Biocontrol Potential of Fungal Endophytes against Fusarium oxysporum f. sp. cucumerinum Causing Wilt in Cucumber
Article information
Abstract
Endophytic fungi have received much attention as plant growth promoters as well as biological control agents against many plant pathogens. In this study, 30 endophytic fungal species, isolated from various plants in China, were evaluated using in vitro dual culture assay against Fusarium oxysporum f. sp. cucumerinum, causing wilt in cucumber. The results of the present study clearly showed that all the 30 endophytic fungal isolates were highly capable of inhibiting the mycelial colony growth of Fusarium oxysporum f. sp. cucumerinum with inhibition % over 66% as compared to control treatments. Among all of them, 5 isolates were highly effective such as, Penicillium sp., Guignardia mangiferae, Hypocrea sp., Neurospora sp., Eupenicillium javanicum, and Lasiodiplodia theobromae, respectively. The Penicillium sp. and Hypocrea sp. were highly effective as compared to other isolates. From in vitro results 10 best isolates were selected for greenhouse studies. The results of the greenhouse studies showed that among all of them 3 endophytic fungal isolates successfully suppressed wilt severity when co-inoculation with pathogen Fusarium. oxysporum f. sp. cucumerinum. The endophytic fungi also enhanced plant growth parameters of the host plants, the antagonistic fungal isolates increased over all plant height, aerial fresh, and dry weight as compared to control.
Cucumber (Cucumis sativus L.) is one of the most important vegetable grown all over the world. However, this crop is attacked by several fungal diseases such as Fusarium wilt caused by Fusarium oxysporum, grey mould (Botrytis cinerea), damping-off (Fusarium spp.), anthracnose (Colletotrichum sp.), and leaf blight caused by Alternaria sp., but the Fusarium wilt caused by F. oxysporum f. sp. cucumerinum (FOC), is one of the most destructive diseases of cucumber crop worldwide (Martínez et al., 2003). This disease has very high importance due to its economic importance and this disease can cause huge losses to cucumber production, as this disease has been reported in many countries of the world and losses are up to 45% reported from many countries (McGovern, 2015). The most common disease symptoms appear as yellowing, stunting and wilting of the leaves and basal stem necrosis. The wilted leaves will dry out and fall off and many times the complete death of the plants may occur (Lim et al., 2006). Due to the soil-borne nature of the fungus and advancement of the pathogen to the parenchymatous cells, the management of cucumber Fusarium wilt is very difficult. Till to date no effective control method has yet been found to control this disease completely in cucumber production. Moreover, there are very few fungicides are available in the market which is effective against this pathogen but due to increasing environmental concerns and other constraints have limited their use (Vethavalli and Sudha, 2012). In addition, it has been shown that there is problem of resistant strains against these fungicides and emergence of new races of the pathogen in many countries have complicated the effective control of this devastating disease (Reis et al., 2005). Due to the side effects of indiscriminate and un-judicious use of chemical fungicides, environmental hazards, concerns to human health and inducing pathogen resistance to fungicides (Fan and Jackson, 1989; Norman, 1988), an alternative is the need of the world in changing global climate and for this, the use of natural biocontrol agents has become a very successful trend in integrated disease management (Backman and Sikora, 2008; Mei and Flinn, 2010). Other approaches are investigated, including the use of biocontrol agents (Singh et al., 1999; Xiang et al., 2016; Youssef et al., 2016; Zhang et al., 2014). Biological control of Fusarium wilt by means of different microorganisms (fungi or bacteria) could be used as a very useful alternative to synthetic fungicides as it has an attractive potential and eco-friendly since several endophytes have the ability to decrease disease severity in a better way through different mechanisms and check the progress of the pathogen within vascular tissues of the plants (Alabouvette et al., 1993; Lu et al., 2016; Patel et al., 2012; Paulitz et al., 1987). The biological control of this disease has become an attractive alternative to chemical fungicides and other conventional control methods. Several biocontrol agents mainly Bacillus, Trichoderma, Pseudomonas, nonpathogenic Fusarium, and Penicillium strains were evaluated to control Fusarium wilt, but still this lethal disease could not be controlled completely (Raza et al., 2017; Vos et al., 2014). In search of the potential biological control agent against several pathogens, one approach is recently being practiced, which is the isolation of different endophytes from diverse host species, because endophytic fungi have been reported to play a key role in numerous functions of the plants due to its nature of producing novel bioactive compounds. It has been repeatedly reported that endophytic fungi can be successfully used to protect plants against pests and diseases as these fungi producing protective metabolites, inducing resistance to the host plants to biotic and a biotic stress by improving their growth and by this directly or indirectly enhanced production and yield (Arnold et al., 2001; Kaul et al., 2012; Rai et al., 2014). These kinds of fungi hold massive potential for the development of eco-friendly and economically viable agricultural products (Lugtenberg et al., 2016). Endophytic fungi interact with their host plants in different ways, with each interaction giving rise to different alterations in both partners. Mutualistic fungi enhance the defense system of the plants to pathogens and improve the nutrient uptake of the host plants; therefore, the interaction between plants and endophytic fungi have the prime importance to understand the potential of fungi in preventing plant diseases (Zeilinger et al., 2016). Due to the ubiquitous nature of endophytic fungi, they may be present in every plant species and can be isolated from different organs of the plants, this group of organisms represents an abundant and dependable source of bioactive and chemically novel compounds with potential for exploitation in a wide variety of medical, agricultural and industrial arenas (Strobel, 2003). Endophytic fungi have been successfully implemented for their positive and sound effects on growth and development of the plants (Rania et al., 2016). And endophytic fungi associated with different host plants are just such a natural resources of biocontrol agents as these endophytic fungi have been explored as bio-factories of novel bioactive substances (Radić and Štrukelj, 2012). Considerable attention has been paid to the inhibitory activity of endophytic fungi against plant pathogenic fungi and their potential as biocontrol agents against plant diseases (Crozier et al., 2015; Hanada et al., 2010; Mejia et al., 2008). The main aim of this novel research work was to assess comprehensively the ability of endophytic fungi isolated from four different host plants to suppress the mycelial growth of FOC causing wilt in cucumber under in vitro and also to evaluate these endophytic fungi to control the cucumber Fusarium wilt in greenhouse conditions.
Materials and Methods
Endophytic fungal species
Sampling site and procedure
Healthy leaves and stems of tomato, mangrove, star anise, and agarwood were collected from various locations in China. For this, samples of these plants were collected from different sites of China. A total of ten healthy plant samples from each species were randomly chosen in the study site. The plants were approximately 20 m away from each other. One branch with leaves was randomly selected from each individual plant species. These samples were placed in zip-lock bags, brought to the laboratory and stored in a refrigerator at 4°C and used within 24 h. The collected plant samples were thoroughly cleaned with tap water and then air-dried on filtered papers. Before processing for isolation, all the samples were surface-sterilized by immersion in 70% ethanol for 1 min, 5% sodium hypochlorite solution for 3 min, and then 70% ethanol for 30 s, then the samples were dipped in sterile distilled water thrice to clean the samples properly and remove the effect of surface sterilization agents and dried on the sterilized filter papers. The isolation of endophytic fungi was conducted as previously described by (Guo et al., 2000; Wiyakrutta et al., 2004).
Isolation and identification
In brief, total of 30 fungal endophytes were isolated and identified from four host plant species such as mangrove, tomato, star anise, and agarwood. the identification of all the fungal isolates was done with the help of microscope and the different keys available in the laboratory, all the procedures were adopted as previously described by Guo et al. (2000) (Table 1).
Antagonistic assay
The biological control ability of the 30 identified fungi (Table 2) was selected for their in vitro activity against FOC causing wilt in cucumber crop by using the dual culture method on potato dextrose agar (PDA) plates. The pure culture of this pathogenic fungus was provided by Prof. Dr. Cai, Lei from State Key Laboratory of Mycology, Institute of Microbiology, Chinese Academy of Sciences, Beijing. These studies were conducted as the methods previously described by (Vethavalli and Sudha, 2012). In brief, for this purpose, a mycelial disk of 5 mm was carefully taken with the help of cork borer from the newly growing margins of the culture for every fungal strain of thirty endophytic taxa, and a same disk of the culture for FOC pathogen was placed 3 cm apart on the surface of PDA and allowed to grow at 25°C. From 7 to 10 days after inoculation, the mycelia colony growth of the pathogen in the presence of endophytic fungus as well as alone in control treatment was calculated. Then, the growth inhibition % of each endophytic fungi versus each pathogen (IR) was calculated using the formula of (Eksteen et al., 2001) which was as under: IR (%) = [(C2 − C1)/C2] × 100, where C2 was diameter of the mycelia colony growth in control treatment, C1 was diameter of the mycelia colony growth of the pathogen in the presence of the antagonist. All the treatments were replicated five times and the whole experiment was repeated two times.
In vivo biocontrol assay
Preparation of spore suspension of endophytic fungi
Among the 30 endophytic fungal species, the 10 best performance fungal species, which gave the best growth inhibition % against FOC of cucumber individually under in vitro conditions were selected for greenhouse studies against cucumber Fusarium wilt pathogen, FOC, as this disease has been considered as one of the most destructive diseases of cucumber worldwide and can cause huge losses to cucumber production all over the world. For this purpose, spore suspension of all the selected endophytic fungi for inoculating cucumber seeds were produced in Erlenmeyer flasks (200 ml) containing 100 ml of potato dextrose broth (PDB) medium which was autoclaved at 121°C for 15 min then allowed to cool down. Mycelia blocks of each endophytic fungus isolate were cut from one-week-old culture on PDA and aseptically transferred to PDB under laminar airflow. Five replicate flasks were used for each endophytic fungi isolate. Two flasks containing non-inoculating PDB were used as control. Flasks were incubated under laboratory conditions by using rotary shaker at 140 rev/min and 25°C for 1 week to allow the fungal sporulation and to disperse spores throughout PDB medium. The fermentation broths of each individual endophytic fungus were mixed thoroughly for few minutes and then added with Tween-20. The fungal spores were harvested by filtering the spore suspension through a cheese muslin cloth to remove mycelia fragments. The spore density of sporulating fungi was then estimated using the hemocytometer and the suspension was standardized to provide a final spore concentration of 1.6 × 106 spores/ml.
Inoculation of seed with spore suspension
The cucumber cultivar, ‘Fuyang F1-35’, susceptible to Fusarium wilt caused by FOC was used throughout this study. For this, seeds were surface-sterilized and properly dried with usual procedure of our lab, then seeds were soaked in spore suspension of each endophyte fungi for 12 h, after seeds were planted on sterilized soil in nursery trays for 1–2 weeks in the greenhouse at 25°C and 85% relative humidity. Nursery trays were watered on alternate days and growth conditions were maintained in greenhouse. Young seedlings at the four-true-leaf growth stage were used throughout this study. These studies were conducted as previously described by (Posada and Vega, 2006).
Re-inoculation of cucumber plants with endophytes
Two weeks old cucumber plants, which were already colonized by each endophytic fungus were transplanted on other pots, then these seedlings were re-inoculated with 100 ml spore suspension of each endophytic fungal species from fermentation broths by soil drenching method, for this three holes were made around the cucumber seedlings near the roots for successful colonization of the endophytes as previously described by Kim et al. (2007) and Costa et al. (2013). Three weeks after inoculation of endophytic fungi, re-isolation of each endophyte was done for confirmation of colonization. For this the few plants were uprooted from the treated plants and re-isolation was made by using same protocol for isolation of endophytes as previously reported by Miles et al. (2012) and Barretti et al. (2009).
Pathogen inoculation
For this purpose, spore suspension of FOC for inoculating cucumber plants was produced in Erlenmeyer flasks (200 ml) containing 100 ml of PDB medium which were autoclaved at 121°C for 15 min then allowed to cool down. Mycelia blocks of FOC were cut from one-week-old culture on PDA and aseptically transferred to PDB under laminar airflow, then these flasks were placed on rotary shaker at 140 rev/min and 25°C for one week to allow the fungal sporulation and to disperse spores throughout PDB medium. The FOC spores were harvested by filtering the spore suspension through a cheese muslin cloth to remove mycelia fragments and then spores were counted and adjusted to 106 conidia/ml. The treated seedlings with each endophytic fungus were artificially inoculated with conidial suspension of FOC (106 conidia/ml at 25 ml per plant) four weeks post endophytic fungal treatment as a soil drench method by making three holes of 5 cm made at the base of cucumber plants treated with endophytes around the root by using 5 cm stick. Plants were left for 24 h after inoculation in the dark at 25°C and 90% RH. Uninoculated control plants were treated with water only (negative control). The plants inoculated only with pathogen spore suspension FOC were termed as positive control. After 24-h plants were transferred to the greenhouse for 1–2 weeks for disease development as described by Kim et al. (2001) and Anith et al. (2015). This individual experiment was conducted in Randomized Complete Design with five replications and the whole experiment was repeated two times.
Disease severity assessment
After 60 days of co-inoculation with biocontrol agents and pathogen the disease severity was recorded. The severity of Fusarium wilt was calculated by using 0–4 disease severity scale:
where, 0, no symptoms of wilting (health leaves); 1, < 25% of leaves showing yellowing and or necrosis; 2, 26–50% of leaves showing yellowing and/or necrosis; 3, 51–75% of leaves with yellowing and/or necrosis; and 4, 76–100% of leaves with yellowing and/or necrosis. After that following parameters were recorded.
Plant growth assessment
At the end of the experiment, plant growth parameters of the host plant were also recorded such as plant height (cm) aerial fresh and dry weights (g) of infected and non-infected plants.
Analysis of data
Statistical analyses were carried out using Statistica software (Statsoft Inc., Tulsa, OK, USA). The ANOVA module was used for analyses of variance and multiple comparisons of means (tests of Neuman and Keuls).
Results
Isolation and identification of endophytic fungi from various host species
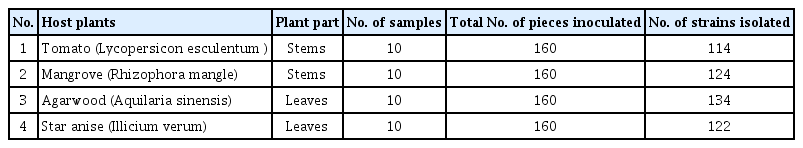
A total of 494 isolates of endophytic fungi were isolated from 640 tissue segments from the four host species of tomato, mangrove, star anise, and agarwood. Of these, 114 isolates were recovered from tomato stems, 124 from mangrove branches, 134 from agarwood leaves and 122 isolated from star anise (Table 1). A total of thirty isolates sporulated properly and identified into different taxa based on its morphological characteristics. Of the 30 endophytic taxa were isolated from four host plant species, mostly all belong to the Ascomycetes. Different endophyte taxa dominated the four host species. In tomato, the Cladosporium sp. was found in the highest frequency followed by Alternaria sp. and Penicillium sp. the stem segments showed higher percentage frequency of colonization of the endophytic fungi, while, Pestalotiopsis sp. was dominant in mangrove, Fusarium sp. was frequently found in agarwood and Trichoderma sp. was mostly found in star anise. The result of this study also showed that endophyte fungi were more prevalent in the stems and twigs as compared to leaves, whereas, Fusarium sp. was commonly present in most of the four-plant species.
In vitro evaluation of antagonistic activity of endophytes against F. oxysporum
Antagonistic activity of all 30 different species of endophytic fungi isolated from four different host species was tested against FOC causing wilt of cucumber under in vitro conditions. The 5-day-old mycelia culture of the endophytic fungi and FOC pathogen by using dual culture techniques on PDA medium and by using the diameter of the growth inhibition % was calculated against each endophyte vis-a-vis pathogen. In the present study, fungal endophytes isolated from all four host species showed considerable antagonistic activity against FOC. The result showed that all the thirty endophytic fungal species were capable of significant inhibition on the mycelia colony growth in culture with control values over 66% inhibition noted after 5–7 days of inoculation and incubated at 25°C as compared to untreated control, but the effects were the highest to the lowest depended upon each pathogen and endophyte species tested (Table 3). The mycelial colony growth of FOC was significantly different within the 30 endophytes but the 10 most effective endophytes fungal species were Penicillium sp., Guignardia mangiferae, Hypocrea sp., Neurospora sp., Eupenicillium javanicum, Lasiodiplodia theobromae, and Trichoderma sp., respectively. The most potent endophytic fungi against FOC as shown in (Table 3), showed that maximum inhibition of mycelia growth of F. oxysporum was observed against Penicillium sp. (66.4%), followed by Gugnardia mangiferae (47%), Hypocrea sp. (44.2%), Neurospora sp. (43.1%), and Eupenicillium sp. (43.3%), respectively. Overall, Penicillium sp. recovered from tomato stems and Hypocrea sp. isolated from agarwood showed the most potent inhibition activity against FOC under in vitro conditions.
Biocontrol efficacy of 10 best performed endophytic fungi in pot experiment
Ten fungal endophytes, which had shown the best antifungal activity against FOC causing wilt in cucumber crop under in vitro conditions, were tested against cucumber Fusarium wilt pathogen plants inoculated with FOC in pot experiment in greenhouse conditions. After analysis of variance, the results showed that there was a significant decrease in the severity of Fusarium wilt noted 60 days after co-inoculation of endophytes with FOC but the effects were depended on each antagonistic fungus tested. Generally, several endophytic fungi have significantly decreased the severity of cucumber Fusarium wilt on 0–4 disease severity scale (Table 4). Two weeks after inoculation of the spore suspension of the pathogen the cucumber plants in infected control treatments started showing the first external wilt symptoms like yellowing of leaves, symptoms of the wilt were appeared as leaf chlorosis and first they were observed in older leaves and then spread to younger leaves (Fig. 1). After seven weeks of inoculation, at this stage 70–85% of the plants in the infected control treatments showed severe wilt symptoms and the disease severity reached up to 3.60 on 0–4 disease severity scale (Fig. 1), but in endophyte treated plants the plantlets showed mild symptoms and the disease severity was much lower 1.20 on 0–4 severity scale as compared to the infected control treatment (Table 4). The results also showed that Penicillium sp. which was isolated from the stems of tomato, Lasiodiplodia theobromae and Hypocrea sp. isolated from agarwood were found to be highly effective in suppressing the Fusarium wilt severity as compared to the infected control treatments (Table 4). The results also showed that there was a highly significant effect (P < 0.05) of endophytic fungi on the plant growth parameters of the cucumber plants as compared to the infected and non-infected control treatments. Sixty days after co-inoculation with FOC, all the endophytic fungi have significantly increased the plant height and aerial fresh and dry weights of the cucumber plants as compared to the infected and non-infected control treatments. The highest plant height was observed in plants treated with Penicillium sp. (85.24 cm) followed by plants treated with Lasiodiplodia theobromae (71.56 cm) and Hypocrea sp. (68.64 cm), respectively. Whereas, the plants treated with Penicillium sp. and Lasiodiplodia theobromae also have the highest aerial fresh and dry weights as compared to the infected and non-infected control treatments. The highest aerial fresh and dry weights increase was achieved by means of Penicillium sp. (17.29 and 1.169) isolated from tomato stems and Lasiodiplodia theobromae (16.18 and 1.413), respectively recovered from agarwood treated plants (Table 4).

Effect of endophytic fungi isolated from four different host species (tomato, mangrove, agarwood, and star anise) tissues on Fusarium wilt severity
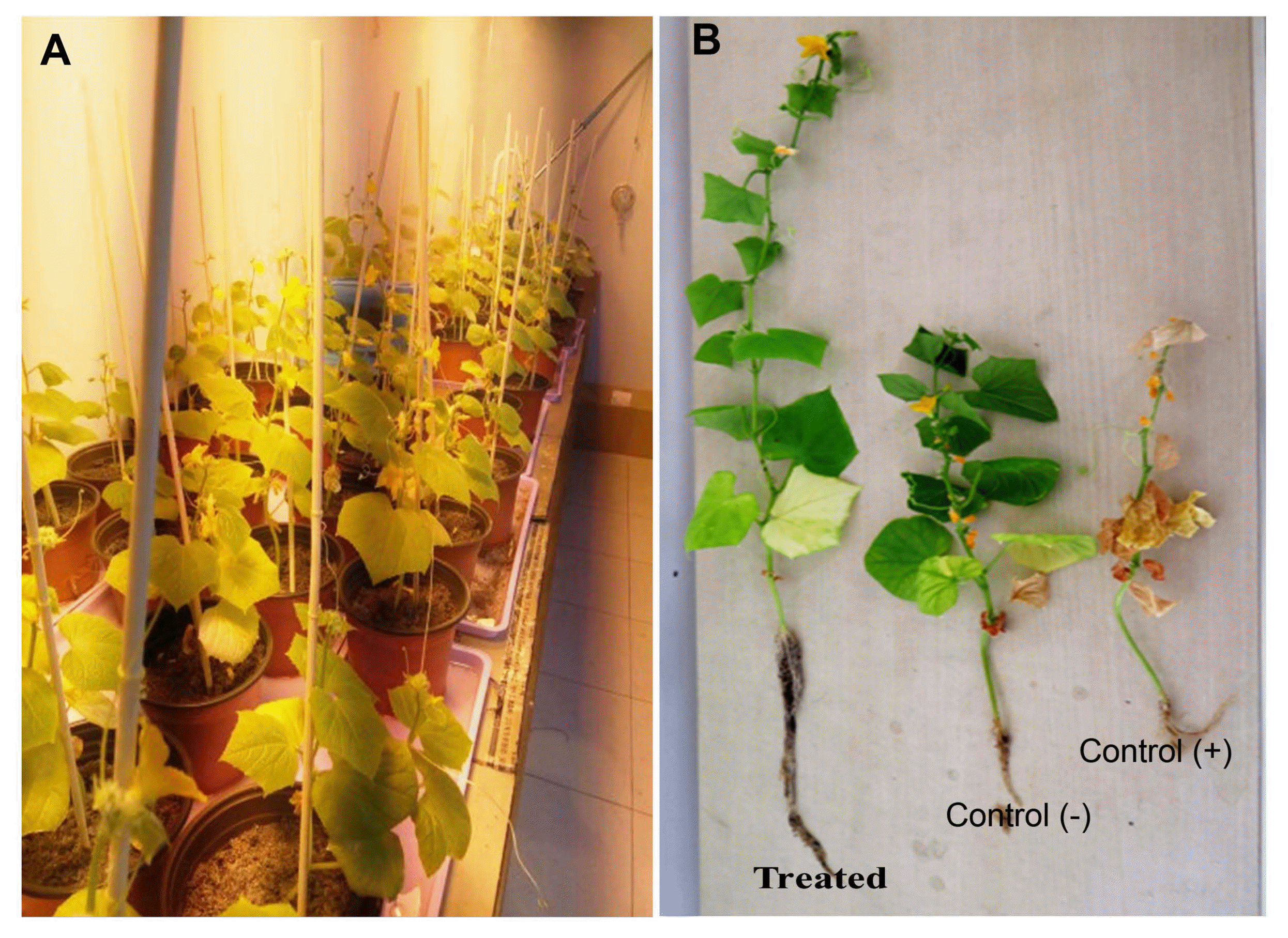
Effect of endophytic fungi on plant growth and development of cucumber plants and suppression of Fusarium wilt disease severity on cucumber ‘Fuyang F1-35’. (A) Plants incubated in greenhouse in controlled conditions. (B) Plants treated with different endophytic fungi. Control (−) uninoculated with pathogen and untreated. Control (+) inoculated with F. oxysporum f. sp. cucumerinum and untreated. All the treated plants were compared to the infected and non-infected controls noted 60 days post inoculation.
Discussion
Several endophytic fungal species are now successfully tested as biocontrol agents against plant pathogenic fungi as well as for plant growth promotion. This strategy is very promising for the improvement of crop productivity and as an alternative to chemical fungicides and synthetic fertilizers (Wang et al., 2016; Wu et al., 2016). Numerous attempts were made to control wilt disease through biocontrol agents using endophytic fungi and bacteria (Aydi Ben Abdallah et al., 2016; Hong et al., 2007; Raza et al., 2017; Saravanakumar et al., 2016). On the other hand, the utilization of different endophytic fungi isolated from diverse host plant species as biocontrol agents against fungal pathogens of cucumber crop in in vitro and in greenhouse conditions was seldom found (Raza et al., 2017). In the present study, endophytic fungi were screened for their potential to inhibit the growth of FOC causing wilt in cucumber and to suppress the cucumber Fusarium wilt and for promotion of plant growth. The novelty and special interest in our studies is that, these endophytic fungi were isolated from four different host species collected from different agro-ecological zones of China. A lot of studies have shown previously that these host plants may be valuable as prospective sources of bioactive compounds and biological control agents against plant diseases (Rai et al., 2014). However, a few reports were available on the use of endophytic fungi isolated from tomato, mangrove, star anise, and agarwood plants as efficient source for the isolation of biocontrol agents. On numerous occasions for example, several endophytic fungi such as Alternaria, Cladosporium, Curvularia, Fusarium, Phaeoacremonium, and Trichoderma have been isolated mainly from the star anise, tomato, mangrove (Debbab et al., 2013), and agarwood (Premalatha and Kalra, 2013) and were used as biocontrol agents against pathogenic fungi.
In the present study, thirty endophytic fungi isolated from four different host species evaluated in in vitro for their antagonistic potential toward FOC causing wilt of cucumber crop, our results clearly showed that, among thirty endophytes tested Penicillium sp., Guignardia mangiferae, Hypocrea sp., Neurospora sp., Trichoderma sp. and has significantly reduced the mycelial growth of FOC with growth inhibition % that ranged up to 66.4% respectively but the effects were depending on each endophyte tested. Similar effects were reported by Saravanakumar et al. (2016), Zhang et al. (2014), and Xiang et al. (2016) using endophytic fungi isolated from cucumber and other medicinal plants and identified as Trichoderma, Alternaria, Phomopsis, Colletotrichum, Phoma, and Acremonium. Endophytic fungi have the ability to control plant pathogens in in vitro conditions with diverse mechanisms. Moreover, in another study an endophytic fungus isolated from Muscodor albus effectively controlled the P. ultimum under in vitro via the production of volatile compounds. Tenday-old cultures of endophyte killed P. ultimum in vitro (Worapong and Strobel, 2009). Moreover, the recent study indicated that when Debaryomyces hansenii was tested against four strains of pathogenic fungi, the results showed that it decreased the mycelia colony growth of the four pathogenic fungi such as Aspergillus sp., Fusarium proliferatum, and F. subglutinans from 97–98.3% by producing volatile compounds (Medina-Córdova et al., 2016). In addition when T. harzianum and S. proteamaculans were screened against Rhizoctonia solani, both endophytic fungi were highly effective to reduce the growth of pathogen in in vitro by overgrown the pathogen mycelia growth (Youssef et al., 2016), likewise, when Beauveria sp. was tested against soil-borne plant pathogenic fungi such as Pythium, Rhizoctonia, and Fusarium in in vitro conditions this endophyte restrict the pathogen growth by producing some bioactive metabolites (Ownley et al., 2010). Some other workers also reported the key role played by endophytes against pathogenic fungi as Miles et al. (2012) investigated the biological control potential of 100 fungal endophytes associated with Espeletia sp. against common crop pathogens, including Rhizoctonia solani, Botrytis cinerea, Fusarium oxysporum, and Phytophthora infestans, their results indicated that all endophytic strains were highly effective against many pathogens tested and the most highly effective strains were identified as Aureobasidium pullulans and Paraconiothyrium sporulosum and that the inhibitory effect was due to the production of endophytic bioactive metabolites. Other species, like well-known biological control agent Trichoderma can inhibit the growth of many pathogenic fungi by producing metabolites (Bae et al., 2016). These results suggest that tomato, mangrove, star anise and agarwood harbors diversify endophytic fungi, from which potential biocontrol agents against destructive plant pathogens like F. oxysporum, Verticillium, and B. cinerea, and for promoting growth of cucumber can be screened as these plants have the potential to harbor the endophytic fungi with promising source of different and structurally unprecedented bioactive natural products is unquestionable and continues to attract considerable attention (Debbab et al., 2013; Premalatha and Kalra, 2013).
Our results also showed that there was highly significant decrease in the severity of Fusarium wilt, observed 60 days after inoculation with FOC, but the effects were depending on the endophytic fungal treatments tested. Penicillium sp. which was isolated from the stems of tomato, Hypocrea sp. and Lasiodiplodia theobromae isolated from agarwood were highly effective in reducing wilt symptoms as compared to FOC inoculated and the infected and non-infected untreated control plants. In the same way, Saravanakumar et al. (2016) screened 100 strains of Trichoderma as potential biocontrol agents against Fusarium wilt in cucumber both under in vitro and in vivo methods, 10 isolates inhibited the growth of the pathogen with more than 85% inhibition and in greenhouse trials 1 strain Trichoderma asperellum was able to decrease the severity of Fusarium wilt by at least 71.67%. Moreover, T. polysporum was highly effective in suppressing melon wilt with highest efficacy to control and enhance the fruit quality and yield under field conditions (Gava and Pinto, 2016). Moreover, Trichoderma sp. have the capability to induce the antioxidant enzymes in plants under stress conditions after pathogen challenge as well as increased the plant fresh weight and seedling growth of tomato plants (Prabhukarthikeyan et al., 2014; Youssef et al., 2016) also found that application of Beauveria sp. effectively reduced wilt disease in tomato plants. In the same sense, some bacterial strains also suppressed the cucumber Fusarium wilt severity as Streptomyces albospinus was found to be the promising biocontrol agent for cucumber wilt (Wang et al., 2016). In another study reported that Bacillus sp. can inhibit the cucumber Fusarium wilt 30 days after inoculation and some strains giving 20–79% control efficacy (Hong et al., 2007). As reported in recent studies, endophytic fungi can successfully suppress the fungal plant pathogens through various means including the promotion of plant growth and by induced resistance to the plants (Lugtenberg et al., 2016; Ownley et al., 2010; Youssef et al., 2016). Meanwhile, we cannot rule out other mechanisms, as some Trichoderma spp. have the ability in triggering plant defense mechanisms as some reports suggested that the expression levels of defense-related genes, chitinase (SlChi3), beta-1,3-glucanase (SlGluA), and PR-1 (SlPR-1a) were significantly increased in the stems and roots of Trichoderma treated, Fusarium oxysporum f. sp. lycopersici infected plants (El Komy et al., 2016).
Conclusion
The evaluation of the endophytic fungi associated with surface-sterilized tissues of four different host species with antifungal potential against FOC causing wilt of cucumber crop under in vitro conditions led to the selection of three the most promising biocontrol agents like Penicillium sp., Hypocrea sp., and Lasiodiplodia theobromae were found very effective in reducing cucumber Fusarium wilt severity and improvement of cucumber plant growth. Moreover, these results also confirmed that tomato, mangrove, star anise, and agarwood are very important host species, which harbor important genera of endophytic fungi and these plants are the potentially major and key source for isolation of most effective endophytic fungi, which has the dual benefits for the plants, both as biocontrol agents and as plant growth promoters. Further studies are required to elucidate the mechanisms involved at cellular and molecular level.
Acknowledgments
This work was financially supported by Chinese Academy of Sciences CAS in the category of visiting Professorship for visiting Scientists Grant No 2016VBB058 and by the Strategic Priority Research Program of the Chinese Academy of Sciences (no. XDB31000000). The authors wish to thank Prof. Cai, Lei from State Key Laboratory of Mycology, CAS, Beijing for providing culture of pathogenic fungi. The authors also wish to thank all the staff of the mycorhizal and fungal endophytes research group at State Key Laboratory of Mycology, Institute of Microbiology, Chinese Academy of Sciences, Beijing, China, for their warm welcome and providing pleasant working conditions.