Isolation and Characterization of Airborne Mushroom Damaging Trichoderma spp. from Indoor Air of Cultivation Houses Used for Oak Wood Mushroom Production Using Sawdust Media
Article information
Abstract
Some species of the Trichoderma genus are reported as the major problem in oak wood mushroom production in Korea. In spite of economic loss by the fungi, scientific information on airborne Trichoderma species is not much available. To generate information for disease management development we analyzed airborne Trichoderma. A total of 1,063 fungal isolates were purely obtained from indoor air sampling of cultivation houses used for oak wood mushroom using sawdust media. Among the obtained isolates, 248 isolates were identified as Trichoderma fungi including T. harzianum, T. atroviride, T. citrinoviride, and T. pseudokoningii, by morphological and molecular analysis. T. harzianum was dominant among the four identified species. All the four Trichoderma species grew fast on solid nutrient media tested (potato dextrose agar [PDA], malt extract agar [MEA], Czapek’s Dox + yeast extract agar [CYA] and cornmeal dextrose agar). Compact mycelia growth and mass spore production were better on PDA and CYA. In addition, T. harzianum and T. citrinoviride formed greenish and yellowish mycelium and spores on PDA and CYA. Greenish and yellowish pigment was saturated into PDA only by T. pseudokoningii. These four Trichoderma species could produce extracellular enzymes of sawdust substrate degradation such as β-glucosidase, avicelase, CM-cellulase, amylase, pectinase, xylanase, and protease. Their mycelia inhibited the growth of oak wood mushroom mycelia of two tested cultivars on dual culture assay. Among of eleven antifungal agents tested, benomyl was the best to inhibit the growth of the four Trichoderma species. Our results demonstrate that the airborne Trichoderma fungi need to be properly managed in the cultivation houses for safe mushroom production.
Oak wood mushroom (Lentinula edodes, called as shiitake in Japaness) is a medicinal and edible mushroom with anticancer and anti-obesity effects as well as with excellent nutrition, good taste and fragrance (Lee and Park, 1998; Lee et al., 2014; Nanba et al., 1987). It is a mushroom frequently consumed in East Asia, especially China, Japan and Korea. At present, the total production and output of this lucrative mushroom are consistently increasing in Korea. Recently, its production and output were 19,398 tons and 184 billion won in 2014, 23,469 tons and 219.1 billion won in 2016, and 3,984 tons and 211.9 billion won in 2017, respectively (Korea Forest Service, 2017). Its export volume in Korea was 60.59 tons ($1.36 million dollars) in 2014, and 118 tons ($1.84 million dollars) in 2018, respectively (Statistics of Forest Product’s Trade, 2018). Domestic production of this mushroom has been performed using the log bed cultivation system and sawdust media cultivation system. Since the mushroom is sensitive to environmental changes, the use of cultivation house that could manage temperature, humidity, light, and ventilation has been preferred. With the development of greenhouse construction technology, the cultivation technology of the sawdust media system has greatly been improved for the mushroom production. Consequently, not only new starters of mushroom growing but also the earlier starters is getting favor to grow the mushroom using the sawdust media system. Currently, oak wood mushroom growing using the sawdust media system in cultivation house facility is increasing for commercial production across the country. This trend of choosing indoor cultivation facility would be better when we consider that climate change is affecting agricultural and horticultural production in Korea.
Trichoderma spp. were reported as the major pathogen which are harmful to oak wood mushroom cultivated in log beds (Huang et al., 1988; Jandaik and Guleria, 1999; Laixuthai et al., 1987; Liao, 1993; Lim et al., 1990). Various Trichoderma spp. could cause contamination in log beds substrates and sawdust media substrate, especially at high humidity condition (Laixuthai et al., 1987). T. harzianum was reported to the major contamination source in Singapore (Lim et al., 1990). According to Togashi’s reports from Japan in 1997, Trichoderma spp. were isolated from indoor air of greenhouses used for oak wood cultivation, frequency of separation was analyzed (Togashi et al., 1997). The control of contamination by Trichoderma spp. was known to be difficult, and their contamination could cause loss of fruit body harvest amounts (Bodine, 1995; Laixuthai et al., 1987). Because Trichoderma spp. produced a lot of conidia that can spread easily, they became detrimental fungi in oak wood mushroom cultivation (Davidse, 1986; Eveleigh, 1985). In Korea, T. citirnoviride and Gliocladium viride (Current name: Trichoderma deliquescens) were reported to new recorded pathogens to oak wood mushroom (Kim et al., 2010; Kim et al., 2012b). In 2016, the damage rate of oak wood mushroom production by green mold disease of Trichoderma spp. took 20% of total damage rate (Korea Rural Economic Institute, 2016).
So far, Trichoderma problem in oak wood mushroom cultivation has been studied mainly with log beds. The presence of Trichoderma problems in the mushroom production with sawdust media has been recognized by the mushroom growers but not much studied on them. Therefore, this study was conducted to obtain basic information on Trichoderma species for sanitary management in oak wood mushroom cultivation houses. For this purpose, we performed fungal isolation, species identification, and characterization of Trichoderma species for 4 years from the indoor air of sawdust media-based cultivation houses.
Materials and Methods
Fungal sampling and isolation
Two cultivation houses of oak wood mushroom located at Cheongyang-gun, Chungcheongnam-do and Jangheung-gun, Jeollanam-do, were selected for air sampling. These cultivation houses have been operated by farmers who have advanced skills in the mushroom production using sawdust media. Indoor air sample of the cultivation houses were collected every month from June 2012 to February 2015 using Andersen samplers (single stage ambient viable sampler, Model 10-880, Tisch Environmental, Cleves, OH, USA) based on ISO16000-18 method. The samplers were set at a height of 1.3 m and run for 1 min with calibrated vacuum pumps (28.3 l/min) for air sampling. Air sampling was replicated at three points of the cultivation houses. Malt extract agar (MEA; Difco, BD Science, France) supplemented with streptomycin 200 μg/ml was used for air exposure in the samplers. The air exposed MEA plates in the samplers were incubated at 25°C for 5 days for fungal growth and isolation.
Fungal classification and identification
Visible colonies with different morphology grown out from the MEA plates were transferred onto new sterile MEA and incubated again at 25°C for 3–5 days. For the pure culture, single spore isolation was performed using a light stereomicroscope (SZX10, Olympus, Seoul, Korea) and spore flooded media. Microstructures of grown fungi were observed by a light microscope (Axioskop 40, Carl Zeiss, Jena, Germany) for morphological identification. Trichoderma fungi were classified based on morphological and molecular characteristics. Morphological identification was followed by the taxonomic key of Domsch et al. (1980), Gams and Bissett (1998), and Rifai (1969).
To classify Trichoderma spp., potato dextrose agar (PDA) was used for cultivation media. Colony morphology and microscopic characteristics on the PDA was observed after cultivation at 25°C for three days. For molecular identification of Trichoderma, we analyzed translation elongation factor 1α (TEF 1α) gene which is known as the better marker for Trichoderma and Hypocrea (sexual stage form of Trichoderma) identification than the internal transcribed spacer (ITS) ribosomal sequence (Samuels, 2006). Fungal genomic DNA was extracted with the drilling method described by Kim et al. (1999). TEF728 (5′-CATCGAGAAGTTCGAGAAGG-3′) and TEF1 (5′-GCCATCCTTGGAGATACCAGC-3′) primers which are targeting for the TEF 1α gene were used for PCR amplification (Dees and Ghiorse, 2001). PCR was performed at the condition described by Kim et al. (2016). The PCR products were purified with the High Pure PCR Purification Kit (Roche, Basel, Swiss), and their nucleotide sequences were determined by Macrogen Inc. (Seoul, Korea). The obtained tef-1α gene DNA sequences were deposited on the GenBank of NCBI (http://www.ncbi.nlm.nih.gow/genbank). tef-1α gene DNA sequence of taxon related reference species were downloaded from the GenBank of NCBI. Phylogenetic analysis was conducted with a neighbor-joining method in MEGA6 program (Tamura et al., 2013). Clade reliability of phylogenetic tree was evaluated with 1,000 times bootstrap resampling. The tef-1α gene sequence of Cladosporium cladosporioides CBS 145.35 was used for outgroup of the phylogenetic analysis. Molecular identification of Trichoderma was performed by putting the obtained tef-1α gene sequence into the TrichoBLAST v.1.0 program at the site of International Subcommission of Trichoderma and Hypocrea Taxonomy (http://isth.info/tools/blast/index.php).
Growth properties
Examination of mycelia growth of selected Trichoderma spp. was performed on media containing different nutrient substrates (Jaklitsch, 2009). Agar plugs (5 mm diameter) of PDA grown Trichoderma spp. were inoculated on PDA, MEA, Czapek’s Dox + yeast extract agar (CYA), and cornmeal dextrose agar (CMD). After incubation at 25°C for 3 days, mycelial length was measured and morphological characteristics including colony color and texture were observed.
Extracellular enzyme activity test
Selected Trichoderma spp. were grown on PDA at 25°C for 2 days to be used as inoculums for extracellular enzyme activity test. For the evaluation of fungal extracellular enzyme activity, the pre-cultured Trichoderma spp. were transferred onto the media containing each of 0.5% CM-cellulose (Sigma, St. Louis, MO, USA), D-cellobiose (Sigma), avicel (Sigma), starch from potato (Sigma), polygalacturonic acid (MP Biomedical, France), xylan from oat spelts (Sigma) as enzymatic carbon source, 0.1/% yeast nitrogen base (Difco) as its fundamental nitrogen source, and 1.5% agar powder. One pointe five percent Congo red dyes (Teather and Wood, 1982) was used for chromogenic reaction due to its better performance in extracellular enzyme activity detection with the tested Trichoderma spp. (Yoon et al., 2007). Ten percent skim milk powder (Sigma) was used for the detection of protease activity. After incubation at 25°C for 3 days, the length of clear zone formed by reaction between the extracellular enzyme secreted by the tested fungi and chromogenic substrates were measured as enzyme activity.
Growth inhibition test of Trichoderma spp. against oak wood mushroom mycelia
To test the ability of Trichoderma spp. in inhibition of mycelia growth of oak wood mushroom strains, two kinds of mushroom cultivars, Chamaram and Sanjo 701ho, were used. The two mushroom strains were pregrown on PDA plate at 25°C for 10 days. Agar plugs of each mushroom cultivars were inoculated one side of PDA and incubated at 25°C for 7 days. Agar plugs of each Trichoderma species pregrown on PDA for 3 days were inoculated on the other side of the PDA plate of each mushroom cultivar grown for 5 days. Thus, each Trichoderma species confronted each oak wood mushroom cultivar on PDA. After dual culturing at 25°C for 14 days, the degree of Trichoderma spp. overlaying to oak wood mycelium was analyzed as the inhibitory effect of Trichoderma on the mycelia growth of the mushroom. The formation of and the degree of darkness of brown line between Trichoderma and the mushroom mycelia were analyzed as the endurance response of the mushroom against Trichoderma.
Sensitivity test of Trichoderma spp. to antifungal agents
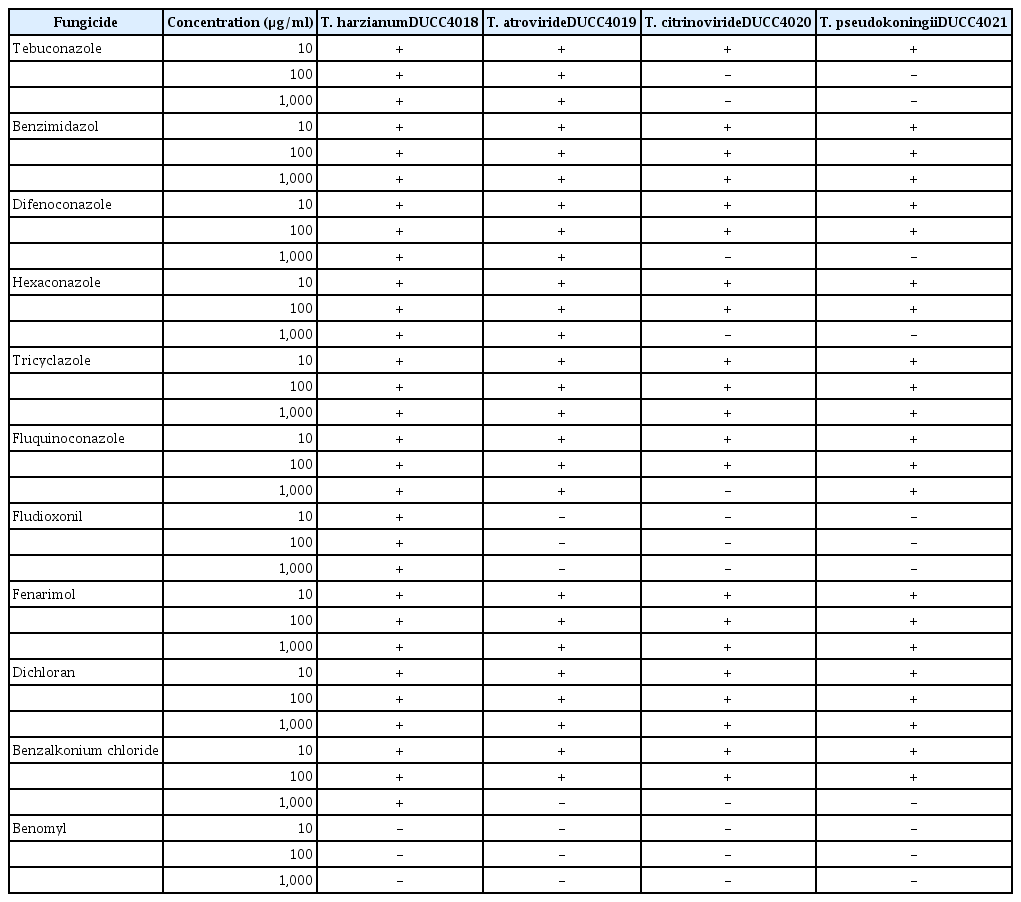
Antifungal agents (Sigma) including tebuconazole, benzimidazol, difenoconazole, hexaconazole, tricyclazole, fluquinoconazole, fludioxonil, fenarimol, dichloran, benzalkonium chloride, and benomyl were tested for their effect on Trichoderma spp. For the sensitivity test, PDA media supplemented with each agent at three different concentration (10 μg/ml, 100 μg/ml, and 1,000 μg/ml) were prepared. Conidia suspension (1 ×106 spores/ml) was prepared in sterile water from the PDA grown test species of Trichoderma. One hundred microliters of conidia suspension of each Trichoderma species was inoculated onto each antifungal agent supplemented PDA. Three days after incubation at 25°C, the presence or absence of grown mycelia from the inoculated spores was determined by naked eyes as the criterion of sensitivity to antifungal agents.
Results and Discussion
Isolation and identification of Trichoderma isolates
Through sequential air sampling for 33 months, a total of 1,063 fungal isolates were obtained from indoor air of the two oak wood mushroom cultivation houses. Among these isolated fungi, Cladosporium spp., Cercospora spp., Epicoccum sp., Mortierella spp., Penicillium spp., Pestalotiopsis sp. and several ascomycete spp. and basidiomycete spp. were found including Trichoderma spp. We recognized Trichoderma fungi by observing their typical colony morphology and anamorphic structures of conidiophores and conidia. The number of Trichoderma isolates was 248 which took 20.16% of the total number of isolates. This percentage indicates Trichoderma takes one fifth of the airborne fungi in the two oak wood mushroom cultivation houses. It is considered to be a high level of Trichoderma presence. Farmers of the two cultivation facilities claimed that they had from time to time contamination of sawdust media by Trichoderma fungi despite that they tried to keep sanitary cultivation with great efforts. Especially, if Trichoderma contamination occurs during production of sawdust mushroom media, all the media should be discarded. In this case, the fungal damage will be very severe and huge economic loss are inevitable. They have been questioned and worried about that how the contamination happens. Our results provide evidence that there were Trichoderma contaminant in the air of the two cultivation houses. Thus, there has been always chance of sawdust media contamination by the Trichoderma buoyant in the air. That’s why they had get Trichoderma problem occasionally.
Currently, to our knowledge, long period air monitoring data on Trichoderma fungi in oak mushroom cultivation houses has not been available because there has been no research report domestically and internationally on the continuous monitoring of Trichoderma fungi for more than 24 months at the same mushroom farms. Therefore, our result is the first and valuable data pointing out the important of airborne Trichoderma in indoor air. Since our data came from 33 months period monitoring, we strongly stress out that air management in cultivation houses should be improved to avoid contamination of the mushroom substrate from Trichoderma. Since we sampled ambient air from inside of the cultivation houses, it is questioned where they came from. They could be originated from outdoor air or indoor environment. Generally, it is known that Trichoderma is a soil-borne fungus (Domsch et al., 1980; Roiger et al., 1991). Thus, we could not rule out that buoyant soil dust associated with Trichoderma spores would be the major source of outdoor air origin. Trichoderma fungi also very well grow saprophytically on organic substrate of plant materials because they have enzyme system such as glucanase and cellulase (Benítez et al., 2004). Thus, airborne Trichoderma spore derived from organic materials of plant debris could be another source of outdoor air origin of the cultivation houses. Regarding indoor environment, Trichoderma spores generated from organic materials of trashed sawdust media after mushroom harvest and/or from pre-contaminated sawdust media from media production sites could be the main source of indoor air origin in the cultivation houses.
Trichoderma genus contains diverse species and some of them could inhibit mushroom growth (Samuel, 2006). Especially, certain species of Trichoderma as green mold disease could give rise to serious damage resulting in mushroom crop loss (Largeteau-Mamoun et al., 2002; Wuest et al., 1996). Thus, to protect the mushroom cultivation from Trichoderma problem it is better to know what Trichoderma species go around in the air of the cultivation houses. Thus, we tried to identify the 248 Trichoderma isolates obtained from the air sampling of this study. Observation of growth pattern on different media and colony properties led us to classify the Trichoderma isolates into four groups (Fig. 1). The ratio of each Trichoderma group was 55.08%, 22.03%, 16.53% and 6.35%, respectively (Fig. 2). For further experiments, each representative isolate was selected from the four Trichoderma groups and coded as DUCC4018, DUCC4019, DUCC4020, and DUCC4021, respectively, and identified following the taxonomic key of Domsch et al. (1980) and Rifai (1969). However, identification of Trichoderma at species level is not easy only with morphological characters, we additionally performed molecular analysis of tef-1α gene which is better marker than ITS DNA for Trichoderma species identification. Using TEF1 primer pairs, 529 bp, 1,249 bp, 845 bp, and 555 bp sizes of PCR amplicons were obtained from each of the four representative isolates. This result implied that the four representative isolates would be genetically different species. To verify this implication, BLAST analysis was performed. BLAST search in the GenBank database revealed that the determined nucleotide sequences of DUCC4018, DUCC4019, DUCC4020 and DUCC4021 isolates share 99% sequence identity with Trichoderma harzianum TMEX33 (accession No. JX650134), T. atroviride CBS 693.94 (accession No. KJ786838), T. citrinoviride DAOM 172792 (accession No. EU280036), and T. pseudokoningii GJS 99-149 (accession No. JN175589), respectively. Sequence search results in the TrichoBLAST are agreed with the BLAST search results in the GenBank. Thus, we conclusively identified the four Trichoderma isolates, DUCC4018, DUCC4019, DUCC4020, and DUCC4021 as T. harzianum, T. atroviride, T. citrinoviride, T. pseudokoningii, respectively. The tef-1α gene sequences of DUCC4018 (accession no. KX431214), DUCC4019 (accession No. KX431215), DUCC4020 (accession no. KX431216), and DUCC4021 (accession No. KX431217) isolates were deposited to the GenBank DNA database. T. harzianum DUCC4018, T. atroviride DUCC4019, T. citrinoviride DUCC4020, and T. pseudokoningii DUCC4021 from this study clearly showed their phylogenetic position with the known reference species in the phylogenic tree constructed with the tef-1α sequences (Fig. 3). According to the identification results, we obtained information that there were four Trichoderma species in the air of the two oak wood cultivation houses. And the portion of Trichoderma species in the two cultivation houses was 39% in Cheonyang and 61% in Jangheung, respectively. In Cheonyang cultivation house T. harzianum, T. atroviride, and T. citrinoviride were detedcted. While, in Jangheung cultivation house, T. harzianum, T. atroviride, T. citrinoviride, and T. pseudokoningii. Thus, more portion and number of species were found in Jangheung cultivation house than Cheongyang cultivation house. In the future, it would be better to monitor more number of cultivation houses to get idea that whether more diverse Trichoderam species go around indoor air of cultivation houses. These four Trichoderma species are found to be known agents that cause damage oak wood mushroom cultivation (Kim et al., 2012a; Pukahuta at al., 2000). And among these four Trichoderma species, dominant species is T. harzianum which is well known agent of green mold disease in button mushroom and oyster mushroom (Komoń-Zelazowska et al., 2007; Wuest et al., 1996). In addition, it is noticeable that T. harzianum, T. atroviride, and T. citrinoviride are mushroom fly carried species that greatly damaged oak mushroom production in Korea (Kim et al., 2016). Therefore, it is strongly recommended to develop a method that efficiently manages indoor air in the cultivation houses.
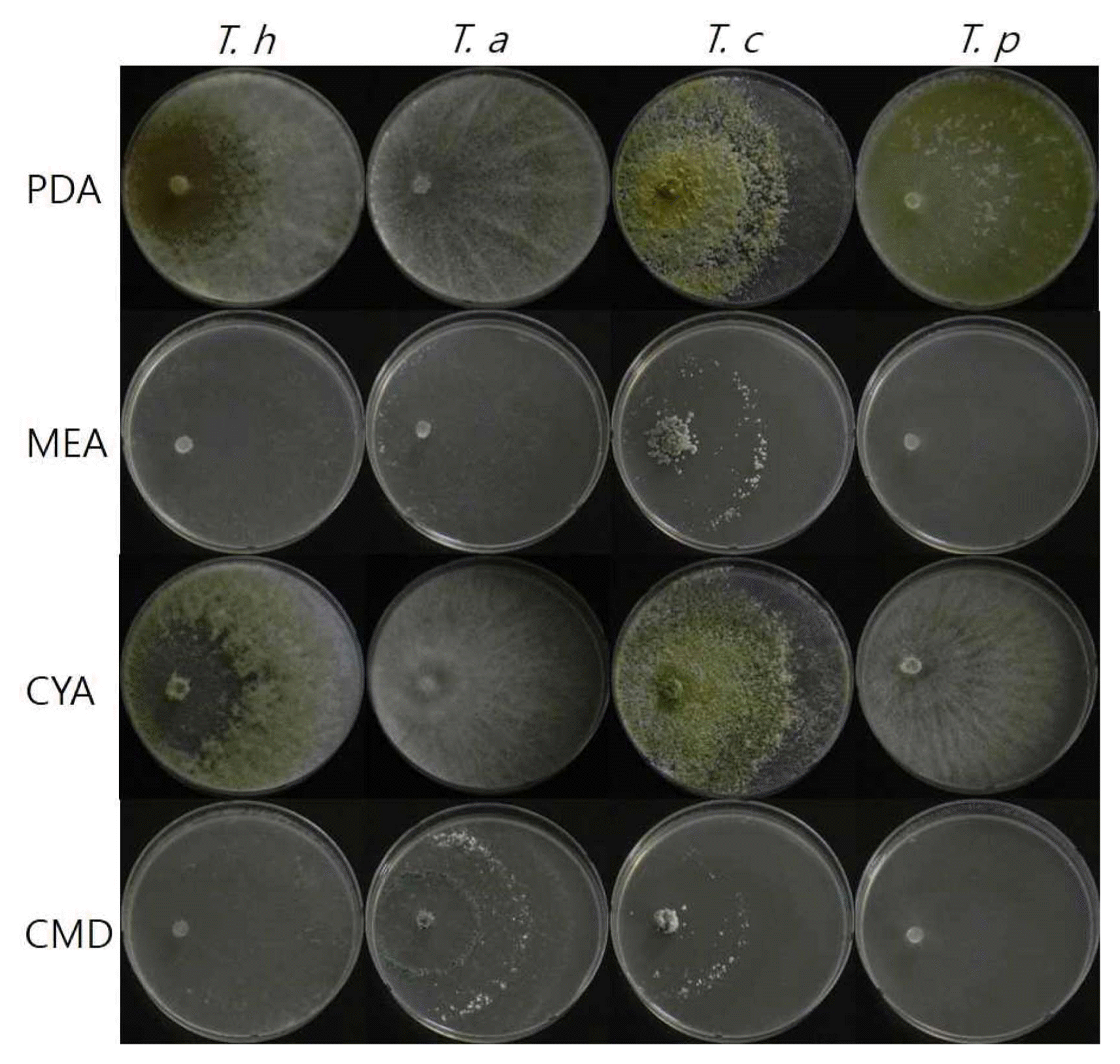
Colony morphology of Trichoderma harzianum DUCC4018, T. atroviride DUCC4019, T. citrinoviride DUCC4020, and T. pseudokoningii DUCC4021 grown on nutrient different media (PDA, MEA, CYA, and CMA) at 25°C for 3 days. T. h, T. harzianum DUCC4018; T. a, T. atroviride DUCC4019; T. c, T. citrinoviride DUCC4020; T. p, T. pseudokoningii DUCC4021; PDA, potato dextrose agar; MEA, malt extract agar; CYA, Czapek’s Dox + yeast extract agar; CMD, cornmeal dextrose agar.
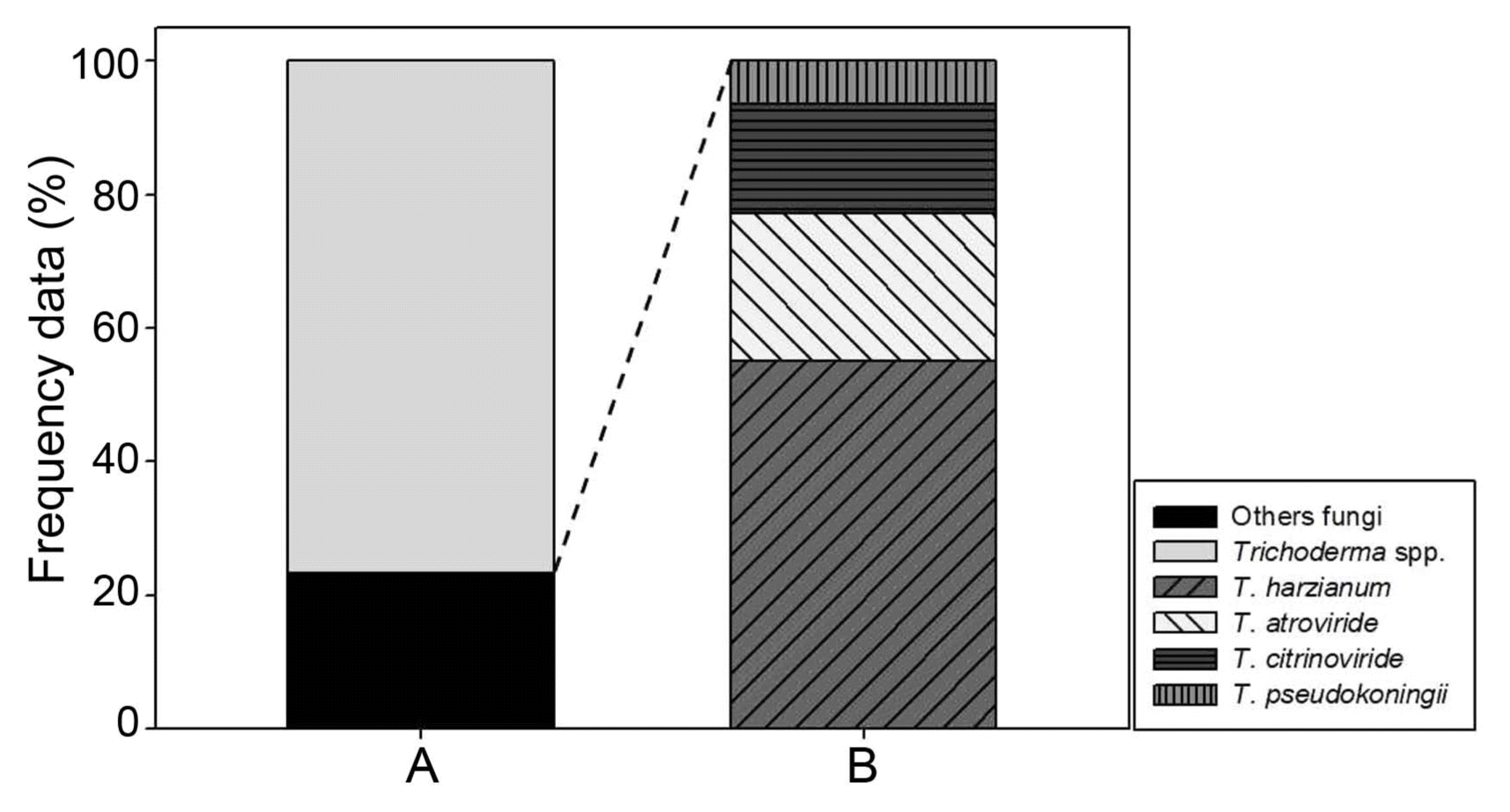
The frequency rate of Trichoderma isolates in the total number of isolates (n = 1,063) from indoor air of oak wood mushroom cultivation houses in this study (A) and relative frequency rate of the isolates of each identified Trichoderma species among the total Trichoderma isolates (n = 248) (B).
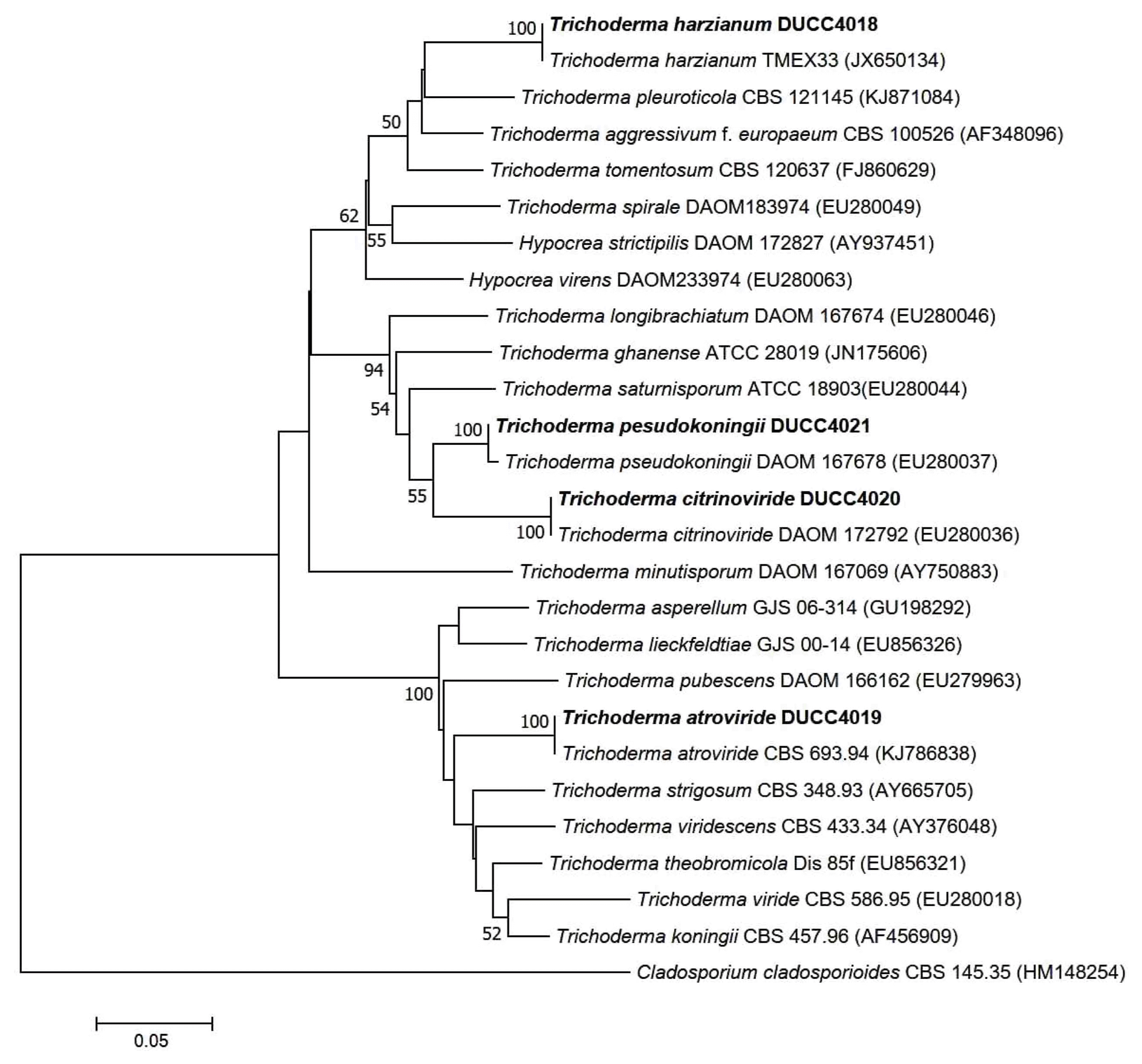
Phylogenetic position of Trichoderma harzianum DUCC4018, T. atroviride DUCC4019, T. citrinoviride DUCC4020, and T. pseudokoningii DUCC4021 isolated from indoor air of oak wood mushroom cultivation houses. Phylogenetic tree was constructed based on tef-1α gene sequences by the neighbor-joining method in Mega program. Bootstrap values based on 1,000 replications are shown above the node.
Colony morphology and growth rate according to different substrates
T. harzianum, T. atroviride, and T. citrinoviride have been reported in Korea regarding their morphology and pathogenicity to shiitake mushrooms (Kim et al., 2012a; Kim et al., 2016). But comparative description between these species has not been described. Thus, we compared T. harzianum DUCC4018, T. atroviride DUCC4019, T. citrinoviride DUCC4020, and T. pseudokoningii DUCC4021 on PDA, MEA, CYA, and CMD (Fig. 1). Mycelium of T. pseudokoningii DUCC4021 matted and embedded yellow pigment on whole media. T. pseudokoningii DUCC4021 formed white colony, matted, and showed floccose hyphal growth on PDA. On MEA and CMD, its mycelia matted with colorless. But on CYA, its mycelium was white and floccose. Mycelium density on CYA was low in the middle part than other parts. After 3 days of culturing at 25°C, the length of grown hypha of T. pseudokoningii DUCC4021 were 63 mm on PDA and CMD, 62.25 mm on MEA, 49.5 mm on CYA (Fig. 4). T. harzianum DUCC4018 and T. citrinoviride DUCC4020 grew 63mm on four different media for 3 days. T. atroviride DUCC4019 grew 50.75 mm on CMD and over than 60 mm on PDA, MEA, and CYA. These results showed that the four Trichoderma species different growth physiology and colony morphology. Thus, we could apply these results for the quick differentiation of these four species from indoor air samples of cultivation houses.
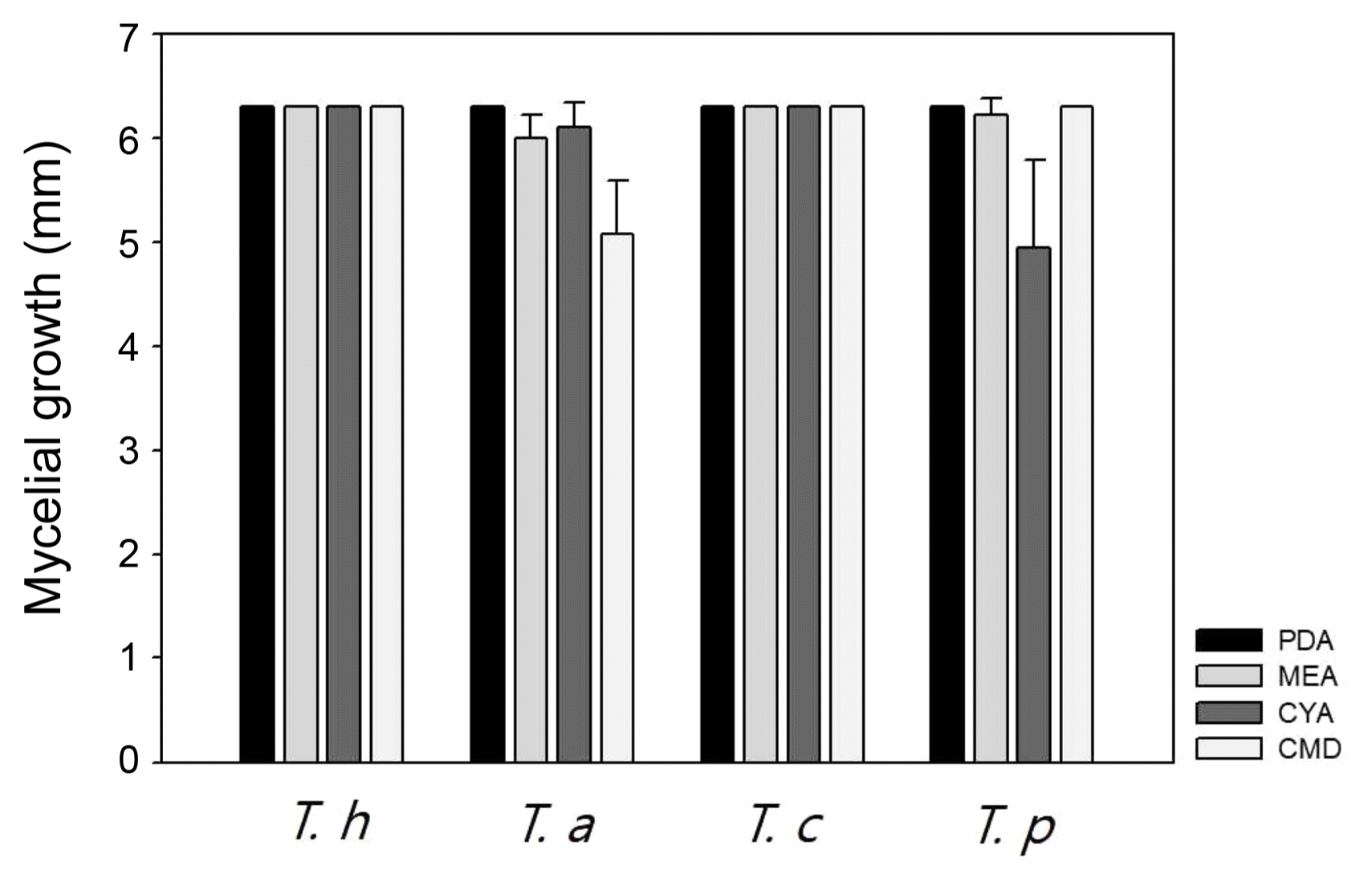
Mycelial growth of Trichoderma harzianum DUCC4018, T. atroviride DUCC4019, T. citrinoviride DUCC4020, and T. pseudokoningii DUCC4021 on different nutrient media (PDA, MEA, CYA, and CMA). T. h, T. harzianum DUCC4018; T. a, T. atroviride DUCC4019; T. c, T. citrinoviride DUCC4020; T. p, T. pseudokoningii DUCC4021; PDA, potato dextrose agar; MEA, malt extract agar; CYA, Czapek’s Dox + yeast extract agar; CMD, cornmeal dextrose agar.
Extracellular enzyme activity test
To further understand at the biochemical level, the four Trichoderma species were examined for their ability to produce seven kinds of extracellular enzymes on chromogenic media (Fig. 5). Size of clear zone of enzyme activity produced by T. harzianum DUCC4018 was 55.22 mm (β-glucosidase), 55.11 mm (avicelase), 36.74 mm (CM-cellulase), 36.54 mm (amylase), 36.74 mm (pectinase), 29.89 mm (xylanase), and 46.12 mm (protease), respectively. Size of clear zone produced by T. atroviride DUCC4019 was 40.16 mm (β-glucosidase), 32.45 mm (avicelase), 38.88 mm (CM-cellulase), 61.29 mm (amylase), 38.88 mm (pectinase), 12.45 mm (xylanase), and 60.01 mm (protease) (Fig. 5). Size of clear zone produced by T. citrinoviride DUCC4020 was 41.56 mm (β-glucosidase), 33.04 mm (avicelase), 39.11 mm (CM-cellulase), 30.59 mm (amylase), 39.11 mm (pectinase), 13.85 mm (xylanase), and 44.60 mm (protease). Size of clear zone produced by T. pseudokoningii DUCC4021 was 61.18 mm (β-glucosidase), 27.32 mm (avicelase), 42.26 mm (CM-cellulase), 49.27 mm (amylase), 42.26 mm (pectinase), 11.79 mm (xylanase), and 58.61 mm (protease). These results demonstrate that the four representative isolates of airborne Trichoderma species are able to produce extracellular enzymes. Since sawdust media for oak wood mushroom cultivation contain woody components and carbohydrates from rice bran, the examined extracellular enzymes could well hydrolyze sawdust media as their substrate. Thus, it is expected that these four extracellular enzyme-producing airborne Trichoderma could easily colonize and damage the substrates of sawdust media causing contamination problems. Trichoderma contamination of sawdust media through air means they could occur not only at the early stage of the cultivation process, especially during spawn running period, but also during fruit body cropping period. Therefore, the potential of huge losses of mushroom crops are considered to be highly possible anytime (Jandaik and Guleria, 1999).

Examples of extracellular enzyme activities shown by Trichoderma harzianum DUCC4018, T. atroviride DUCC4019, T. citrinoviride DUCC4020, and T. pseudokoningii DUCC4021 on chromogenic media. T. h, T. harzianum DUCC4018; T. a, T. atroviride DUCC4019; T. c, T. citrinoviride DUCC4020; T. p, T. pseudokoningii DUCC4021.
Dual culture with oak wood mushroom strains
Antagonism of Trichoderma spp. with oak wood mushroom mycelium is related enzyme activity. This enzyme was reported to lysis the cell wall of oak wood mushroom mycelium. And Trichoderma spp. produced colorless antifungal metabolites (Ishikawa et al., 1980; Tokimoto and Komatsu, 1979). But shiitake mycelia endure to Trichoderma spp. infection, and brown confronted line was created between Trichoderma spp. mycelium and shiitake (oak woof mushroom) mycelium (Ishikawa et al., 1980; Kawamura et al., 1980; Tokimoto and Komatsu, 1979). Therefore, in addition to their ability to degrade the substrate of mushroom cultivation media, we further examined whether the four airborne Trichoderma species could inhibit the growth of oak wood mushroom strains using dual culture assay on solid media which has been successively used for interactions between Trichoderma and oak wood mushroom strain (Lentinula edodes) (Kim et al., 2012a; Wang et al., 2016). Mushroom competitors of Trichoderma hinder the colonization of oak wood mushroom strain onto sawdust media or log bed substrate, resulting in no further growth of the mushroom strain. Consequently, fruit body doest not form and mushroom production reduces.
All four Trichoderma species showed antagonistic behavior against two mushroom strains, Chamaram and Sanjo 701ho, by invading the mycelial blocks of the two mushroom strains on PDA (Fig. 6). Enduring reaction by the mycelia of two mushroom strains to invasion of the four Trichoderma spp. produced brown confrontation line between each Trichoderma species mycelium and oak wood mushroom mycelium. The size, color, and sharpness of the reaction derived confrontation line varied depending on Trichoderma species, indicating there is difference in the ability of invasion among the four Trichoderma species. The degree of overlap area by the four Trichoderma species’invasion to the two mushroom strains was the highest by T. harzianum DUCC4018 (30 mm) and T. atroviride DUCC4019 (30 mm), followed by T. citrinoviride DUCC4020 (20 mm) and T. pseudokoningii DUCC4021 (10 mm). These data implied that all the Trichoderma isolates of the four species from indoor air source are likely competitors of oak wood mushroom strains. T. harzianum, T. atroviride, and T. citrinoviride were reported as competitors of oak wood mushroom strains in Korea (Kim et al., 2012a). Thus, based on our results in Figs. 5 and 6, we would like to report T. pseudokoningii as a new competitor of oak wood mushroom strains in sawdust media cultivation in Korea.
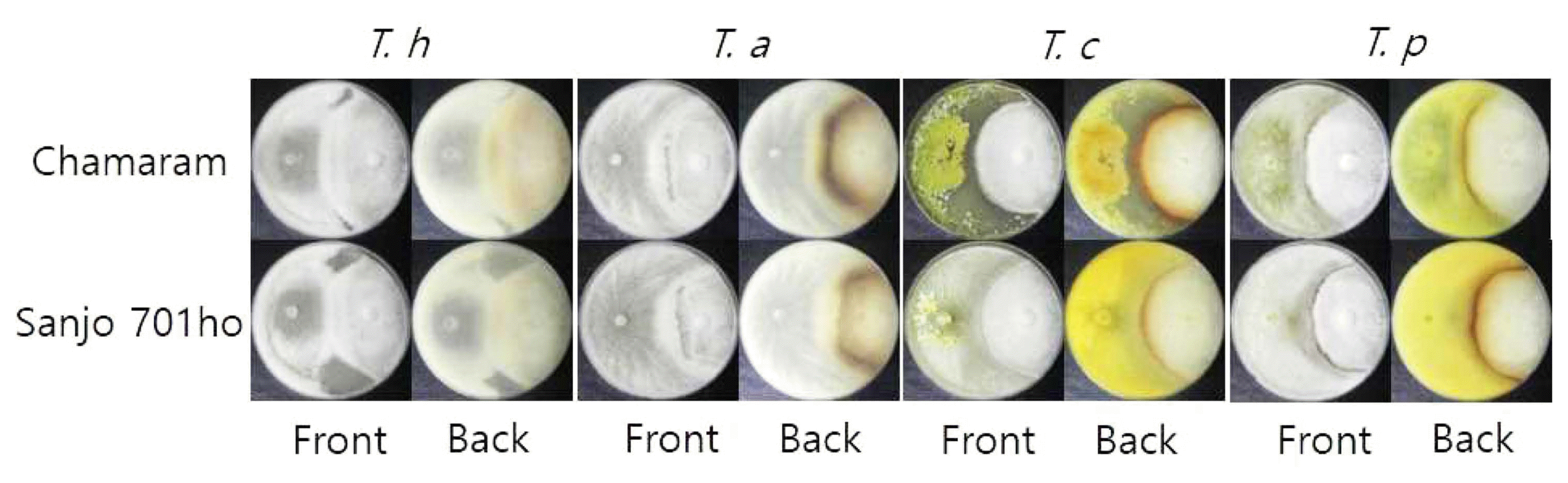
Dual culture results of Trichoderma harzianum DUCC4018, T. atroviride DUCC4019, T. citrinoviride DUCC4020, and T. pseudokoningii DUCC4021 with Korean oak wood mushroom cultivars (Chamaram and Sanjo 701ho) on PDA. T. h, T. harzianum DUCC4018; T. a, T. atroviride DUCC4019; T. c, T. citrinoviride DUCC4020; T. p, T. pseudokoningii DUCC4021; PDA, potato dextrose agar. Dual cultures were incubated at 25°C for 10 days.
Antifungal agents sensitivity test of Trichoderma spp
To help the potent choice of sanitary compounds that could be used for mushroom cultivation facility as preventive measures, the sensitivity of four Trichoderma species were assessed to twelve antifungal agents; tebuconazole, benzimidazol, difenoconazole, hexaconazole, tricyclazole, fluquinoconazole, fludioxonil, fenarimol, dichloran, benzalkonium chloride, and benomyl. T. harzianum DUCC4018 could grow at all concentration of test antifungal agents, except every concentration of benomyl (10 μg/ml, 100 μg/ml, and 1,000 μg/ml) (Table 1). T. atroviride DUCC4019 was sensitive to all concentration of fludioxonil and benomyl, and 1,000 μg/ml of benzalkonium chloride. T. citrinoviride DUCC4020 showed broad sensitivity to tebuconazole (10 μg/ml, 100 μg/ml), 1,000 μg/mL of difenoconazole, hexaconazole, fluquinoconazole and benzalkonium chloride and every concentration of fludioxonil and benomyl. T. pseudokoningii DUCC4021 also showed sensitivity to tebuconazole (10 μg/ml, 100 μg/ml), 1,000 μg/mL of difenoconazole, hexaconazole, and benzalkonium chloride and every concentration of fludioxonil and benomyl. These results show there is difference in the sensitivity to antifungal agent among the four Trichoderma species. Overall, among of tested antifungal agents, benomyl inhibited mycelial growth and conidia germination of the four Trichoderma species at all tested concentrations. Benomyl was reported to inhibit assembly of fungal microtubules (Davidse, 1986), rapidly degrade into carbendazim in aqueous (Tang et al., 1992) or organic (Chiba and Cherniak, 1978) solution, or induce hydroxylation in the microsomal monooxygenase system (Danielson and Davey, 1973). While, mushroom strains of Chamaram and Sanjo 701ho could grow at all the fungicide concentration (data not shown). Thus, we found benomyl is the best choice to disinfect the oak wood mushroom cultivation facility to deter Trichoderma spp. contamination.
Acknowledgments
This work was supported by a grant National Institute of Horticultural and Herbal Science (PJ01328102), Rural Development Administration and by Golden Seed Project (Center for Horticultural Seed Development, No. 213007-05-3-WTH32), Ministry of Agriculture, Food and Rural Affairs (MAFRA), Ministry of Oceans and Fisheries (MOF), Rural Development Administration (RDA) and Korea Forest Service (KFS).