|
 |
| Plant Pathol J > Volume 39(6); 2023 > Article |
|
Abstract
Fusarium root rot is an increasingly severe problem in soybean cultivation. Although several Fusarium species have been reported to infect soybean roots in Heilongjiang province, their frequency and aggressiveness have not been systematically quantified in the region. This study aimed to investigate the diversity and distribution of Fusarium species that cause soybean root rot in Heilongjiang province over two years. A total of 485 isolates belonging to nine Fusarium species were identified, with F. oxysporum and F. solani being the most prevalent. Pot experiments were conducted to examine the relative aggressiveness of different Fusarium species on soybean roots, revealing that F. oxysporum and F. solani were the most aggressive pathogens, causing the most severe root rot symptoms. The study also assessed the susceptibility of different soybean cultivars to Fusarium root rot caused by F. oxysporum and F. solani. The results indicated that the soybean cultivar DN51 exhibited the most resistance to both pathogens, indicating that it may possess genetic traits that make it less susceptible to Fusarium root rot. These findings provide valuable insights into the diversity and distribution of Fusarium species that cause soybean root rot and could facilitate the development of effective management strategies for this disease.
Heilongjiang province, situated in the northeast of China, is recognized as the birthplace of soybean cultivation and a major contributor to the global soybean production (Hymowitz and Shurtleff, 2005). The region’s black soil, with its dark color and crumbly texture, is renowned for its fertility and nutrient richness, accumulated over thousands of years of organic matter (Zhao et al., 2014). Moreover, the high water-holding capacity of Heilongjiang’s black soil makes it an ideal place for cultivating different crops, including soybeans, corn, wheat, and rice (Cai et al., 2022b). Additionally, the region’s soil harbors a diverse range of microorganisms that play a crucial role in its ecosystem (Zhou et al., 2017).
Despite being rich in nutrients and microorganisms, the soil in Heilongjiang has been undergoing severe degradation over the years, primarily due to monoculture planting systems and the extensive use of fertilizers and pesticides (Du et al., 2021, Mei et al., 2022). This has caused soil compaction and a decline in biodiversity, allowing certain soil-borne pathogens like Fusarium spp. to dominate (Cai et al., 2022a; Husaini et al., 2018). The prevalence of Fusarium presents a new threat to soybean production, making it imperative to conduct a comprehensive investigation of its distribution in the region and develop better management strategies to combat the pathogen.
The genus Fusarium is notorious for housing numerous plant pathogenic fungi, and several species within this genus have been known to cause severe soybean diseases globally, such as root rot, sudden death syndrome, blight, wilt, and damping off (Arias et al., 2013; Cai et al., 2020; Lin et al., 2022). While using fungicidal seed treatment is a common approach to managing Fusarium infection in soybean, it is not environmentally friendly and can lead to soil degradation and reduced soil biodiversity (Ayesha et al., 2021). An alternative solution is to cultivate soybean varieties that are resistant to Fusarium, but this requires a thorough understanding of the distribution and virulence of the different Fusarium species.
To improve the management of Fusarium diseases in soybean in Heilongjiang province, a two-year survey of soybean root rot was conducted. The study aimed to identify the Fusarium species responsible for soybean root rot, assess the virulence of these species on soybean, and evaluate the resistance of soybean cultivars to the major Fusarium species. The information gathered from this study will facilitate the development of more effective management strategies against Fusarium in the region and promote sustainable soybean production in Heilongjiang province.
For agricultural production purposes, Heilongjiang Province has been classified into six accumulated temperature zones (ATZs), with each zone utilizing specific soybean cultivars to achieve optimal yield (Lu et al., 2017). Over a period of two years, soybean roots affected by the disease were sampled, resulting in the collection of 206 root samples from across the six ATZs, which represent the primary soybean production areas.
The pathogen was isolated using tissue separation method. First, the diseased plant samples were washed with sterilized distilled water. Then, the infected root tissues of the plants were cut into small pieces of about 1 cm, and sterilized with 75% ethanol for 1 min. After rinsing with sterile water for 5 times, they were treated with 10% sodium hypochlorite for 5 min, and then rinsed with sterile water for 5-8 times until clean. The surface moisture of the tissue was absorbed with sterilized filter paper. Root pieces were embedded in antibiotic-amended Nash-Snyder medium (15 g peptone, 1 g KH2PO4, 0.5 g MgSO4·7H2O, 20 g agar, 0.75 g PCNB, 33 mg/ml streptomycin sulfate solution, 40 mg/ml neomycin sulfate solution per liter) and incubated at a constant temperature of 28°C for 5 days. After the fungal colonies grew, the isolates were purified by single-spore isolation and grown on potato dextrose agar for morphological identification, molecular identification, and pathogenicity testing.
DNA extraction and purification were performed as described previously (Arias et al., 2013). Briefly, the mycelium of each isolate was collected by scraping the surface of growing colonies on potato dextrose agar medium incubated for 1 week at 28°C. The fungal mycelia were ground in liquid nitrogen, then the genomic DNA was extracted using the Fungal DNA mini kit (Tiangen Biotech, Beijing, China) as described by the manufacturer. The DNA purity and concentration were determined by using a NanoDrop ND1000 spectrophotometer (NanoDrop Technologies Inc., Waltham, MA, USA). The complete rDNA-ITS and the highly conserved mitochondrial and nuclear ribosomal small subunit (mtSSU rRNA genes) of Fusarium were amplified. The primers used were ITS1/ITS4 (5′-TCCGTAGGTGAACCTGCGG-3′, 5′-TCCTCCGCTTA TTGATATGC-3′) and MS1/MS2 (5′-CCAGCAGTCAAGAATATTAGTCAATG-3′, 5′-GCGGATTATCGAATTAAATAAC-3′), respectively (O’Donnell et al., 1998; White et al., 1990). These primers were synthesized by Sangon Biotech (Shanghai) Co., Ltd. Polymerase chain reaction (PCR) products were sequenced at the Sangon Biotech (Shanghai) Co., Ltd. Sequences were submitted for BLAST searches for comparison to known DNA sequences in the NCBI. Random amplified polymorphic DNA (RAPD)-PCR was carried out in a total volume of 25 μl containing 12.5 μl 2× SanTaq PCR mix, 8.5 μl double distilled water, 1 μl primer (10 pmol/μl) and 1 μl template DNA (40 ng/μl). Amplification was run with the following profile: an initial denaturation at 94°C for 5 min, followed by 45 cycles of denaturation at 94°C for 30 s, annealing at 36°C for 1 min, extension at 72°C for 2 min, and a final extension at 72°C for 10 min.
For pathogenicity assay of nine Fusarium species, soybean cv. Xiaojinhuang was used. Soybean seeds were sterilized with 75% ethanol for 3 min and then washed three times with sterile water. Two seeds were planted per pot containing a 3:1 mixture of sterile soil and vermiculite. Inoculation was performed about two weeks after emergence of plants, as described previously (Cai et al., 2020). Six strains from different ATZs were used for pathogenicity test for each Fusarium species. Spore suspensions for inoculation were prepared by flooding cultures with distilled water, then filtering them through sterile cloth. The final conidia concentration was adjusted to 106 conidia/ml. Nine plants per treatment were immersed in a spore suspension for 2 h. Then the roots were washed with distilled water and put back into the pots for further growth. Disease index was calculated according to Chang et al. with some modifications (Chang et al., 2015). Briefly, after 21 days of inoculation, soybean seedlings were gently removed from the pot by turning it upside down, the soybean plants are repeatedly rinsed with clean water, and then the surface of the plant is dried with filter paper. Based on the symptoms of the main and lateral roots and the degree of rot, soybean root rot caused by Fusarium is divided into five levels; Level 0: no disease symptoms; Level 1: the main root is lightly browned, the lateral root growth point is diseased, but the plant can grow normally; Level 2: the main root is diseased and turns from brown to black, the tip of the lateral root turns black, but the plant can still grow normally; Level 3: most of the main root turns black and cannot continue to grow, the lateral roots are reduced or there are no lateral roots, and the plant grows slowly; Level 4: the roots are completely rotten, and the plant cannot continue to grow and may even wilt and die. By observing the root disease situation of Fusarium interacting with soybean plants, referring to the grading standards, the disease grades of different Fusarium infections are recorded, and the disease index is calculated according to the formula as follows.
Disease index = ∑ (number of plants × number of degree in symptoms)/(total number of plants × 5(maximum degree in symptoms)×100. Plant height and root length was also measured, and the shoot dry weights and root dry weights recorded for each plant after oven drying for 24 h at 60°C. Three replications were conducted to assess the pathogenicity of each Fusarium isolate.
For the soybean cultivar resistance test, a total of 21 cultivars were included in the study. The specific cultivars used are HN48, HN51, HN53, HN64, HF35, HF47, HF51, DN44, DN51, DN54, DN57, HN63, HN75, BD48, SN30, SN31, SN35, SN37, LD5, KF21, and HL44. As the most frequent species identified in this study, F. oxysporum and F. solani were selected. Soybean seeds were sterilized with 75% ethanol for 3 min and then washed three times with sterile water. Inoculation was conducted by dipping 200 ml 106 conidia/ml spores to soybean seed surface. Then the seeds were put in dark condition for seven days. Disease severity was measured by dividing length of infected brown root to whole root length.
Over the course of a two-year survey, a total of 485 isolates belonging to the Fusarium genera were recovered, and nine species were identified based on characteristics of colony and spore structure, as well as ITS and mtSSU rRNA gene sequences (Fig. 1, Supplementary Figs. 1 and 2). Among all pathogenic Fusarium, a total of 227 strains of F. oxysporum were isolated, with an isolation frequency of 46.80%, ranking first in proportion, followed by F. solani with an isolation frequency of 19.38%. F. equiseti was the third most abundant species, with a relative abundance of 11.75%. The other Fusarium species identified were F. proliferatum, F. graminearum, F. tricinctum, F. brachygibbosum, F. globosum, and F. incarnatum. Among these, F. globosum was first discovered in China from diseased soybean plants with root rot. These findings highlight the diverse nature of Fusarium species present in the surveyed region.
The distribution of the isolated strains in different ATZ is shown in Fig. 2. F. oxysporum, F. solani, and F. equiseti are distributed in all regions; F. brachygibbosum, F. incarnatum, and F. proliferatum are only distributed in the first and second ATZs; F. globosum is found in the first, second, and third ATZs; among the six ATZs, only F. tricinctum was not found in the sixth ATZ, while it is distributed in all other regions. F. graminearum was not found in the fifth ATZ among the six ATZs. The data also shows that the distribution of Fusarium species is significantly reduced in the fifth and sixth ATZs, the second ATZ has the most diverse distribution of Fusarium species and the highest number of pathogenic strains. F. oxysporum and F. solani were found to be the dominant species in almost all six ATZs, indicating their widespread distribution throughout the region. This suggests that these two species have the potential to cause significant damage to agricultural production and warrant further study. Intriguingly, ATZ2 had the highest abundance of Fusarium spp. in terms of both species diversity and quantity, suggesting that temperature plays a crucial role in the pathogen’s distribution. The result is in agreement with prior investigations that have illustrated the impact of temperature on the growth and survival of Fusarium spp. Understanding the distribution and environmental factors influencing the prevalence of Fusarium species is critical for developing effective strategies to control their spread and minimize the impact on crop yields.
Out of the 30 primers that were tested, only 10 RAPD primers yielded PCR products with clear and vibrant polymorphic bands. Conversely, the remaining 20 primers resulted in negligible band formation in the PCR products. The 10 different primers consistently amplified multiple polymorphic bands, ranging from 5 to 11 bands (Supplementary Fig. 3). In total, 79 RAPD markers were acquired, out of which 43 were polymorphic, constituting 54.43% of the total markers obtained (Table 1).
To examine the relative aggressiveness of different Fusarium species on soybean roots, a series of pot experiments were conducted. Nine Fusarium species were tested, and the results revealed significant differences in their ability to cause root rot (Fig. 3). F. oxysporum and F. solani were found to be the most aggressive pathogens, causing the most severe root rot symptoms and being the most frequently isolated species. F. tricinctum, F. proliferatum, F. graminearum, F. brachygibbosum, F. equiseti, F. globosum, and F. incarnatum also caused significant damage to plant growth and development, as evidenced by reductions in plant height, root length, shoot dry weight, and root dry weight compared to the control (Fig. 4). Further analysis of the data showed that the different Fusarium species had varying effects on plant characteristics. F. oxysporum was found to be the most severe species in terms of its impact on plant growth, indicating that it is the most aggressive isolate. These findings are consistent with previous studies that have shown F. oxysporum and F. solani to be highly virulent pathogens that can cause significant yield losses in soybean (Hafez et al., 2021).
The results of disease susceptibility testing indicated significant variation in the response of different soybean cultivars to F. oxysporum and F. solani infections (Fig. 5). Most cultivars showed greater susceptibility to F. oxysporum infection than to F. solani infection, with F. oxysporum causing more severe disease symptoms. However, for some cultivars, including BD48, SN30, SN35, LD5, KF21, and HL44, F. solani infection resulted in more severe disease symptoms than F. oxysporum infection. Interestingly, there were no significant differences in disease severity between F. oxysporum and F. solani infection for cultivars HN53, DN44, and DN51. Moreover, the soybean cultivar DN51 showed the most resistance to both F. oxysporum and F. solani infection, indicating that it may possess genetic traits that make it less susceptible to Fusarium root rot.
Pathogens and plants are not independent entities. Disease development requires the presence of three key factors: a susceptible plant host, a virulent pathogen, and favorable environmental conditions. If any of these factors are absent or unfavorable, disease will not occur (Velásquez et al., 2018). Numerous studies have shown that temperature can affect both pathogen growth and reproduction, as well as the severity of diseases caused by Fusarium spp. (Abbas et al., 2022; Cruz et al., 2019; Kaur et al., 2022). Climate has also been identified as a significant factor in determining the distribution of Fusarium spp. in soil worldwide, implying that temperature may play a critical role in the incidence and severity of Fusarium-caused plant diseases (Nikitin et al., 2023; Poole et al., 2013).
Climate parameters such as accumulated temperature during soybean growth, relative humidity, precipitation, sunlight duration, and soil surface temperature significantly impact the distribution of Fusarium pathogens. The occurrence of Fusarium root rot primarily occurs in warm and humid regions, with the severity of the Fusarium infection increasing with higher precipitation and relative humidity (Abbas et al., 2022). The climate in Heilongjiang province spans a large range, featuring limited heat resources and significant differences in accumulated temperature (Li et al., 2022). The first ATZ has an accumulated temperature of over 2,700°C and a warm climate. The second ATZ has a moderate climate with an accumulated temperature of between 2,500-2,700°C, the third ATZ has a cool climate with an accumulated temperature of between 2,300-2,500°C, the fourth ATZ has a cold climate with an accumulated temperature of between 2,100-2,300°C, the fifth ATZ has a very cold climate with an accumulated temperature of between 1,900-2,100°C, and the sixth ATZ has a high and cold temperature with an accumulated temperature of below 1,900°C (Cao et al., 2014). Lower soil surface temperatures and high humidity also promote the formation of plant tissue, leading to an increase in spore density and the accumulation of pathogenic Fusarium fungi, which in turn affects soybean disease (Yan and Nelson, 2022). Additionally, environmental factors influence both the pathogenic mechanism of the pathogen and the resistance pathways of the plant (Cheng et al., 2019).
This study provides the first systematic reports on the distribution, prevalence, and aggressiveness of Fusarium root rot soybean disease among the six ATZs in Heilongjiang province. Our results confirmed that the diversity of Fusarium spp. varies among different ATZs, but F. oxysporum and F. solani are the most frequently isolated species. Using a combination of morphological characterization and molecular analysis, we identified nine Fusarium species associated with soybean root rot, all of which can infect soybean plants with varying severity and affect plant biomass. F. oxysporum was found to be the most aggressive in terms of disease severity and impact on plant biomass, while F. brachygibbosum showed the second most inhibitory effect on root length after F. oxysporum.
RAPD analysis allows a better understanding of the pathogen’s virulence, evolution, and population genetic variation. Our results revealed relatively small genetic differentiation within populations, indicating significant genetic homogeneity within the six ATZ populations. This homogeneity is also reflected in the similar aggressiveness of the same species from different ATZs. These findings are consistent with previous studies, which demonstrated that F. oxysporum isolates exhibited less polymorphism and a high degree of genetic similarity among them (Nasr Esfahani, 2018, Brizuela et al., 2021). The low genetic diversity within F. oxysporum populations may be attributed to the narrow region in this study or the strictly asexual reproduction of F. oxysporum and limited gene flow between populations (Sillo, 2022).
In addition to temperature, numerous other environmental factors can influence the interactions between plants and microbes and disease development (Liu et al., 2020). Our study demonstrated that neither warmer nor colder conditions increased the diversity or richness of Fusarium spp., as reflected in ATZ1 or ATZ6. Instead, moderate temperatures appeared to favor the growth of Fusarium spp., as reflected in ATZ2. Many studies have reported that, under both low and high temperature regimes, Fusarium spp. exhibit low average mycelial growth rates (Saleh et al., 2021; Scott et al., 2010). Different Fusarium spp. exhibit diverse responses to temperature, indicating the complex genetic and evolutionary mechanisms underlying Fusarium species. Further research is necessary to explore the relationship between temperature, the pathogen, and the host, as this information will play a crucial role in managing diseases more effectively.
In conclusion, our study confirms that F. oxysporum is the dominant species causing soybean root rot in Heilongjiang province, distributed across six ATZs. For hemibiotrophic pathogens such as F. oxysporum and F. solani, cultivating resistant soybean cultivars would be the best control strategy. In our study, we tested 21 soybean cultivars for their resistance to the dominant and most aggressive species, F. oxysporum and F. solani. The same cultivar may exhibit a different response to F. oxysporum and F. solani infections, suggesting that different infection mechanisms may exist for different Fusarium spp. DN51 demonstrated resistance to both F. oxysporum and F. solani infections, making it a potential resistance material for future breeding programs or further studies on Fusarium-soybean interactions.
Acknowledgments
This study was supported by The National Key R&D Program of China (2022YFE0203300) and Heilongjiang Postdoctoral Fund (LBH-Z20160).
Electronic Supplementary Material
Supplementary materials are available at The Plant Pathology Journal website (http://www.ppjonline.org/).
Fig. 1
Colony appearance on potato dextrose agar medium of 9 Fusarium species isolated from soybean roots in ATZ2 after seven days of culture. (A) F. oxysporum. (B) F. solani. (C) F. equiseti. (D) F. proliferatum. (E) F. graminearum. (F) F. tricinctum. (G) F. brachygibbosum. (H) F. globosum. (I) F. incarnatum.
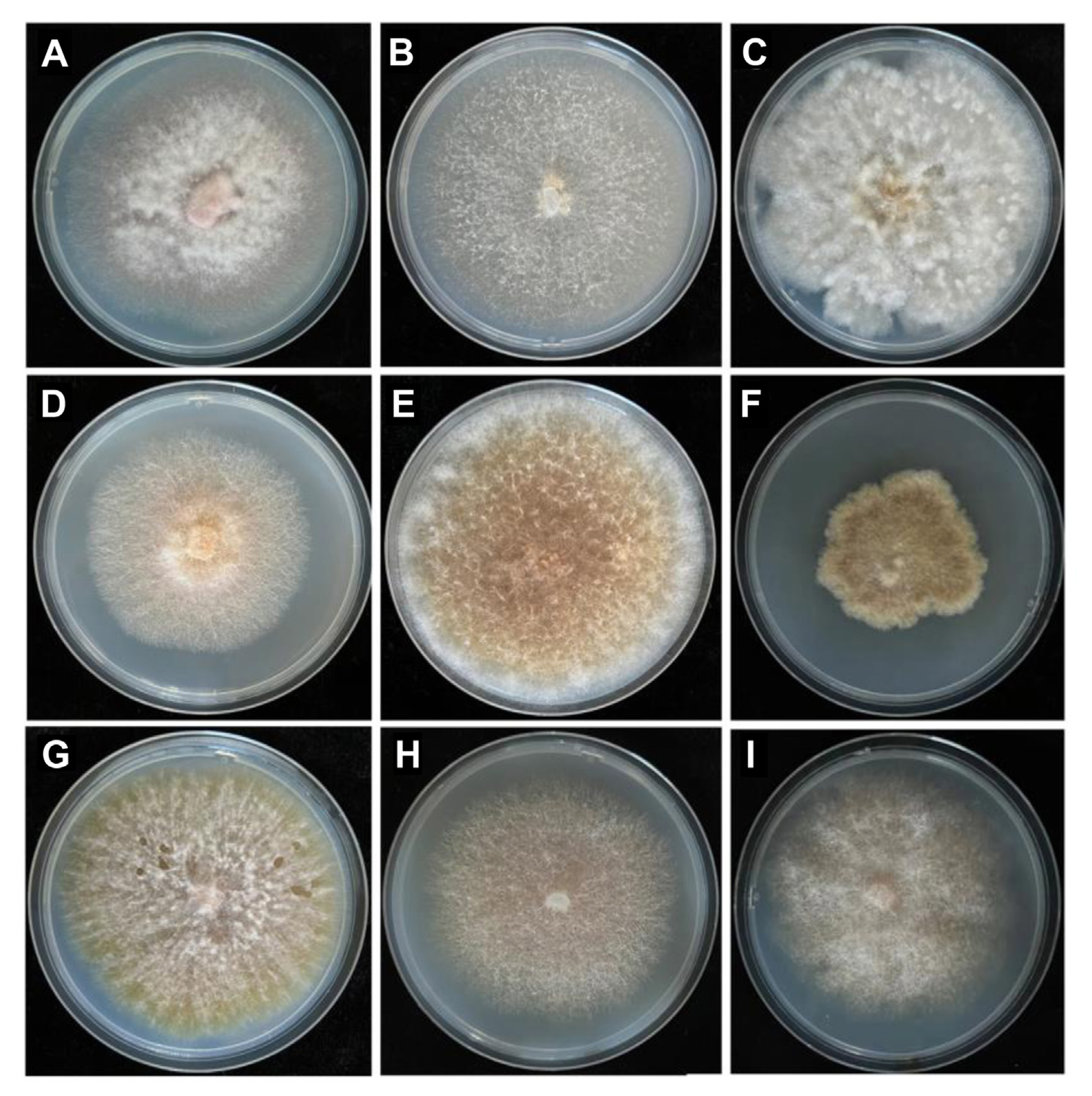
Fig. 2
Frequency of isolation of Fusarium species recovered from soybean root rot at six accumulative temperature zones from 2021 to 2022. FB, F. brachygibbosum; FE, F. equiseti; FGl, F. globosum; FGr, F. graminearum; FI, F. incarnatum; FO, F. oxysporum; FP, F. proliferatum; FS, F. solani; FT, F. tricinctum.
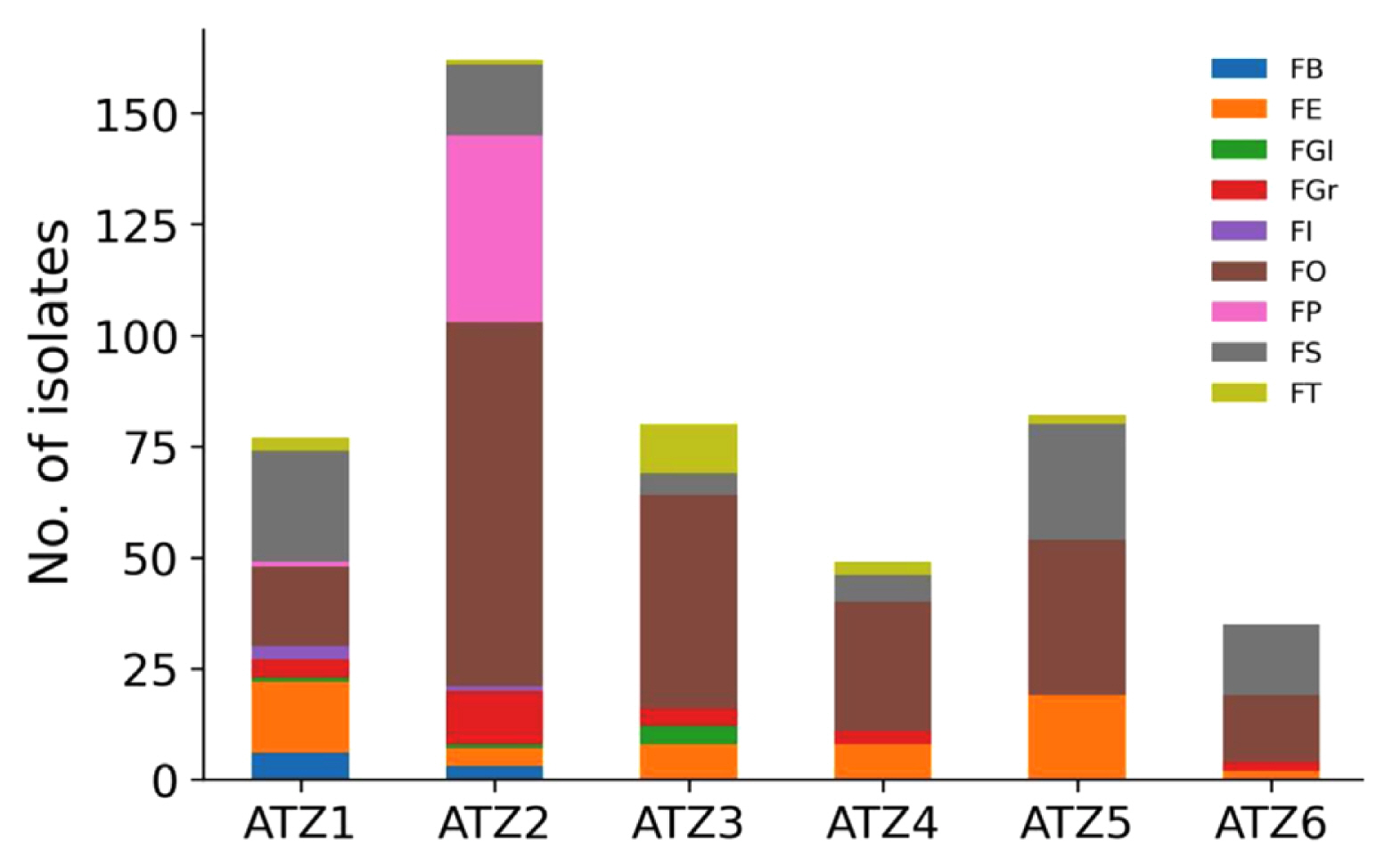
Fig. 3
Phenotype variation in response to infection by different Fusarium species in soybean roots. (A) Typical symptoms of root rot in soybean plant infected with nine different Fusarium species in greenhouse conditions: (a) control, (b) F. solani (FS), (c) F. graminearum (FGr), (d) F. incarnatum (FI), (e) F. equiseti (FE), (f) F. globosum (FGl), (g) F. proliferatum (FP), (h) F. tricinctum (FT), (i) F. brachygibbosum (FB), and (j) F. oxysporum (FO). (B) Disease index scores of soybean plants infected with Fusarium spp. The data shown are presented as mean ± standard deviation. Means with the same letter are not significantly different at a significance level of P = 0.05.

Fig. 4
Effects of Fusarium species on plant growth characteristics under greenhouse conditions. (A) Plant height (cm). (B) Root length (cm). (C) Shoot dry weight (g). (D) Root dry weight (g). NI, not inoculated; FB, F. brachygibbosum; FE, F. equiseti; FGl, F. globosum; FGr, F. graminearum; FI, F. incarnatum; FO, F. oxysporum; FP, F. proliferatum; FS, F. solani; FT, F. tricinctum. The data shown are presented as mean ± standard deviation. Means with the same letter are not significantly different at a significance level of P = 0.05.
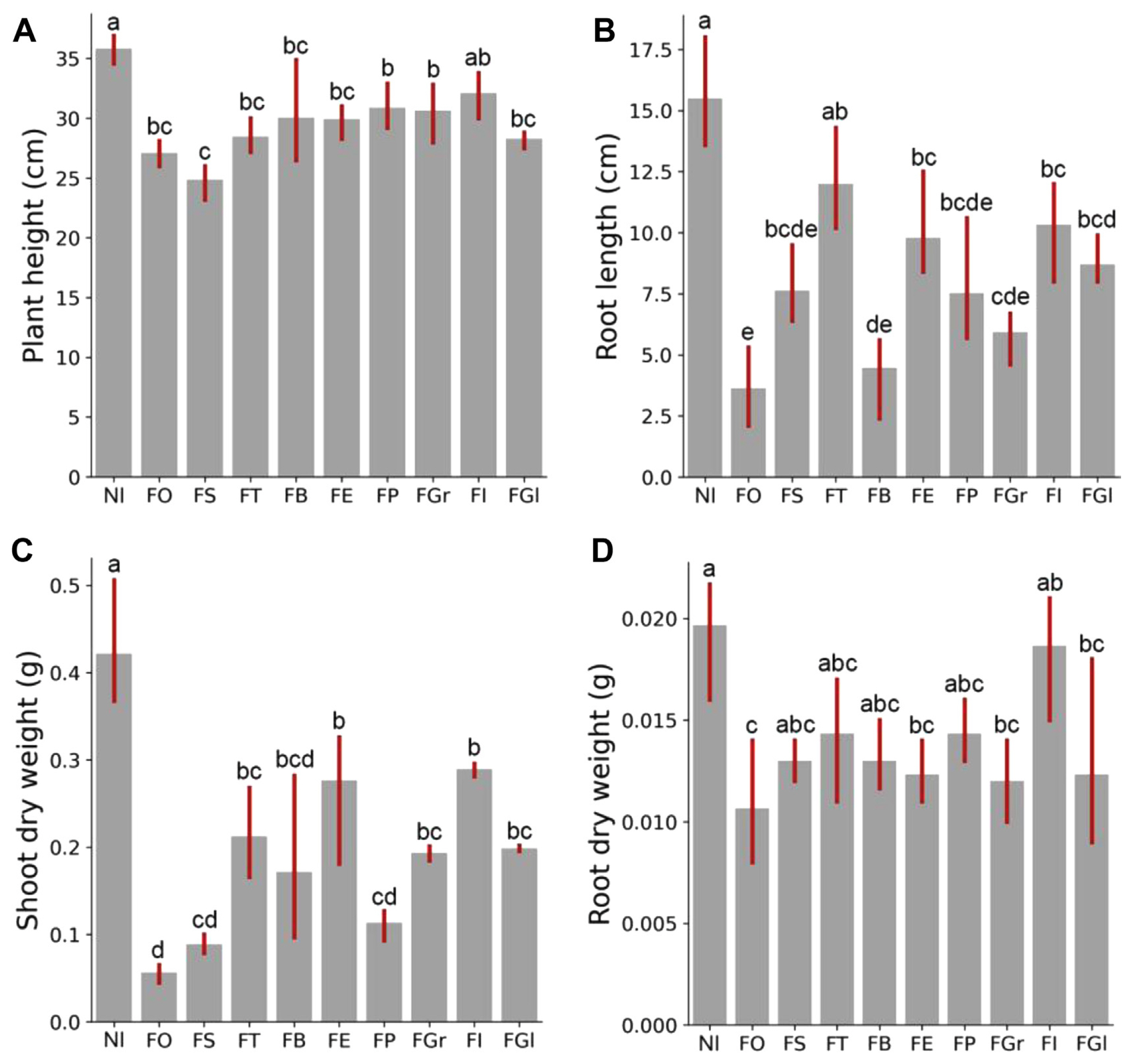
Fig. 5
The severity of root rot in 21 soybean cultivars in responses to Fusarium oxysporum (FO) and F. solani (FS) infections. Means with the same letter are not significantly different at a significance level of P = 0.05. Lowercase letters indicate significant differences among the severity of FO infection, while uppercase letters indicate significant differences among the severity of FS infection. The data shown are presented as mean ± standard deviation.
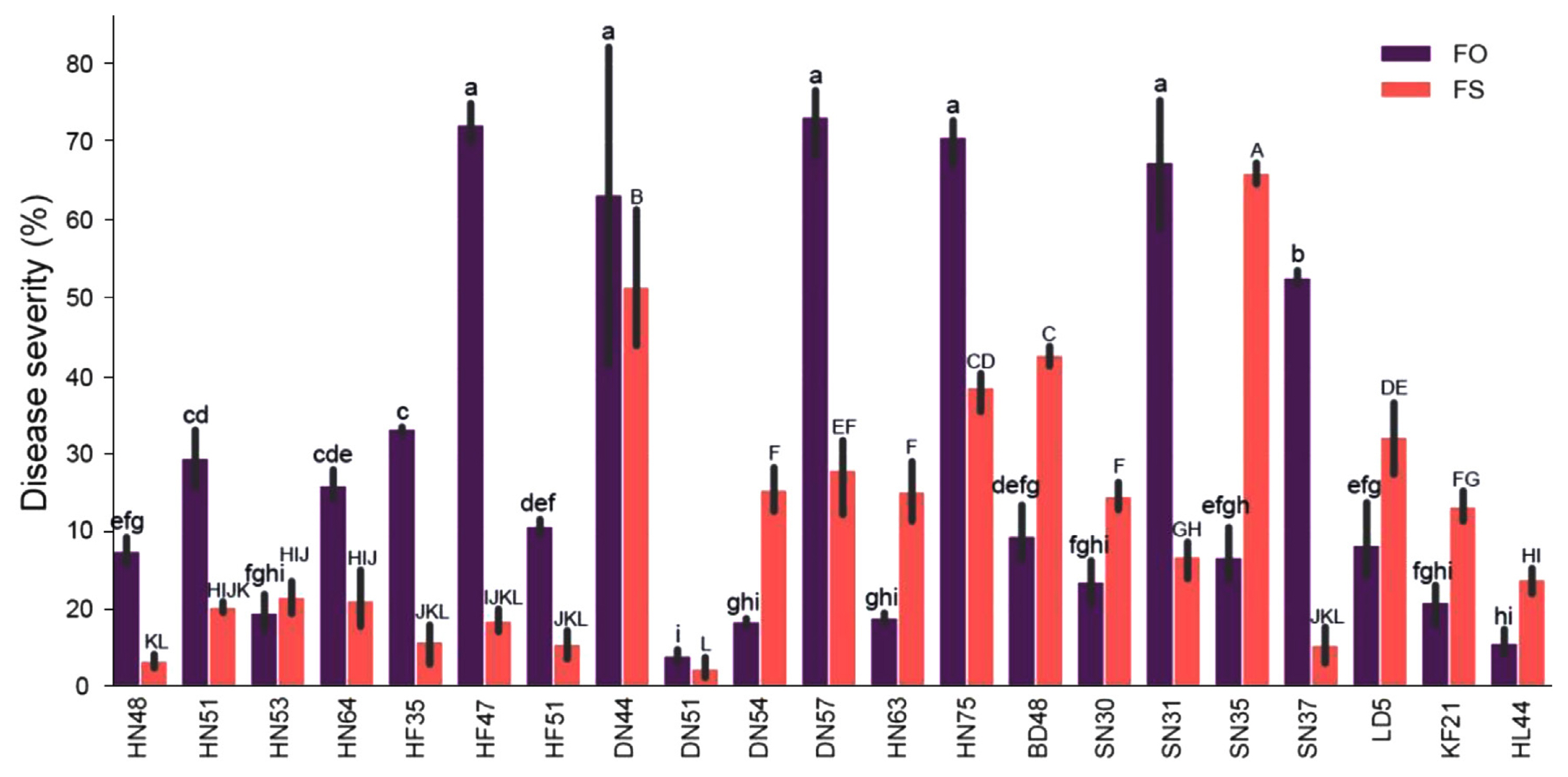
Table 1
RAPD primers used to assess intraspecific genetic diversity among the Fusarium oxysporum isolated from soybean
References
Abbas, A., Mubeen, M., Sohail, M. A., Solanki, M. K., Hussain, B., Nosheen, S., Kashyap, B. K., Zhou, L. and Fang, X. 2022. Root rot a silent alfalfa killer in China: distribution, fungal, and oomycete pathogens, impact of climatic factors and its management. Front. Microbiol. 13:961794.



Arias, M. M. D., Leandro, L. F. and Munkvold, G. P. 2013. Aggressiveness of Fusarium species and impact of root infection on growth and yield of soybeans. Phytopathology 103:822-832.


Ayesha, M. S., Suryanarayanan, T. S., Nataraja, K. N., Prasad, S. R. and Shaanker, R. U. 2021. Seed treatment with systemic fungicides: time for review. Front. Plant Sci. 12:654512.



Brizuela, A. M., Lalak-Kańczugowska, J., Koczyk, G., Stępień, Ł., Kawaliło, M. and Palmero, D. 2021. Geographical origin does not modulate pathogenicity or response to climatic variables of Fusarium oxysporum associated with vascular wilt on asparagus. J. Fungi (Basel) 7:1056.



Cai, H., Tao, N. and Guo, C. 2020. Systematic investigation of the effects of macro-elements and iron on soybean plant response to Fusarium oxysporum infection. Plant Pathol. J. 36:398-405.




Cai, H., Yu, N., Liu, Y., Wei, X. and Guo, C. 2022a. Meta-analysis of fungal plant pathogen Fusarium oxysporum infection-related gene profiles using transcriptome datasets. Front. Microbiol. 13:970477.


Cai, Z., Li, S., Du, G. and Xue, R. 2022b. Linking smallholder farmers to the Heilongjiang Province Crop Rotation Project: assessing the impact on production and well-being. Sustainability 14:38.

Cao, M. M., Li, Q., Zhang, L. Y., Gao, J., Li, W. H., Ding, W. M. and Sun, Y. K. 2014. Accumulated temperature variation and accumulated temperature rezone in Heilongjiang province. Chinese J. Agrometeorol 35:492-496.
Chang, K. F., Hwang, S. F., Conner, R. L., Ahmed, H. U., Zhou, Q., Turnbull, G. D., Strelkov, S. E., McLaren, D. L. and Gossen, B. D. 2015. First report of Fusarium proliferatum causing root rot in soybean (Glycine max L.) in Canada. Crop Prot. 67:52-58.

Cheng, Y. T., Zhang, L. and He, S. Y. 2019. Plant-microbe interactions facing environmental challenge. Cell Host Microbe 26:183-192.



Cruz, D. R., Leandro, L. F. S. and Munkvold, G. P. 2019. Effects of temperature and pH on Fusarium oxysporum and soybean seedling disease. Plant Dis. 103:3234-3243.


Du, Z., Gao, B., Ou, C., Du, Z., Yang, J., Batsaikhan, B., Dorjgotov, B., Yun, W. and Zhu, D. 2021. A quantitative analysis of factors influencing organic matter concentration in the topsoil of black soil in northeast China based on spatial heterogeneous patterns. ISPRS Int. J. Geo-Inf 10:348.

Hafez, M., Abdelmagid, A., Aboukhaddour, R., Adam, L. R. and Daayf, F. 2021. Fusarium root rot complex in soybean: molecular characterization, trichothecene formation, and cross-pathogenicity. Phytopathology 111:2287-2302.


Husaini, A. M., Sakina, A. and Cambay, S. R. 2018. Host-pathogen interaction in Fusarium oxysporum infections: where do we stand? Mol. Plant-Microbe Interact 31:889-898.


Hymowitz, T. and Shurtleff, W. R. 2005. Debunking soybean myths and legends in the historical and popular literature. Crop Sci. 45:473-476.


Kaur, S., Barakat, R., Kaur, J. and Epstein, L. 2022. The effect of temperature on disease severity and growth of Fusarium oxysporum f. sp. apii races 2 and 4 in celery. Phytopathology 112:364-372.


Li, D., He, L., Qu, J. and Xu, X. 2022. Spatial evolution of cultivated land in the Heilongjiang Province in China from 1980 to 2015. Environ. Monit. Assess 194:444.



Lin, F., Chhapekar, S. S., Vieira, C. C., Da Silva, M. P., Rojas, A., Lee, D., Liu, N., Pardo, E. M., Lee, Y.-C., Dong, Z., Pinheiro, J. B., Ploper, L. D., Rupe, J., Chen, P., Wang, D. and Nguyen, H. T. 2022. Breeding for disease resistance in soybean: a global perspective. Theor. Appl. Genet. 135:3773-3872.




Liu, H., Brettell, L. E., Qiu, Z. and Singh, B. K. 2020. Microbiome-mediated stress resistance in plants. Trends Plant Sci. 25:733-743.


Lu, Z.-j., Song, Q., Liu, K.-b., Wu, W.-b., Liu, Y.-x., Xin, R. and Zhang, D.-m. 2017. Rice cultivation changes and its relationships with geographical factors in Heilongjiang Province, China. J. Integr. Agric. 16:2274-2282.

Mei, L., Zhang, N., Wei, Q., Cao, Y., Li, D. and Cui, G. 2022. Alfalfa modified the effects of degraded black soil cultivated land on the soil microbial community. Front. Plant Sci. 13:938187.



Nasr Esfahani, M. 2018. Genetic and virulence variation in Fusarium oxysporum f. sp. cepae causing root and basal rot of common onion in Iran. J. Phytopathol. 166:572-580.


Nikitin, D. A., Ivanova, E. A., Semenov, M. V., Zhelezova, A. D., Ksenofontova, N. A., Tkhakakhova, A. K. and Kholodov, V. A. 2023. Diversity, ecological characteristics and identification of some problematic phytopathogenic Fusarium in soil: a review. Diversity 15:49.

O’Donnell, K., Kistler, H. C., Cigelnik, E. and Ploetz, R. C. 1998. Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. U. S. A. 95:2044-2049.



Poole, G. J., Smiley, R. W., Walker, C., Huggins, D., Rupp, R., Abatzoglou, J., Garland-Campbell, K. and Paulitz, T. C. 2013. Effect of climate on the distribution of Fusarium spp. causing crown rot of wheat in the Pacific Northwest of the United States. Phytopathology 103:1130-1140.


Saleh, A. A., Sharafaddin, A. H., El Komy, M. H., Ibrahim, Y. E. and Hamad, Y. K. 2021. Molecular and physiological characterization of Fusarium strains associated with different diseases in date palm. PLoS ONE 16:e0254170.



Scott, J. C., Gordon, T. R., Shaw, D. V. and Koike, S. T. 2010. Effect of temperature on severity of Fusarium wilt of lettuce caused by Fusarium oxysporum f. sp. lactucae. Plant Dis. 94:13-17.


Sillo, F. 2022. Genetic analysis of plant pathogens natural populations. Methods Mol. Biol. 2536:405-422.


Velásquez, A. C., Castroverde, C. D. M. and He, S. Y. 2018. Plant-pathogen warfare under changing climate conditions. Curr. Biol. 28:R619-R634.



White, T. J., Bruns, T., Lee, S. and Taylor, J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR protocols: a guide to methods and applications, eds. by M. A. Innis, D. H. Gelfand, J. J. Sninsky and T. J. White, pp. 315-322. Academic Press, San Diego, CA, USA.

Yan, H. and Nelson, B. Jr 2022. Effects of soil type, temperature, and moisture on development of Fusarium root rot of soybean by Fusarium solani (FSSC 11) and Fusarium tricinctum. Plant Dis. 106:2974-2983.


- TOOLS
-
METRICS

-
- 1 Crossref
- 0 Scopus
- 1,273 View
- 104 Download
- ORCID iDs
-
Hongsheng Cai

https://orcid.org/0000-0002-9968-8719 - Related articles
-
Incidence of Alternaria Species Associated with Watermelon Leaf Blight in Korea2021 August;37(4)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Supplement1
Supplement1 Print
Print