race worldwide and race 5 having been observed in the NGP, were selected for this study (Abdullah et al., 2016; Ali et al., 1999). SD13-101 isolate of race 1 and SD13-103 isolate of race 5 were used to evaluate two-week old seedlings of all rye genotypes grown in containers (5 × 23 cm) filled with Sunshine Mix (Sun Gro Horticulture, Agawam, MA, USA). The two isolates used in this study were recovered from wheat grown in South Dakota (Abdullah et al., 2016). We planted three seedlings per container (per replication) and a total of nine seedlings (3 replications) of each genotype were tested. The seedlings were watered and fertilized as needed throughout the experimentation. Experiments were conducted in the greenhouse at South Dakota State University with an average temperature of 21°C and 18°C during the day and night, respectively, and a 16-h photoperiod was imposed. All 211 and 171 genotypes were evaluated for their reaction to race 1 and race 5, respectively by following the procedure described by Ali and Francl (2001). In brief, 2-week-old seedlings of all rye genotypes were inoculated individually with race 1 and race 5 by spraying with a spore suspension adjusted to 2,500 spore/ml until runoff with a hand held sprayer (Preval Sprayer; Chicago Aerosol, Coal City, IL, USA). Inoculated seedlings were placed in a humidity chamber set at 100% humidity, using a humidity controller set to mist 16 s every 5 min for 24 h. Thereafter, seedlings were placed on a greenhouse bench for seven days until plants were scored for symptom development using a 1–5 disease rating scale where 1–2 = resistant to moderately resistant; 3–5 = moderately susceptible to susceptible (Lamari and Bernier, 1989). To confirm the success of the inoculation procedure, the wheat differential lines Glenlea (susceptible to race 1), 6B365 (susceptible to race 1), 6B662 (susceptible to race 5), and Salmouni (resistant to race 1 and race 5) were included in the experiment as checks.
Reaction of rye genotypes to Ptr ToxA and Ptr ToxB
All 211- and 171-rye genotypes tested for against race 1 and race 5 were also screened for their reaction to Ptr ToxA and Ptr ToxB, respectively. Seedlings of all genotypes were raised in plastic containers. Three fully expanded second leaves of each genotype were infiltrated individually with purified Ptr ToxA at 10 μg/ml and Ptr ToxB culture filtrates about 10 μl/leaf using a needleless syringe as described by Effertz et al. (1998). The infiltrated leaf area was marked with a permanent non-toxic black marker. Infiltrated leaves were rated for sensitivity three days post-infiltration of Ptr ToxA for presence/absence of necrosis and five days post-infiltration of Ptr ToxB for presence/absence of chlorosis in the infiltrated area. Purified Ptr ToxA and Ptr ToxB culture filtrates were kindly provided by Dr. S. Mienhardt, Department of Plant Pathology, North Dakota State University, Fargo, ND 58102, USA and Dr. T. Friesen, USDA-ARS, Fargo, ND 58102, USA, respectively.
Recovery of PTR isolates from rye leaves
Diseased leaf samples exhibiting tan spot like symptoms were randomly collected from commercial fields and South Dakota State University (SDSU) experimental field plots during 2013 and 2014 growing seasons. Leaf samples were collected from 4 locations in 2013 and five locations in 2014. The samples were collected when the crop was at late milk to soft dough stage. PTR isolates were recovered from the collected leaf samples by following the procedure described by Ali and Francl (2001). Thereafter, leaves of each sample were cut into 1.5 to 2 cm long segments and surface-disinfected with 5% bleach (NaClO). Forty-five randomly selected leaf segments from each sample were placed in a petri dish (9 cm diameter) containing three layers of moist Whatman #1 filter paper. Plates were incubated for 24 h under light at room temperature (21–22°C) and 24 h in dark at 16°C to induce conidial formation. The incubated leaf segments were examined with a stereoscope, and single conidia were collected using a flamed steel needle and then placed on a fresh V8PDA (150 ml of V8 juice, 10 g of potato dextrose agar, 10 g of Difco agar, 3 g of CaCO3, and 850 ml of distilled water) plate (Lamari and Bernier, 1989). In total, 103 isolates were recovered and stored at −20°C until characterized for their race structure.
Genotyping of PTR isolates from rye for Ptr ToxA and Ptr ToxB genes
DNA of all 103 PTR isolates recovered from rye was isolated by growing them individually on V8PDA in 9 cm petri dishes for 5 days (Supplementary Table 2). Mycelia were scraped from the agar surface using a flamed scalpel and placed in a 2 ml microcentrifuge tube. The mycelia were then dried overnight in a water bath at 37°C and were ground into a fine powder using a first prep machine Retsch MM 301 (Retsch, Clifton, NJ, USA). DNA was extracted from mycelia of each isolate by following the procedure of Moreno et al. (2008). The DNA concentration was adjusted to 25 ng/μl using a Nanodrop Spectrophotometer (Counterpane Inc., Tacoma, WA, USA) and run in a 0.8% agarose gel to verify quality. The PTR isolates were genotyped for Ptr ToxA and Ptr ToxB genes by using the Ptr ToxA and Ptr ToxB specific primers developed by Andrie et al. (2007). Conformity of the isolates was determined by using two PTR mating type genes (MAT1-1 and MAT1-2) specific primers suggested by Lepoint et al. (2010). PCRs for specific markers were performed in 20 μl volume including; 2 μl genomic DNA (25 ng/μl), 0.8 μl of each primer (10 mM), 0.5 μl dNTP (200 μM), 2 μl 10× thermophol buffer, 0.2 μl 10 U/ml Taq polymerase and 13.7 μl of molecular biology grade water. PCR reaction for individual primers was conducted in a S-1000 thermal cycler (BioRad, Hercules, CA, USA) using amplification steps of 94°C for 3 min, followed by 30 cycles of 94°C for 45 s, 55°C for 30 s and 72°C for 1 min and a final extension of 72°C for 7 min. We pooled PCR products from housekeeping genes (MAT1-1 or MAT1-2) with Ptr ToxA or Ptr ToxB specific PCR products as a positive amplification control for each isolate. The PCR products were electrophoresed on 1.5% agarose gels and scored with reference to 1 kb ladder (New England Biolabs, Ipswich, MA, USA).
Phenotyping of PTR isolates
For race characterization of all 103 isolates, 2-week-old seedlings of the tan spot differential genotypes Glenlea, 6B365, 6B662, and Salamouni were inoculated individually with each isolate using spore suspensions (2,500 spore/ml). Inoculum preparation and inoculation were performed as described by Ali and Francl (2001). Inoculated seedlings with each isolate were rated for necrosis and chlorosis symptom development and the isolates were grouped under appropriate race (Lamari et al., 2003).
Results and Discussion
Reaction of rye genotypes to tan spot (race 1) and Ptr ToxA
All 211 rye genotypes evaluated against tan spot responded differentially to PTR race 1 by exhibiting reactions that ranged from moderately susceptible (MS) to susceptible (S) and moderately resistant (MR) to resistant (R). Of the eight rye cultivars screened only ND5 exhibited a moderately susceptible (lesion type 3) reaction to race 1 while others showed a moderately resistant reactions. Nearly 20.9% (n = 44) of the genotypes showed susceptibility (lesion type 3–5) to race 1 (Table 1, Supplementary Table 1) while the other 79.1% (n = 167) exhibited resistance (lesion type 1–2). The ratio of susceptible to resistant genotypes against race 1 was about 1:3 in genotypes from Africa (2 susceptible and 6 resistant), Europe (22 susceptible and 67 resistant), North America (10 susceptible and 32 resistant), and South America (4 susceptible and 12 resistant) (Table 1); whereas, about 1:7 (6 susceptible and 46 resistant) in genotypes from Asia suggesting higher prevalence of resistant germplasm or varieties in Asia as compared to other continents.

Reaction of 211 rye genotypes from six continents to Pyrenophora tritici-repentis race 1 and Ptr ToxA
All 211 rye accessions and eight cultivars from NGP, when infiltrated with Ptr ToxA exhibited insensitivity as they did not develop necrosis in the toxin infiltrated areas (Fig. 1). As expected, tan spot differentials Glenlea and 6B365 on inoculation with race 1 developed necrosis and chlorosis symptoms, respectively; whereas, 6B662 and Salamouni remained symptomless. Also, Glenlea exhibited sensitivity (developed necrosis) and Salamouni insensitivity (no necrosis) in the toxin-infiltrated area, validating the inoculation procedure and conformity of race 1 and the toxin viability.
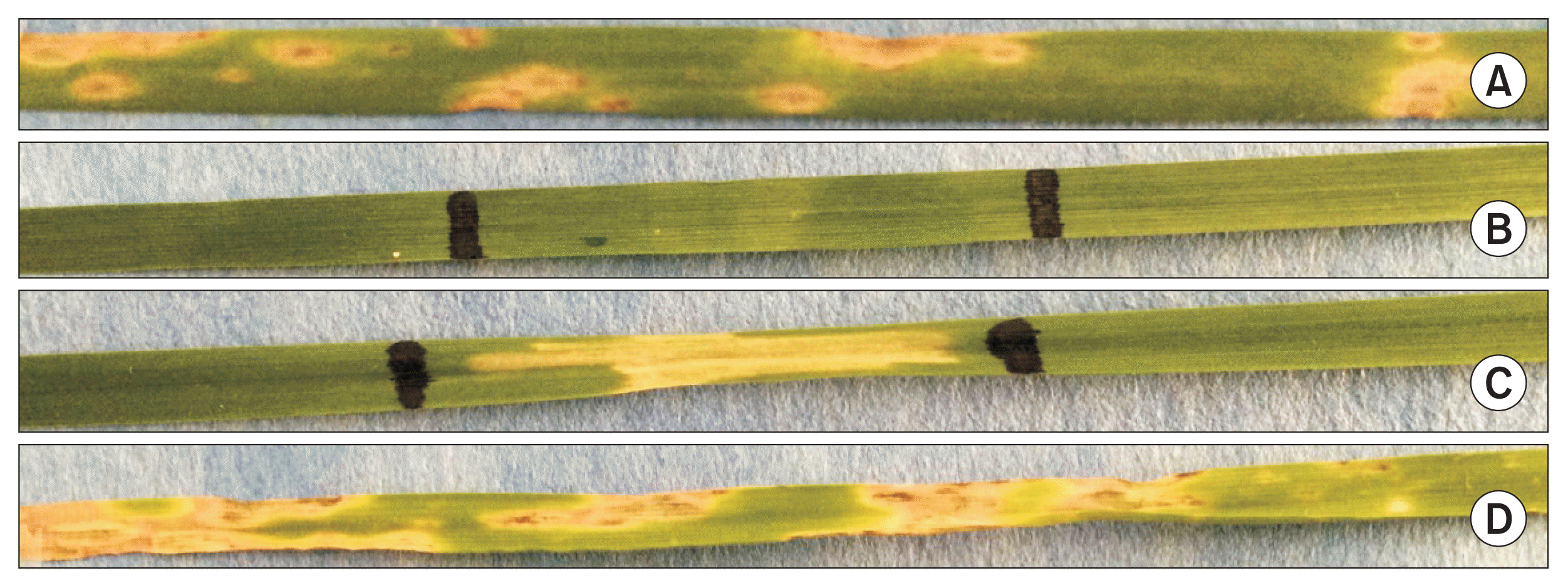
Reaction of rye genotype to Pyrenophora tritici-repentis race 1 and Ptr ToxA (top to bottom). (A) Rye genotype (PI446514) inoculated with race 1 (necrosis), (B) rye genotype (PI446514) infiltrated with Ptr ToxA (no necrosis), (C) wheat genotype Glenlea infiltrated with Ptr ToxA (necrosis), and (D) Glenlea inoculated with race 1 (necrosis).
We observed susceptible to resistant reactions on a large number of rye genotypes to PTR (tan spot) race 1, suggesting variability for resistance to tan spot exists in rye. Our results with a global collection of 211 rye genotypes validate previous studies where a small number of rye accessions were screened and differential response to tan spot was observed (Cox et al., 2005; Hosford, 1971; Oliver et al., 2008a; Postnikova and Khasanov, 1998). Cox et al. (2005) evaluated 10 perennial grass species, including two perennial rye genotypes for tan spot using PTR race 1 and found them resistant, similar to the resistant wheat cultivar ‘Karl 92’ used as a check in their study. Similarly, Oliver et al. (2008a) evaluated 199 wheat-alien species derivatives for tan spot and Stagonospora nodorum blotch resistance and found some of the derivatives from rye were resistant to both diseases and concluded that resistance to tan spot in these wheat alien species derivatives came from the rye. In contrast to these two studies, rye was found equally susceptible as the wheat genotype used as tan spot susceptible check in two independent studies for PTR host range (Hosford, 1971; Postnikova and Khasanov, 1998). However, a single genotype was evaluated in each study to confirm whether rye could be an alternative host of PTR. It is not surprising to observe diverse reactions to tan spot in rye, similar observations have been reported in other alternative hosts of PTR that exhibited from susceptible to resistant reactions (Ali and Francl, 2001; Ali and Langham, 2015; Krupinsky, 1982, 1992). Both resistant and susceptible responses to race 1 were observed among genotypes from all six continents; however, a higher ratio of resistant to susceptible genotypes was observed in all continents (susceptibe:resistant, 1:3) except in Asia (1:7). This could be due to less selection pressure on the pathogen surviving on alternative hosts. Interestingly, all 211 genotypes and 8 cultivars evaluated irrespective of the fungal susceptibility or resistance showed insensitivity to Ptr ToxA, indicating a lack of Tsn1 (Ptr ToxA sensitivity gene). This suggests that Ptr ToxA may not play a significant role in disease development on rye and that perhaps other factors such as unknown toxins may be responsible for tan spot on rye. Similar observations have been reported (Ali et al., 2010; Faris and Friesen, 2005; Noriel et al., 2011; Oliver et al., 2008b; Zhang and Jin, 1998) where wheat and its wild relatives showed susceptibility to Ptr ToxA producing races 1 and 2 but remained insensitive to Ptr ToxA. Our earlier studies along with others have also suggested the possibile involvement of some other potential toxins or mechanisms for compatible tan spot-wheat host-pathogen interaction (Ali et al., 2010; Faris and Friesen, 2005; Noriel et al., 2011; Oliver et al., 2008b; Zhang and Jin, 1998). Very recently, Virdi et al. (2016) studied the role of Ptr ToxA in tan spot development on durum wheat and concluded that role of Ptr ToxA was insignificant. Additionally, the fungal isolates lacking the Ptr ToxA gene, but virulent on both Ptr ToxA sensitive and insensitive wheat genotypes indicates a minimal role of Ptr ToxA; however, the role of Ptr ToxA in tan spot development may be genotype dependent. Alternatively, it is possible that Ptr ToxA lacking isolates may carry additional virulence factors causing disease induction (Ali et al., 2010).
Reaction of rye genotypes to tan spot (race 5) and Ptr ToxB
We screened 171 rye genotypes for their reaction to tan spot (race 5) and observed a range of reactions from moderately susceptible to susceptible and moderately resistant to resistant (Table 2, Supplementary Table 1). Nearly 38.6% (n = 66) of the genotypes exhibited susceptibility while the other 61.4% (n = 105) genotypes were resistant to race 5 (Table 2, Supplementary Table 1). All the 66 genotypes that exhibited susceptibility to race 5 did not exhibit sensitivity to Ptr ToxB (Fig. 2). Tan spot susceptible and resistant rye genotypes were observed from all continents except Australia (Table 2) but the percentage of susceptible to resistant genotypes varied among continents. Of the genotypes with European origin, 47.4% (n = 36) were susceptible and 52.7% (n = 40) were resistant to race 5, whereas, among genotypes from Asia, only 25.0% (n = 11) were susceptible and nearly 75.0% (n = 33) were resistant to race 5 (Table 2). Four (Derb 15, Fredrick, Muskter, and Spooner) of the eight cultivars grown in NGP showed susceptibility with lesion type ranging from 3–5, whereas the other four cultivars were rated as resistant (Supplementary Table 1). Overall, the percent of susceptible genotypes to race 5 was lower (38.6%) as compared to resistant genotypes (61.4%) across all the continents although European genotypes were most susceptible (47.4%). Higher level susceptibility in European rye germplasm to race 5, is expected because Europe (Belarus, Germany, Poland, Ukraine, and western Russia) produces 80% of the rye grown in the world (Bushuk, 2001). Most likely this originates from the use of race 5 susceptible germplasm in the breeding programs. Similar observations were reported by Lamari et al. (2005) in wheat in Canada when they studied the reasons of increased susceptibility in Canadian wheat cultivars to race 5 in the 1960s. They were able to trace it back to one susceptible of the parent “Kanred Winter” of cultivar Thatcher that had been heavily utilized in developing high yielding and stem rust resistance cultivars in Canada and North Dakota, USA in 1960s. Further, 14 of 211 rye genotypes exhibited susceptibility to both race 1 and race 5 (Table 3). The presence of genotypes susceptible to both races may help in the survival of race 1 and race 5 on rye and may lead to the evolution of new races. As expected, the wheat differential genotype 6B662 inoculated with race 5 and infiltrated with Ptr ToxB toxin developed chlorosis to both spore inoculations and the toxin infiltration, while Glenlea, 6B365, and Salamouni exhibited resistance to race 5 and insensitivity to Ptr ToxB. This verified the successful inoculation process, and confirmed race 5 isolate and Ptr ToxB viability. The presence of race 5 susceptibility in about 1/3 of the rye genotypes from across the globe and half of the commercially grown cultivars in the region is very surprising because race 5 was not observed until 1995, when it was first reported from Algeria (Lamari et al., 1995) and thereafter in Asia, and North America (Abdullah et al., 2016; Ali et al., 1999; Lamari et al., 2003). Widespread susceptibility to race 5 in rye genotypes suggests that race 5 has been present much longer than 22 years and perhaps earlier than race 1 in most of wheat growing countries. In contrast, susceptibility to race 5 could be due to use of rye germplasm with good agronomic characters but susceptible to tan spot race 5 in the cultivars development as it has been observed in wheat cultivars susceptible to race 5 developed in the 1960s (Lamari et al., 2005), even though the race 5 had not been reported until recently in North America (Ali et al., 1999; Lamari et al., 2003). Based on our results, it can be hypothesized that rye could be a contributor to race 5 resistance and/or susceptibility, especially in Ptr ToxB insensitive wheat genotypes where rye has been used in wheat improvement. Triticale serves as a bridge between rye and the modern wheat. Evaluation of triticale germplasm against multiple races of PTR may provide additional insight into the role rye has played in susceptibility or resistance to tan spot race 5.

Reaction of 171 rye genotypes from six continents to Pyrenophora tritici-repentis race 5 and Ptr ToxB

Reaction of rye genotypes to Pyrenophora tritici-repentis race 5 and Ptr ToxB (top to bottom). (A) Wheat genotype 6B662, (B) rye cultivars ‘Fredric’ (chlorosis susceptible), (C) ‘Muskater’ (moderately susceptible), (D) ‘ND 5’ (resistant), (E) wheat genotype Salamouni (resistant) inoculated with P. triticirepentis race 5, (F) wheat genotype 6B662, (G) rye cultivar Fredric, and (H) wheat genotype Salamouni infiltrated with Ptr ToxB.
Recovery of PTR isolates, their genotyping for Ptr ToxA and Ptr ToxB genes and phenotyping for race characterization
All rye leaf samples collected from nine South Dakota locations were analyzed for harboring PTR and the frequency of recovery ranged from 1% to 20% on the plated leaf segments. In 2013, we recovered 20 PTR isolates, whereas 83 isolates were recovered in 2014. All 103 isolates were evaluated for the presence of Ptr ToxA and Ptr ToxB genes; however, only 23 isolates amplified Ptr ToxA and the other 80 isolates lacked in both Ptr ToxA and Ptr ToxB (Table 4). As expected the positive controls (race 1 and race 5) amplified bands of 585 bp and 295 bp corresponding to the Ptr ToxA and Ptr ToxB respectively, thus validating the primers and the PCR procedure (Fig. 3). The genotypic data suggests that the isolates that harbor Ptr ToxA could possibly be race 1 or race 2. The other 80 isolates lacking in both Ptr ToxA and Ptr ToxB could potentially be race 3 or race 4 as neither of these races carry the genes. However, due to unavailability of a Ptr ToxC gene-specific DNA marker, it is not possible to successfully discriminate these isolates as race 3 or race 4. Presently the only method to characterize such isolates is through phenotyping with the tan spot differential set. Further, isolates that lack in both toxins genes may not be even PTR; however, we confirmed the identity of all 103 isolates through amplification of PTR specific housekeeping genes and eliminated this possibility. We phenotyped 23 isolates that harbored Ptr ToxA on wheat differentials and all isolates were grouped as race 1 because they incited necrosis on wheat genotype Glenlea, chlorosis on 6B365, and no symptoms on 6B662 and Salamouni. The 80 isolates that lacked both Ptr ToxA and Ptr ToxB did not produce necrosis and/or chlorosis on any of the four differential genotypes and were therefore characterized as race 4 (Table 4).

Genotypic and phenotypic characterization of Pyrenophora tritici-repentis isolates recovered from rye in 2013 and 2014
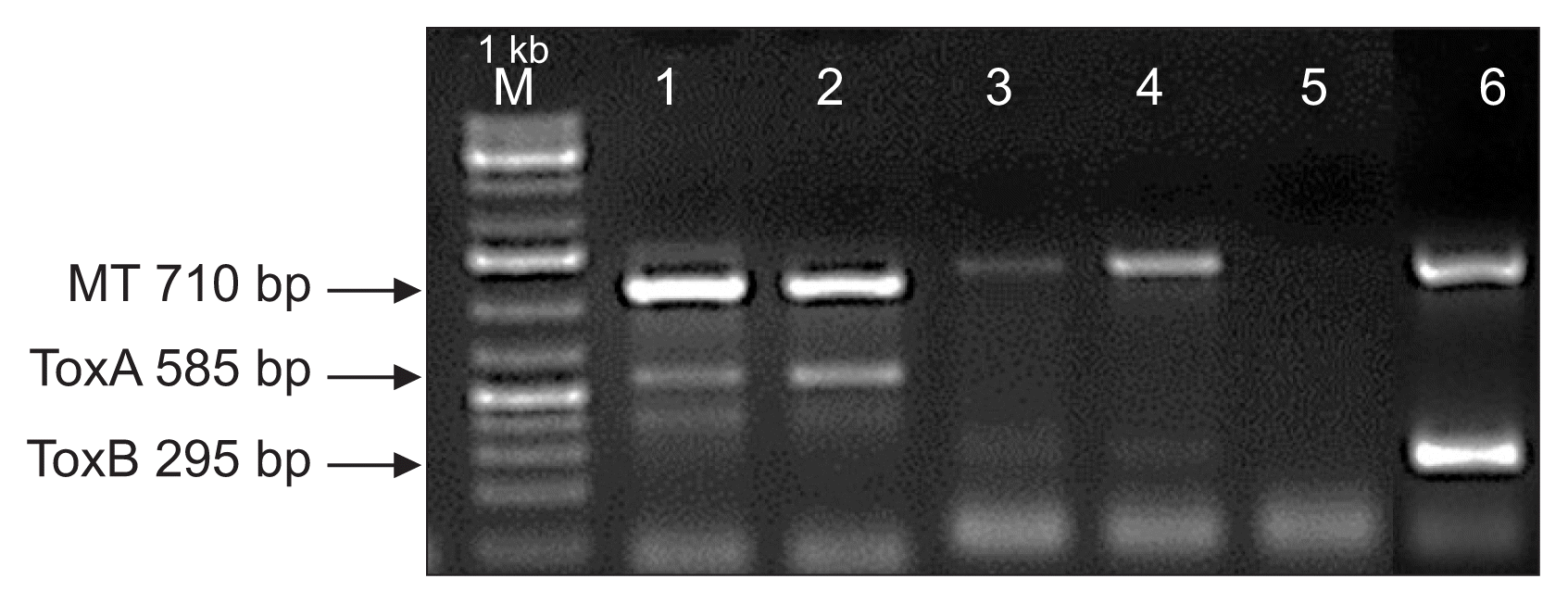
Gel picture of the PTR isolates showing the presence of ToxA, ToxB and mating type (MAT1-1) gene of PTR as an internal control. Lane 1, ToxA (from wheat race 1 [SD13–101] positive control); lane 2, ToxA (from rye race 1 [13-3-P1.1]); lane 3, race 4 (isolates from wheat, 13-103-P4.30) lacking ToxA and ToxB; lane 4, race 4 (from rye, 14-40-P6) lacking ToxA and ToxB; lane 5, water as negative control; lane 6, ToxB (from wheat race 5 [SD13-103] positive control). M, marker; MT, mating type.
Prevalence of PTR race 4 on rye is not surprising, as this had been observed in abundance on other alternative host plant species (Ali and Francl, 2001, 2003). However, the reasons for the existence of race 4 in high frequency are not clear. Race 4 has never been tested for virulence on a large number of wheat or alternative host genotypes. The colony growth of race 4 from rye was green olivecious in color and low in spore production (data not shown) as compared to race 1 isolates recovered from rye and wheat (Supplementary Fig. 2). Rye and wheat are generally cultivated in the same niche during the cropping season in the NGP. However, to our knowledge, rye has never been studied in the past for its role, if any, in the diversity of PTR races as has been done on wheat in the region and anywhere else. In our study, we analyzed 103 PTR isolates from rye and identified race 1 and race 4 were prevalent on rye, which is not surprising because race 1 is the most prevalent race worldwide including the U.S. northern Great Plains (Ali and Francl, 2003; Benslimane et al., 2011; Lamari et al., 2003). It is possible that PTR is surviving as an opportunistic pathogen/saprophyte on necrotic area caused by rye leaf spot pathogens, as most collected samples were also heavily infected with Bipolaris sorokiniana, Stagnospora nodorum, and Alternaria spp. (data not shown). Earlier prevalence of B. sorokiniana, and S. nodorum on rye has also documented by American Phytopathological Society (1993). Previously we have reported race 1 on barley, another alternative host of PTR and concluded its survival as a facultative saprophyte (Ali and Francl, 2001). Prevalence of race 4 on rye in the region validates our earlier results (Ali and Francl, 2003), observing race 4 population at a higher frequency on alternative hosts such as non-cereal grasses. Conversely, the pathogen’s isolates with high (race 1) and low aggressiveness (race 4) from alternative hosts such as Alti wild rye and smooth brome grass have also been documented in other studies (Hosford, 1971, 1982; Howard and Morrall, 1975; Krupinsky, 1982, 1986, 1992). However, S. cereale was not included in these studies.
In a first comprehensive study, we evaluated 211 rye genotypes representative of six continents against the tan spot pathogen’s races 1 and 5 and their host selective toxins Ptr ToxA and Ptr ToxB. Our results suggest that rye genotypes exhibit diversity in reaction to tan spot caused by PTR race 1 and race 5. However, susceptibility to race 1 and 5 in the evaluated genotypes is probably not due to Ptr ToxA and Ptr ToxB sensitivity as they lacked in TSn1 and TSc2 sensitivity genes needed for the two toxins. This suggests the role of unknown toxins or other factors in disease development. Further, the existence of race 1 and 4 on rye grown in South Dakota indicates it may have a minimal role in diversification of PTR population and in the disease epidemiology in the region. However, planting race 5 susceptible rye germplasm in the region could change this scenario in the future. Therefore, it is imperative that tan spot resistant rye cultivars should be planted and periodic monitoring of the fungal population on rye crops would help in better management of tan spot throughout the region.
Supplementary Information
Acknowledgments
We like to thank Mr. Richard Gappert and Ms. Navjot Kaur for their help in greenhouse and lab work. The South Dakota Wheat Commission and USDA NIFA through SDSU Agricultural Experiment Station (AES) provided financial support for this research.
