 |
 |
| Plant Pathol J > Volume 39(1); 2023 > Article |
|
Abstract
Plant pathogenic Pectobacterium species cause severe soft rot/blackleg diseases in many economically important crops worldwide. Pectobacterium utilizes plant cell wall degrading enzymes (PCWDEs) as the main virulence determinants for its pathogenicity. In this study, we screened a random mutant, M29 is a transposon insertion mutation in the metC gene encoding cystathionine β-lyase that catalyzes cystathionine to homocysteine at the penultimate step in methionine biosynthesis. M29 became a methionine auxotroph and resulted in growth defects in methionine-limited conditions. Impaired growth was restored with exogenous methionine or homocysteine rather than cystathionine. The mutant exhibited reduced soft rot symptoms in Chinese cabbages and potato tubers, maintaining activities of PCWDEs and swimming motility. The mutant was unable to proliferate in both Chinese cabbages and potato tubers. The reduced virulence was partially restored by a complemented strain or 100 μM of methionine, whereas it was fully restored by the extremely high concentration (1 mM). Our transcriptomic analysis showed that genes involved in methionine biosynthesis or transporter were downregulated in the mutant. Our results demonstrate that MetC is important for methionine biosynthesis and transporter and influences its virulence through Pcc21 multiplication in plant hosts.
Soft-rot Pectobacteriaceae (SRP), composed of Pecto- bacterium spp. and Dickeya spp., are widespread in agricultural regions around the world as well as in a variety of ecological niches from soils and water to insects (Bell et al., 2004; Liu et al., 2022; Rossmann et al., 2018). These bacteria are necrotrophic phytopathogens that cause wilt, rot, and blackleg diseases in agriculturally important crops, particularly potato, carrot, tomato, onion, pineapple, maize, rice, hyacinth, chrysanthemum, and calla lily with significant losses in crop production (Adeolu et al., 2016; Charkowski, 2018; Charkowski et al., 2012; Perombelon and Kelman, 1980). Pectobacteria were ranked within the Top10 plant pathogenic bacteria, due to their negative impact on potato production in the field and during transportation and storage (Mansfield et al., 2012; Perombelon and Kelman, 1980; Toth et al., 2003). There are three main species, P. atrosepticum, P. carotovorum, and P. wasabiae have been commonly found on potato. Both P. carotovorum subsp. carotovorum (Pcc) and P. brasiliensis (syn. P. carotovorum subsp. brasiliensis, Pcb) are mostly found on potatoes (Duarte et al., 2004; Ma et al., 2007; Malko et al., 2019; Marković et al., 2021; Muturi et al., 2018; Portier et al., 2019; van der Merwe et al., 2010).
The disease severity by SRP depends on environmental conditions, host susceptibility, and abundance of pathogen inoculum (Charkowski, 2007; Eisfeld et al., 2022; Jee et al., 2020). Pectobacteria utilize plant cell wall degrading enzymes (PCWDEs), such as polygalacturonase (Peh), pectate lyase (Pel), cellulose (Cel), xylanase, and protease (Prt) as major pathogenic determinants for successful host infection and encode all six key secretion systems involving in attacking hosts and competing with other bacteria (Bell et al., 2004; Charkowski, 2018; Charkowski et al., 2012; Islam et al., 2019; Kim et al., 2022; Rossmann et al., 2018). During infection, these enzymes break down plant structures, such as pectin, Cel, and hemicellulose, causing plant cell necrosis and tissue maceration (Bell et al., 2004; Charkowski et al., 2012; Rossmann et al., 2018). Pcc uses a cell-to-cell communication system called quorum sensing (QS) to regulate many virulence genes and pathogenesis from the initial infection process (Bellieny-Rabelo et al., 2020; Mole et al., 2007). It has been known that the production of PCWDEs in Pcc is tightly modulated by QS system LuxI/LuxR (ExpI/ExpR in the species) through a signal molecule 3-oxo-C6-HSL and the secretion is mainly through the type II secretion system (Abbott and Boraston, 2008; Bell et al., 2004; Bellieny-Rabelo et al., 2020; Lee et al., 2013; Mole et al., 2007; Torres et al., 2017). In addition to PCWDEs, other virulence determinants and factors contributing to bacterial invasion, colonization, proliferation, and host resistance evasion in plants have been characterized. These include flagellar system (Hossain et al., 2005; Mulholland et al., 1993), siderophores (Bell et al., 2004; Monson et al., 2013), biofilm formation (Yi et al., 2010), exopolysaccharides (Islamov et al., 2021), efflux pumps (Valecillos et al., 2006), the type III secretion system (Holeva et al., 2004), and plant immunity (López-Solanilla et al., 1998). Additionally, the PCWDEs and other virulence determinants are regulated by QS system through a complex set of transcription factors and posttranscriptional regulators including the regulator of secondary metabolism system (Mole et al., 2007). Environmental factors such as water availability, oxygen level, and temperature are also important for infection process and bacterial multiplication (Eisfeld et al., 2022; Jee et al., 2020; Pérombelon, 2002). Recent studies suggest that type VI secretion system (T6SS) functions in bacterial competition and pathogenesis (Bernal et al., 2018; Shyntum et al., 2019; Wang et al., 2018).
Nutrients are essential for the growth of all living organisms. Plant pathogenic bacteria infect host plants to obtain nutrients. Nutrient availability and environmental conditions inside hosts are important for the growth and multiplication of pathogens during infection process (Fatima and Senthil-Kumar, 2015). Necrotrophs, such as Pectobacteria, secrete PCWDEs to break down the components of host cell wall, causing host cell death, which in turn acquire nutrients released from the dead cells (Barras et al., 1994). Many studies have focused on the acquisition strategies and regulatory systems of Pectobacteria (Bell et al., 2004; Charkowski, 2018; Charkowski et al., 2012; Mole et al., 2007; Perombelon and Kelman, 1980; Rossmann et al., 2018). However, there are relatively few studies on nutrients and bacterial metabolic pathways important for pathogenicity and multiplication of Pectobacteria in hosts.
In this study, we performed transposon mutagenesis of P. carotovorum subsp. carotovorum Pcc21 and screened random mutants with altered virulence on Chinese cabbage. A random mutant disrupted in metC gene by Tn5 grew poorly and reduced soft rot symptoms on Chinese cabbage and potato tuber, compared to wild-type. The reduced virulence is independent of well-known virulence determinants, such as PCWDEs, biofilm formation, and motility activities. The metC gene codes for cystathionine β-lyase to catalyze cystathionine to homocysteine in methionine biosynthetic pathway. The mutant became be auxotrophy in methionine-limited conditions, leading to impaired multiplication in hosts. Moreover, our transcriptomic data indicated that genes involved in methionine biosynthesis and transport were downregulated in the mutant. Here, we reported that MetC is important for methionine biosynthesis and acquisition in the host, eventually contributing to multiplication and virulence.
Pcc21 wild-type and its mutant library were obtained from the Rural Development Administration (Korea) (Lee et al., 2013). Pcc21 strains were routinely grown at 28°C with aeration (160 rpm) in Luria-Bertani (LB) medium or M9 basal medium (30 g of Na2HPO4, 15 g of KH2PO4, 10 g of NH4Cl, 3 g of NaCl, 0.1 ml of 1 M CaCl2, 2 ml of 1 M MgSO4 per liter) supplemented with glucose (1.0%). Kanamycin (50 μg/ml) was used for the growth of transposon mutants, and ampicillin (100 μg/ml) and tetracycline (20 μg/ml) were used to maintain the complemented strain and green fluorescence protein (GFP)-harboring strains, respectively.
To investigate genetic factors related to virulence, we evaluated the severity of soft rot symptoms caused by Pectobacterium transposon mutations in Chinese cabbage, compared to Pcc21 wild-type (Lee et al., 2013). Fresh and intact Chinese cabbage was sterilized with 70% ethanol and rinsed twice with autoclaved water. Pcc21 strains were grown in LB liquid medium at 28°C for 16 h, and the cultures were adjusted to OD600 of 0.01. A small scar was made on Chinese cabbage with sterilized forceps, and 3 μl of Pcc21 cultures was inoculated onto the wounded injured section. The samples were placed in sterilized plastic boxes with wet filter paper to keep moisture and stored at 28°C for 72 h.
Transposon insertion of the metC gene was verified according to the manufacturer’s protocol (EZ-Tn5 <R6kcori/KAN-2>Tnp Transposome kit, Epicentre Biotechnologies, Madison, WI, USA). Briefly, a genomic DNA was isolated using an AccuPrep Genomic DNA Extraction kit (Bioneer, Daejeon, Korea), digested with EcoRI, and then self-ligated immediately using a T4 ligase. The self-ligated product was introduced into Escherichia coli DH5α by electroporation using Micropulser (Bio-Rad, Hercules, CA, USA), and kanamycin-resistant transformants were selected. The transposon insertion site was amplified with a primer pair, KAN-2 FP-1 (5′-ACC TAC AAC AAA GCT CTC ATC AAC C-3′) and R6KAN-2 RP-1 (5′-CTA CCC TGT GGA ACA CCT ACA TCT-3′). The polymerase chain reaction (PCR) condition was as follows: 94°C for 10 min, followed by 35 cycles at 94°C for 45 s, 58°C for 45 s, 72°C for 45 s, and 1 final cycle of 72°C at 10 min. The PCR product was purified and sequenced with the primers at Bioneer. The transposon-flanking region was identified via BLAST searches (http://www.ncbi.nlm.nih.gov/) of the sequenced DNA.
The complemented strain of the metC mutant, ΔmetC-CP, was generated using the lac promoter in pBBR1 MCS-4 vector in E. coli Top10 cells (Kovach et al., 1995). The full-length metC gene was amplified with PCR using primers of MetC-CP1 (5′-CGC CTC GAG CCC CGC CCT ACG ATT CCC TCT G-3′) and MetC-CP2 (5′-CGC GAG CTC GTT TTA TGG CCG TCA CAC TTT CTA-3′). The PCR condition was as follows: 94°C for 10 min, followed by 35 cycles at 94°C for 45 s, 60°C for 45 s, 72°C for 45 s, and 1 final cycle at 72°C for 10 min. The PCR product was cloned at XhoI and SacI sites of pBBR1 MCS-4 vector. The constructed plasmid was introduced by electroporation into E. coli Top10. The final construct was transformed into ΔmetC strain from E. coli Top10 by a tri-parental conjugation method as described previously (Lee et al., 2008). GFP-harboring Pcc21 wild-type (Pcc21-gfp) and ΔmetC (ΔmetC-gfp) were constructed using a pRK415-gfp vector (Lee et al., 2011) for use in visualization of Pcc21 colonization on plants. The vector was then conjugated into Pcc21 strains as described above.
The growth of Pcc21 wild-type, ΔmetC, and ΔmetC-CP was evaluated in four different conditions (M9, M9 with 100 μM cystathionine, M9 with 100 μM homocysteine and M9 with 100 μM methionine) (Jochim et al., 2019; Lee et al., 2020). Overnight-grown bacterial cells in LB were harvested and washed twice with M9 basal medium to remove residual LB medium. 1.0 × 107 cfu (colony-forming-unit)/ml of bacterial cells was inoculated into the media, and bacterial multiplication was determined by measuring absorbance of 600 nm for 18 h at 1-hour intervals using an Epoch 2 Microplate Spectrophotometer (BioTek, Winooski, VT, USA). Six biological replicates for each strain were used. To address whether ΔmetC restored the growth-defective phenotype by exogenous methionine, the growth of Pcc21 strains was also measured in M9 medium with different methionine concentrations (15, 30, 50, and 100 μM) (Jochim et al., 2019).
To assess the ability to attach to abiotic surfaces, Pcc21 strains were inoculated with 40-fold dilution in fresh LB media overnight. Two hundred μl of cultures were incubated in 96-well polystyrene plates at 28°C for 18 h in the static state. The cultures were removed, and the wells were gently rinsed twice with deionized water. Biofilm cells were stained with 0.1% of crystal violet solution for 10 min. The wells were washed twice with deionized water and eluted with 200 μl of 95% EtOH. The amount of biofilm was determined by measuring the absorbance at 540 nm using an Epoch2 microplate reader (BioTek) (Lee et al., 2009). Swimming motility assay was performed as previously described (Lee et al., 2008). Three μl of Pcc21 strains grown at 28°C in LB was inoculated on semi-solid LB agar (0.3% Bacto agar) by stabbing, followed by incubation with the lid side up at 28°C for 14 h.
To evaluate the role of MetC in the virulence activity of Pcc21, we performed the pathogenicity test using Chinese cabbage (Lee et al., 2013) and potato tuber (Torres et al., 2017) as described above. To evaluate the bacterial multiplication on Chinese cabbages, the lesion was cut into 1 × 1 cm2 size and resuspended in 10 ml of M9 basal medium at designated time points. The suspension (100 μl) was spread onto LB agar plates with rifampicin (50 μg/ml) or kanamycin (50 μg/ml) for Pcc21 wild-type or ΔmetC, respectively. After 2 days of incubation at 28°C, bacterial multiplication was determined by counting cfu. All experiments were performed three times.
Extracellular enzymatic assays were performed according to the previous study with a slight modification (Lee et al., 2013, 2014). After Pcc21 strains were grown overnight at 28°C in LB medium, cell-free supernatants were obtained by centrifugation at 13,500 rpm for 10 min and sterilized by 0.22-μm-pore-size filter. The supernatant (3 μl) was spotted onto each assay plate. The PCWDEs assay media were prepared as follows: 1% skim milk, 0.1% yeast extract, and 0.8% agarose for Prt assay; 0.5% carboxymethyl cellulose, 0.1% yeast extract, 0.3 mM calcium chloride, 2 mM magnesium sulfate, and 0.8% agarose for Cel assay; 1% polygalacturonic acid, 1% yeast extract, 2.5 mM EDTA, 50 mM sodium acetate, 0.2% sodium azide, and 0.8% agarose for Peh assay; 1% polygalacturonic acid, 1% yeast extract, 0.38 mM calcium chloride, 100 mM Tris/HCl (pH 8.8), and 0.8% agarose for Pel assay. The assay was fulfilled for 16 to 18 h. Pel and Peh assay media were developed with 4 N HCl, and clear zones were measured. Cel assay plates were developed with 0.1% Congo red for 5 min and washed several times with 1 M NaCl until clear zones were observed. Prt assay plates were assessed without any further treatment after incubation for 36 h.
For RNA-seq analyses, Pcc21 strains grown overnight in LB were inoculated with a 100-fold dilution in M9 minimal medium containing 50 μM methionine and incubated at 28°C for 24 h. Bacterial cells were harvested, washed twice with fresh M9 basal medium, and diluted to 1 × 108 cfu/ml in 20 ml of M9 basal medium. Four Chinese cabbage slices having a size of 1 × 1 cm2 were transferred into the culture, and the cultures were incubated at 28°C for 4 h with gentle shaking (80 rpm). The cultures were then gently vortexed to detach bacterial cells from the slices. The slices were removed with sterilized forceps, and the cells were collected by centrifugation at 4,000 rpm for 10 min at 4°C. The pellet was resuspended in 1 ml of RNAlater (Invitrogen, Carlsbad, CA, USA), centrifuged at 13,500 rpm for 5 min at 4°C, and stored at −80°C until further analysis.
Total RNA was isolated using a Bacterial RNA extraction kit (Bioneer), according to the manufacturer’s instructions. RNA samples were treated with RNase-free Dnase I (Qiagen, Valencia, CA, USA) to remove DNA contamination. RNA quality and quantity were measured with an Epoch 2 Microplate Spectrophotometer (BioTek), and all samples were immediately frozen in liquid nitrogen and stored at −80°C before analyses. After rRNA was removed by a Ribo-Zero rRNA removal kit (Qiagen), mRNA sequencing using the Illumina NovaSeq 6000 system at Bioneer. Metabolic mapping of sequencing reads to all coding sequences (CDSs) was performed quantitatively using the Burrows-Wheeler Alignment tool (Li and Durbin, 2009). The level of relative gene expression was analyzed as RPKM values (Read numbers per kilobase of each CDS, per million mapped reads) of all mapped sequencing reads (Chun et al., 2019). The false discovery rate (FDR) ≤ 0.05 and the absolute value of log2 fold change > 1 were used as the threshold to determine the statistically significant differences in gene expression (Liu et al., 2020). Gene transcriptional profiles were graphically rendered by GraphPad Prism (https://www.graphpad.com/scientific-software/prism/).
All experiments described above were performed at least three times independently with three to six replicates. Error bars represented standard error of mean. Statistical analysis was performed using GraphPad Prism 9 (GraphPad Software, San Diego, CA, USA). Independent samples t-test and one-way ANOVA were used to analyze statistical significance (P < 0.05).
We screened random mutants with altered pathogenicity from a transposon mutant library of Pcc21 to investigate virulence determinants. Among 5,184 transposon mutants, 13 mutants were selected with 10 mutants (M2, M6, M9, M13, M26, M27, M29, M33, M43, and M44) and 3 mutants (M4, M15, and M23) of reduced and increased soft rot symptoms on Chinese cabbage, respectively, compared to wild-type. We further characterized that M29 carried a Tn5 transposon insertion in the metC gene encoding cystathionine β-lyase (PCC21_RS01820, at 528-bp of 1,191-bp ORF), known to catalyze cystathionine to homocysteine in methionine biosynthetic pathway in bacteria (Supplementary Fig. 1) (Andersen et al., 1998; Weissbach and Brot, 1991).
To identify the methionine biosynthetic pathway in Pcc21, all functionally annotated genes in its genome (GenBank accession no. NC_018525.1) (Park et al., 2012) were cumulatively mapped to the Kyoto Encyclopedia of Genes and Genomes (KEGG) database (http://www.genome.jp/keeg/). The result showed that the methionine biosynthetic pathway of Pcc21 is highly similar to those of E. coli (Weissbach and Brot, 1991) and Salmonella (Ejim et al., 2004). As shown in Fig. 1A, Pcc21 has a conserved methionine biosynthetic pathway to catalyze cystathionine to homocysteine at the penultimate step via MetC (Andersen et al., 1998; Ferla and Patrick, 2014; Weissbach and Brot, 1991). Inactivation of genes involved in the biosynthetic pathways becomes auxotrophic under methionine depletion conditions (Husna et al., 2018; Jochim et al., 2019; Taté et al., 1999). Therefore, we functionally verified the mutant in the metC gene disrupted by Tn5 by measuring growth in methionine-limited M9 minimal medium. As expected, the metC mutant grew poorly in the limited condition, while the growth was similar to that of Pcc21 wild-type in LB medium which is a nutritionally rich medium (Fig. 1B and C). Impaired growth in minimal media was restored by exogenous supply of homocysteine and methionine but not cystathionine, demonstrating a methionine auxotroph phenotype (Fig. 1C). Moreover, the complemented strain harboring a full-length metC gene (ΔmetC-CP) was able to grow as wild-type in the methionine-limited condition without any amino acids (Fig. 1D). Thus, we reckoned that metC mutant compromised methionine biosynthesis and bacterial growth.
To investigate the roles of MetC on multiplication and virulence of Pcc21, we monitored sort rot disease symptom in Chinese cabbage and potato tuber slices by Pcc21. Pcc21 strains carrying a pRK415-gfp with the constitutively expressed gfp gene were used for visualization of bacterial multiplication (Lee et al., 2011). Pcc21 wild-type (Pcc21-gfp) apparently caused soft rot symptom on both host plants 24 h of post-inoculation (hpi) (Fig. 2A and B Top). However, the symptom was significantly decreased in the plants infected with ΔmetC (ΔmetC-gfp) (Fig. 2A and B bottom). Consistent with soft rot symptom, the amount of GFP expression and viable cells of ΔmetC was lower than Pcc21 wild-type at the symptomatic area in cabbages (Fig. 2A). Reduced symptom by ΔmetC might be due to its reduced multiplication in Chinese cabbage and potato tuber slices (Fig. 2C). Thus, we reckoned that MetC contributes to Pcc21 mediated soft rot disease development.
We then monitored virulence activities of Pcc21 dependent on PCWDEs (Peh, pectinase, cellulase, xylanase, and Prt), flagellar motility, and biofilm formation (Bell et al., 2004; Charkowski, 2018; Charkowski et al., 2012; Hossain et al., 2005; Rossmann et al., 2018; Yi et al., 2010). Notably, no differences of PCWDEs activity and swimming motility were observed between Pcc21 wild-type and ΔmetC (Fig. 3A). Interestingly, unlike the previously known auxotrophs (Jochim et al., 2019), the biofilm formation was rather increased in ΔmetC, compared to Pcc21 wild-type (Fig. 3B). The increased biofilm formation was restored with methionine (100 μM) to the level of Pcc21 wild-type (Fig. 3B). Together, our results indicated that ΔmetC reduced Pcc21 virulence independent of well-known virulence determinants such as PCWDEs, bacterial motility, and biofilm formation.
In other bacteria, methionine auxotrophic mutants (metA, metB, metC, metX, metY, or metI) exhibited growth defects in methionine-restricted conditions and required for high amounts of free methionine to overcome the auxotrophy (Jochim et al., 2019). Similarly, the impaired growth of metC mutant was restored in a dose-dependent manner (Fig. 4A). The growth was partially restored with 15 to 30 μM methionine, higher amount of methionine (50 to 100 μM) facilitated the growth of metC mutant strain similar to Pcc21 wild-type. Higher concentrations above 50 μM methionine did not affect the growth of Pcc21 (Fig. 4A).
Based on this observation, we addressed whether reduced virulence of ΔmetC could be caused by the multiplication defect in the host environment. We examined disease symptoms caused by Pcc21 strains with high concentration of methionine in potato tubers. Contrary to the growth pattern in methionine-limited medium (Fig. 4A), the disease severity by ΔmetC was partially restored by the addition of 100 μM of exogenous methionine or by the complemented strain (ΔmetC-CP) (Fig. 4B and C). More importantly, a higher concentration of exogenous methionine (1 mM) was sufficient to restore the disease severity of soft rot region size as Pcc21 wild-type. This strongly suggested that the methionine biosynthesis in Pcc21 is essential for bacterial multiplication in vitro and in planta to lead successful disease development.
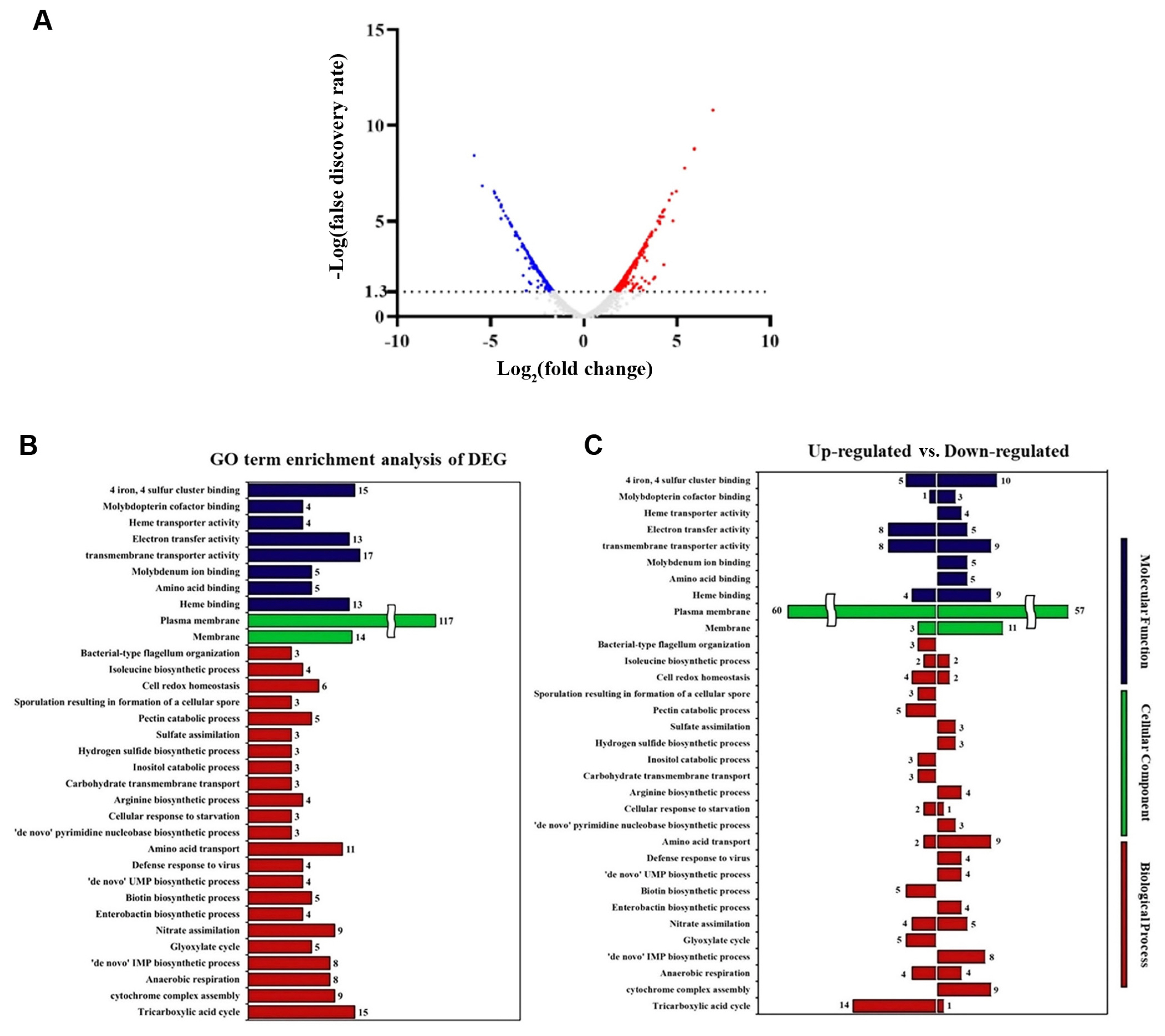
Our results demonstrated that ΔmetC mutant did not affect well-known virulence activities of Pcc21 with less multiplication in planta, leading to reduced soft-rot disease symptom in host plants (Figs. 1,-4). This led us to investigate putative virulence factors dependent on methionine biosynthesis of Pcc21. To predict the biological and cellular processes affected by MetC, we performed a whole transcriptomic analysis with the gene ontology classification of Pcc21 wild-type and ΔmetC. To identify genes differentially expressed in ΔmetC at the early infection stage, 1 × 108 cfu/ml of Pcc21 strains was inoculated into M9 basal medium containing Chinese cabbage slices, and we collected samples to perform transcriptomic profiling when early soft rot symptoms were observed in the plants inoculated with wild-type Pcc21 (4 hpi).
Total 4,460 genes were detected, and about 10.4% of genes (464) were differentially expressed (log2-fold changed ≥ 1.0, FDR < 0.05) at 4 hpi. Among them, 266 and 199 differentially expressed genes were upregulated and downregulated in ΔmetC, respectively, compared to Pcc21 wild-type (Fig. 5, Supplementary Table 1). As shown in Supplementary Table 2, genes involved in the methionine biosynthesis (metX, metB, metE, and metF) as well as transporter system (metL and metN) were downregulated in ΔmetC. Bacteria possess two types of methionine transporter systems: the methionine ABC uptake transporter (MUT) family and a secondary transporter termed BcaP (den Hengst et al., 2006; Hullo et al., 2004; Merlin et al., 2002). In E. coli, the MUT system consists of MetQ (substrate binding protein), MetL (transmembrane permease), and MetN (cytoplasmic ATP-hydrolyzing protein, ATPase) (Merlin et al., 2002). Both metL (D5081_01345) and metN (D5081_04150) genes responsible for the MUT system were downregulated in ΔmetC, indicating that disruption of metC leads to defects in biosynthesis and acquisition of methionine, supporting our results shown above. As we observed no phenotypic differences in virulence activity between Pcc21 wild-type and ΔmetC, we could not discern significant differences in gene expression for PCWDEs and flagellar-dependent motility (Supplementary Table 2).
In this study, we screened Tn-5 mutant library and identified Pcc21 ΔmetC that exhibited methionine auxotrophic phenotype with growth defect in the absence of methionine. Pcc21 ΔmetC mutant compromised soft-rot disease symptom and development in Chinese cabbage and potato tuber. The soft-rot disease was reestablished by exogenous treatment of high concentration of methionine. Transcriptomic analysis revealed that key genes involved in Met biosynthesis pathway were mainly downregulated in ΔmetC mutant consistent with methionine auxotrophic phenotype.
In pathogenic bacteria, the efficient nutrient uptake from host is essential for the multiplication and successful infection. Methionine, a proteinogenic amino acid, is necessary for all organisms and also acts as a component of the cofactor, S-adenosyl methionine (Ferla and Patrick, 2014). Most bacteria possess conserved methionine biosynthetic pathways well characterized (Ferla and Patrick, 2014). Methionine has been known to interaction with hosts in many bacteria (Basavanna et al., 2013; Coplin et al., 1974; Ejim et al., 2004; Jochim et al., 2019). A recent study showed that methionine auxotrophic mutants in Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli were defective in multiplication and virulence in human serum and had reduced biofilm formation and maintenance (Jochim et al., 2019). In P. atrosepticum, the metJ gene is a repressor of the methionine biosynthesis regulon, and many genes involved in both methionine biosynthesis and virulence were upregulated in the mutant (Cubitt et al., 2013). The report addressed that the modulatory mechanisms of methionine biosynthesis may be linked to pathogenicity in Pectobacteria. Based on our results, we assume that methionine biosynthesis in Pectobacteria may closely link to bacterial virulence activities.
We observed that Pcc21 ΔmetC mutant retained well-known virulence activities such as PCWDEs production and flagella-dependent motility (Fig. 3), which is demonstrated in RNA-seq analysis as well (Fig. 5, Supplementary Table 2). Therefore, a simple conclusion that can explain the reduced soft-rot disease symptoms in Chinese cabbage and potato tuber is due to multiplication defect in ΔmetC mutant. Methionine is a proteinogenic amino acid well known for its roles in the initiation elongation stages of translation and is essential in all organisms. In addition, methionine in the form of S-adenosyl-L-methionine acts as a methyl donor in several key biochemical reactions, such as DNA methylation, protein methylation, and polyamine biosynthesis. Thus, its limitation is expected to have a broad impact on bacterial physiology (Ferla and Patrick, 2014). The ability to synthesize methionine and uptake the host resources is essential for the multiplication of bacterial pathogens inside the host to successfully interact with and infect the host (Basavanna et al., 2013; Cubitt et al., 2013; Jochim et al., 2019; Plener et al., 2012). Methionine is a less abundant amino acid in prokaryotes. For example, the intracellular amount of free methionine in glucose-fed E. coli was estimated to be 1.5 × 10−4 mol/l (Bennett et al., 2009). Disruption of the metC gene became to be methionine auxotrophic which was not able to grow in methionine-limited media, and the auxotrophic phenotype was fully restored by exogenous methionine at concentrations above 50 μM or in the complemented strain carrying the full-length metC gene (Fig. 2). In contrast, the reduced virulence of ΔmetC was partially restored with 100 μM methionine and the complemented strain (Fig. 4). A 10-fold higher concentration (1 mM) of exogenous methionine was required for full recovery. Like our results (Fig. 1), methionine auxotrophic mutants in human pathogens exhibited several phenotypic defects of multiplication, virulence, biofilm formation and maintenance, and application of exogenous methionine more than concentrations reported in human serum allowed to overcome the auxotroph phenotype (Jochim et al., 2019). In addition, our transcriptomic data indicated that genes involved in the methionine biosynthesis and ABC uptake transporter (MUT) family (metL and metN) were downregulated in ΔmetC (Supplementary Table 2). These observations suggest that impaired methionine biosynthesis is difficult to completely recover by free methionine available in host environments. Pcc21 ΔmetC mutant showed normal growth in LB media (Fig. 1B), suggesting that the auxotrophic phenotype can be complemented in this growth condition. The soft-rot disease symptom and bacterial multiplication in Chinese cabbage infected with Pcc21 ΔmetC were like wild-type 24 h post-infection, and only wild-type strain could successfully grow and develop disease symptoms from 36 h after infection. This difference is relatively less than the auxotrophic phenotype confirmed in vitro (Fig. 1), suggesting that Pcc21 ΔmetC mutant can colonize in the host plant by utilizing methionine in the plant cells at the early stage of infection. However, the concentration of methionine required for multiplication and virulence of pathogens may be limited, which results in reduced colonization and disease symptoms by Pcc ΔmetC mutant.
The amounts and types of nutrients are known to influence the development of biofilms (Bowden and Li, 1997). A previous study revealed that sufficient nutrient concentrations increased the rate and extent of biofilm formation, but at higher concentrations, biofilm formation was decreased due to increased detachment shown in Pseudomonas putida (Rochex and Lebeault, 2007). Thus, the nutrient-rich LB media may have masked the potential effect of MetC on biofilm formation, and the role of MetC in Pcc21 biofilm formation is still elusive to be examined further. In our results, the biofilm formation was not responsible for the reduced virulence of ΔmetC due to the increased amount in ΔmetC (Fig. 4B). We speculate that ΔmetC may be involved in the production of metabolites secreted to the biofilm even though further investigation is needed.
The genes involved in the type II secretion systems contributing to PCWDEs secretion and flagellar motility (Bell et al., 2004; Charkowski et al., 2012), were not affected dramatically in ΔmetC mutant (Supplementary Table 2). This is consistent with our observation of PCWDEs and motility phenotype of ΔmetC (Fig. 3). Interestingly, the T6SS-related genes were downregulated in ΔmetC. The T6SS functions commonly in Gram-negative bacteria to modulate the interaction with other organisms such as animals, plants, and mainly other bacteria for symbiosis, adherence, and/or competition with other microorganisms (Ryu, 2015). In P. atrosepticum, T6SS cluster genes and its effectors, hcp and vgrG, were upregulated in the presence of potato tuber extract, and a mutation in the hcp genes led to a slight reduction in virulence (Mattinen et al., 2007, 2008). The previous study indicated that T6SS and its effectors were functionally associated during the interaction with host plant. Our transcriptomic data showed that hcp (D5081_00825 and D5081_10915) and vgrG homologous genes (D5081_10660) were downregulated in ΔmetC, like the T6SS genes. Therefore, we speculate that the reduction of T6SS and its effectors may lead to reduced virulence in ΔmetC, but it remains to be elucidated.
Taken together, our study represented that the inactivation of the metC gene resulted in the typical methionine auxotrophic phenotype, impaired multiplication in the methionine-limited condition, and reduced virulence on Chinese cabbage and potato tuber, proposing that MetC enzymatically could involve in the methionine biosynthesis and methionine uptake during the infectious process. When the methionine biosynthesis pathway is compromised, it is difficult to recover even with methionine available in the environment. Therefore, our study suggests the importance of the requirement of micronutrient for Pectobacterium to infect and colonize in host plants.
Acknowledgments
We are very grateful to Kyung Hye Lee and Hee Yeon Kim for their technical support and helpful comments. This research was supported by Basic Science Research Program through the National Research Foundation of Korea (Y.L: 2018R1D1A1A02085563) funded by the Ministry of Education, Republic of Korea, Korea University Grant (E.-H.C: K2021521 and K2106871), and the BK21 FOUR program (E.-H.C: 4299991014324).
Electronic Supplementary Material
Supplementary materials are available at The Plant Pathology Journal website (http://www.ppjonline.org/).
Fig. 1
Methionine auxotrophy of ΔmetC in Pcc21. (A) Predicted methionine biosynthetic pathway of Pcc21 based on genome mapped Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway. MetA, homoserine o-acetyltransferase; MetB, cystathionine γ-synthase; MetC, cystathionine β-lyase; MetE, cobalamin-independent methionine synthase; MetH, cobalamin-dependent methionine synthase. Growth of ΔmetC in Luria-Bertani medium (B) or in a methionine-limited M9 minimal medium (C) with three different sources (100 μM); cystathionine, homocysteine, or methionine. (D) Growth of ΔmetC with exogenous methionine (100 μM) or in the complemented strain for 18 h in methionine-limited M9. The means ± standard deviations are displayed. Results were representative of three independent experiments with six replicates per group. ***P < 0.001.

Fig. 2
Disease and multiplication of ΔmetC on Chinese cabbage and potato tuber. Development of soft rot symptoms (upper panel) and bacterial multiplication of Pcc21 strains harboring green fluorescence protein on Chinese cabbage (A) and potato tuber (B). (C) Bacterial multiplication on Chinese cabbage. The means ± standard deviations are displayed. Results were representative of three independent experiments with four replicates per group. *P < 0.05, **P < 0.005.
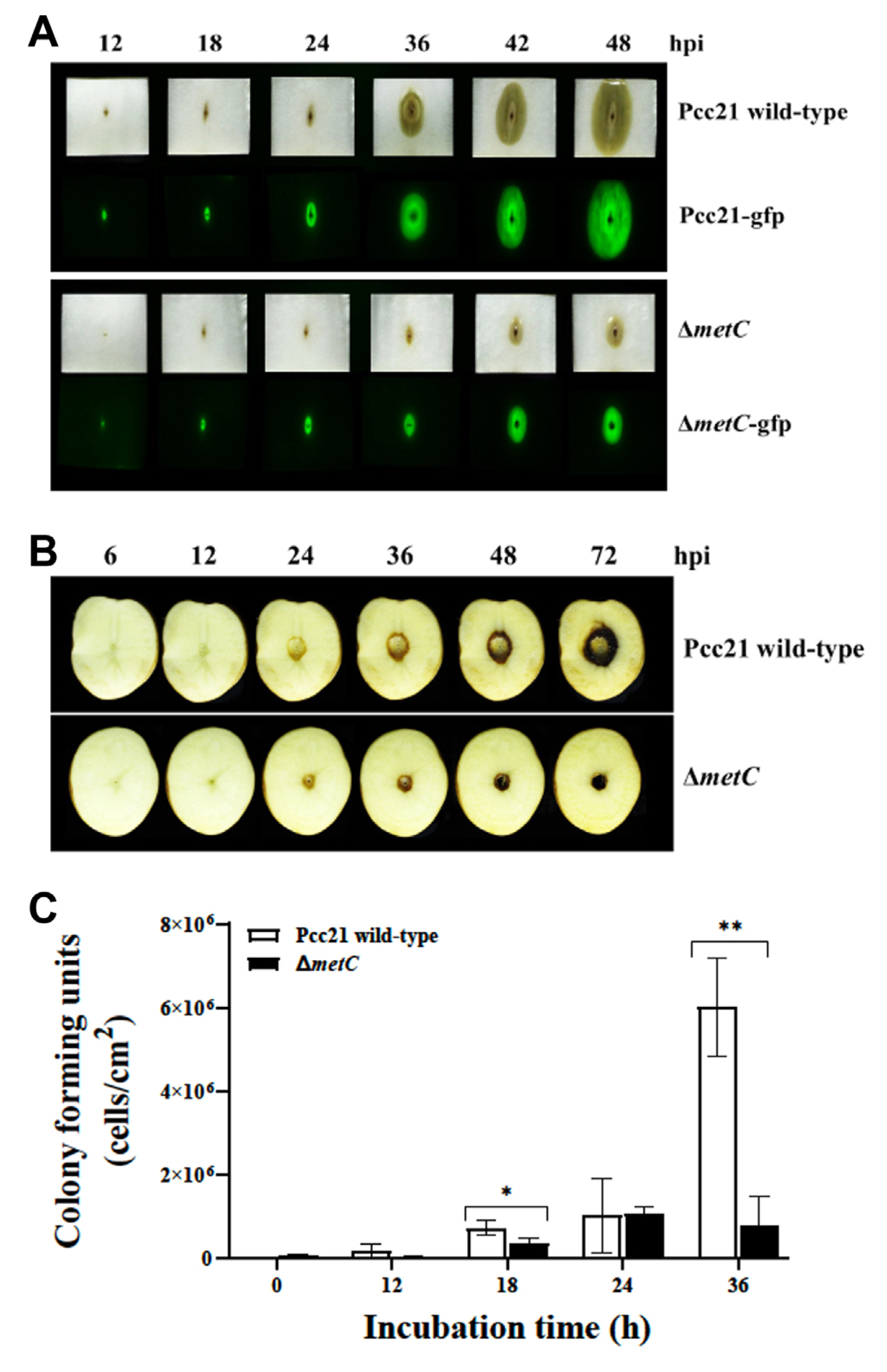
Fig. 3
Activities of plant cell wall degrading enzymes (PCWDEs), flagellar motility (A), and biofilm formation (B) of ΔmetC. The supernatant of Pcc21 strains was spotted onto Prt (protease), Pel (pectate lyase), Cel (cellulase), and Peh (polygalacturonase) assay media and developed at 28°C. Swimming motility was determined at 28°C on semi-solid Luria-Bertani (LB) agar plates (0.3% Bacto agar) at 14 h. These photographs are representative of five independent experiments. Biofilm cells were stained with 1.0% of crystal violet and observed at 540 nm at 18 h of inoculation in LB medium. The means ± standard deviations are displayed. Results are representative of three independent experiments with six replicates per group. *P < 0.05, **P < 0.005, ***P < 0.001.
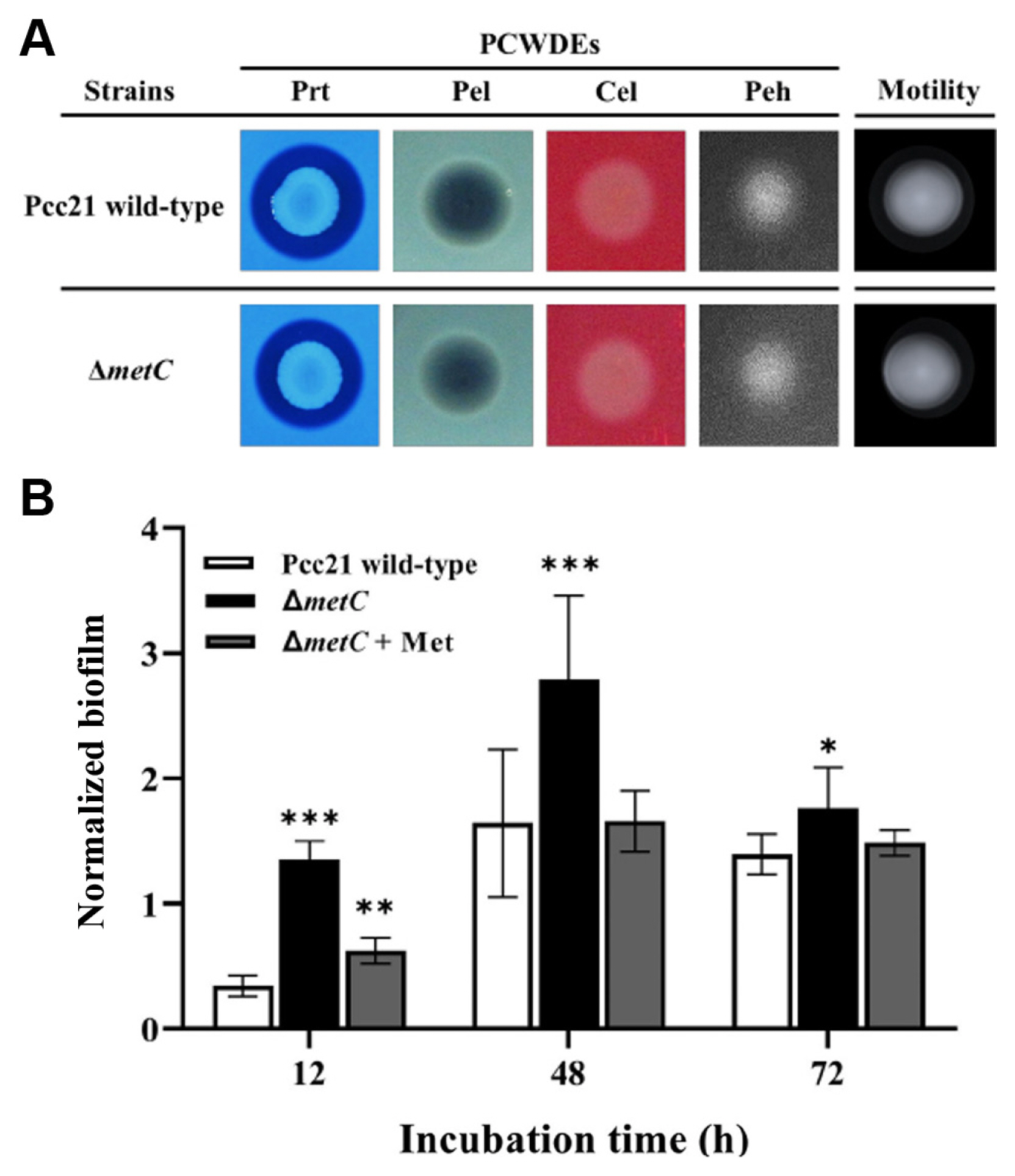
Fig. 4
Requirement of exogenous methionine for ΔmetC growth in vitro and in planta. Growth of ΔmetC in a methionine-limited M9 minimal medium with different concentrations of exogenous methionine (A). The means ± standard deviations are displayed. Results are representative of three independent experiments with four replicates per group. *P < 0.05, ***P < 0.001. Visualization (B) and rotting area (C) of the symptoms on potato tuber. The rotting region was analyzed by ImageJ program (version 1.52a). These photographs were representative of three independent experiments. The mean ± standard deviations were displayed. Results are representative of three independent experiments with four replicates per group. ***P < 0.001; in comparison to Pcc21 wild-type, #P < 0.05; ##P < 0.005; ###P < 0.001; in comparison to ΔmetC.

Fig. 5
Comparative transcriptomic analysis of ΔmetC and Pcc21 wild-type infecting Chinese cabbage at the early infection stage. (A) Volcano plot of the transcriptome of ΔmetC, compared to Pcc21 wild-type. Statistical significance (-log10 of P-value; y-axis) was plotted with log2-fold change (x-axis). The gene ontology (GO) classification (B) and the number of significantly differentially expressed genes (DEGs) (C) (ratio of log2-fold ≥ 1).
References
Abbott, D. W. and Boraston, A. B. 2008. Structural biology of pectin degradation by Enterobacteriaceae
. Microbiol. Mol. Biol. Rev 72:301-316.




Adeolu, M., Alnajar, S., Naushad, S. and Gupta, R. S. 2016. Genome-based phylogeny and taxonomy of the ‘Enterobacteriales’: proposal for Enterobacterales ord. nov. divided into the families Enterobacteriaceae, Erwiniaceae fam. nov., Pectobacteriaceae fam. nov., Yersiniaceae fam. nov., Hafniaceae fam. nov., Morganellaceae fam. nov., and Budviciaceae fam. nov. Int. J. Syst. Evol. Microbiol 66:5575-5599.


Andersen, G. L., Beattie, G. A. and Lindow, S. E. 1998. Molecular characterization and sequence of a methionine biosynthetic locus from Pseudomonas syringae
. J. Bacteriol 180:4497-4507.




Barras, F., van Gijsegem, F. and Chatterjee, A. K. 1994. Extracellular enzymes and pathogenesis of soft-rot Erwinia
. Annu. Rev. Phytopathol 32:201-234.

Basavanna, S., Chimalapati, S., Maqbool, A., Rubbo, B., Yuste, J., Wilson, R. J., Hosie, A., Ogunniyi, A. D., Paton, J. C., Thomas, G. and Brown, J. S. 2013. The effects of methionine acquisition and synthesis on Streptococcus pneumoniae growth and virulence. PLoS ONE 8:e49638.



Bell, K. S., Sebaihia, M., Pritchard, L., Holden, M. T. G., Hyman, L. J., Holeva, M. C., Thomson, N. R., Bentley, S. D., Churcher, L. J. C., Mungall, K., Atkin, R., Bason, N., Brooks, K., Chillingworth, T., Clark, K., Doggett, J., Fraser, A., Hance, Z., Hauser, H., Jagels, K., Moule, S., Norbertczak, H., Ormond, D., Price, C., Quail, M. A., Sanders, M., Walker, D., Whitehead, S., Salmond, G. P. C., Birch, P. R. J., Parkhill, J. and Toth, I. K. 2004. Genome sequence of the enterobacterial phytopathogen Erwinia carotovora subsp. atroseptica and characterization of virulence factors. Proc. Natl. Acad. Sci. U. S. A 101:11105-11110.



Bellieny-Rabelo, D., Nkomo, N. P., Shyntum, D. Y. and Moleleki, L. N. 2020. Horizontally acquired quorum-sensing regulators recruited by the PhoP regulatory network expand the host adaptation repertoire in the phytopathogen Pectobacterium brasiliense
. mSystems 5:e00650-19.




Bennett, B. D., Kimball, E. H., Gao, M., Osterhout, R., Van Dien, S. J. and Rabinowitz, J. D. 2009. Absolute metabolite concentrations and implied enzyme active site occupancy in Escherichia coli
. Nat. Chem. Biol 5:593-599.




Bernal, P., Llamas, M. A. and Filloux, A. 2018. Type VI secretion systems in plant-associated bacteria. Environ. Microbiol 20:1-15.



Bowden, G. and Li, Y. H. 1997. Nutritional influences on biofilm development. Adv. Dent. Res 11:81-99.



Charkowski, A., Blanco, C., Condemine, G., Expert, D., Franza, T., Hayes, C., Hugouvieux-Cotte-Pattat, N., López Solanilla, E., Low, D., Moleleki, L., Pirhonen, M., Pitman, A., Perna, N., Reverchon, S., Rodríguez Palenzuela, P., San Francisco, M., Toth, I., Tsuyumu, S., van der Waals, J., van der Wolf, J., van Gijsegem, F., Yang, C.-H. and Yedidia, I. 2012. The role of secretion systems and small molecules in soft-rot Enterobacteriaceae pathogenicity. Annu. Rev. Phytopathol 50:425-449.


Charkowski, A. O. 2007. The soft rot Erwinia
. In: Plant-associated bacteria, eds. by S. S. Gnanamanickam, pp. 423-505. Springer, Dordrecht, Germany.

Charkowski, A. O. 2018. The changing face of bacterial soft-rot diseases. Annu. Rev. Phytopathol 56:269-288.


Chun, B. H., Han, D. M., Kim, K. H., Jeong, S. E., Park, D. and Jeon, C. O. 2019. Genomic and metabolic features of Tetragenococcus halophilus as revealed by pan-genome and transcriptome analyses. Food Microbiol 83:36-47.


Coplin, D. L., Sequeira, L. and Hanson, R. S. 1974.
Pseudomonas solanacearum: virulence of biochemical mutants. Can. J. Microbiol 20:519-529.


Cubitt, M. F., Hedley, P. E., Williamson, N. R., Morris, J. A., Campbell, E., Toth, I. K. and Salmond, G. P. C. 2013. A metabolic regulator modulates virulence and quorum sensing signal production in Pectobacterium atrosepticum
. Mol. Plant-Microbe Interact 26:356-366.


den Hengst, C. D., Groeneveld, M., Kuipers, O. P. and Kok, J. 2006. Identification and functional characterization of the Lactococcus lactis CodY-regulated branched-chain amino acid permease BcaP (CtrA). J. Bacteriol 188:3280-3289.




Duarte, V., De Boer, S. H., Ward, L. J. and De Oliveira, A. M. R. 2004. Characterization of atypical Erwinia carotovora strains causing blackleg of potato in Brazil. J. Appl. Microbiol 96:535-545.


Eisfeld, C., Schijven, J. F., van der Wolf, J. M., Medema, G., Kruisdijk, E. and van Breukelen, B. M. 2022. Removal of bacterial plant pathogens in columns filled with quartz and natural sediments under anoxic and oxygenated conditions. Water Res 220:118724.


Ejim, L. J., D’Costa, V. M., Elowe, N. H., Loredo-Osti, J. C., Malo, D. and Wright, G. D. 2004. Cystathionine beta-lyase is important for virulence of Salmonella enterica serovar Typhimurium. Infect. Immun 72:3310-3314.




Fatima, U. and Senthil-Kumar, M. 2015. Plant and pathogen nutrient acquisition strategies. Front. Plant Sci 6:750.



Ferla, M. P. and Patrick, W. M. 2014. Bacterial methionine biosynthesis. Microbiology (Reading) 160(Pt 8):1571-1584.


Holeva, M. C., Bell, K. S., Hyman, L. J., Avrova, A. O., Whisson, S. C., Birch, P. R. and Toth, I. K. 2004. Use of a pooled transposon mutation grid to demonstrate roles in disease development for Erwinia carotovora subsp. atroseptica putative type III secreted effector (DspE/A) and helper (HrpN) proteins. Mol. Plant-Microbe Interact 17:943-950.


Hossain, M. M., Shibata, S., Aizawa, S.-I. and Tsuyumu, S. 2005. Motility is an important determinant for pathogenesis of Erwinia carotovora subsp. carotovora
. Physiol. Mol. Plant Pathol 66:134-143.

Hullo, M.-F., Auger, S., Dassa, E., Danchin, A. and Martin-Verstraete, I. 2004. The metNPQ operon of Bacillus subtilis encodes an ABC permease transporting methionine sulfoxide, D- and L-methionine. Res. Microbiol 155:80-86.


Husna, A. U., Wang, N., Cobbold, S. A., Newton, H. J., Hocking, D. M., Wilksch, J. J., Scott, T. A., Davies, M. R., Hinton, J. C., Tree, J. J., Lithgow, T., McConville, M. J. and Strugnell, R. A. 2018. Methionine biosynthesis and transport are functionally redundant for the growth and virulence of Salmonella Typhimurium. J. Biol. Chem 293:9506-9519.



Islam, R., Brown, S., Taheri, A. and Dumenyo, C. K. 2019. The gene encoding NAD-dependent epimerase/dehydratase, wcaG, affects cell surface properties, virulence, and extracellular enzyme production in the soft rot phytopathogen, Pectobacterium carotovorum
. Microorganisms 7:172.



Islamov, B., Petrova, O., Mikshina, P., Kadyirov, A., Vorob’ev, V., Gogolev, Y. and Gorshkov, V. 2021. The role of Pectobacterium atrosepticum exopolysaccharides in plant-pathogen interactions. Int. J. Mol. Sci 22:12781.



Jee, S., Choi, J.-G., Lee, Y.-G., Kwon, M., Hwang, I. and Heu, S. 2020. Distribution of Pectobacterium species isolated in South Korea and comparison of temperature effects on pathogenicity. Plant Pathol. J 36:346-354.




Jochim, A., Shi, T., Belikova, D., Schwarz, S., Peschel, A. and Heilbronner, S. 2019. Methionine limitation impairs pathogen expansion and biofilm formation capacity. Appl. Environ. Microbiol 85:e00177-19.




Kim, H., Kim, M., Jee, S.-N., Heu, S. and Ryu, S. 2022. Development of a bacteriophage cocktail against Pectobacterium carotovorum subsp. carotovorum and its effects on Pectobacterium virulence. Appl. Environ. Microbiol 88:e0076122.



Kovach, M. E., Elzer, P. H., Hill, D. S., Robertson, GT., Farris, M. A., Roop, R. M. 2nd and Peterson, K. M. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175-176.


Lee, D. H., Kim, J.-B., Lim, J.-A., Han, S.-W. and Heu, S. 2014. Genetic diversity of Pectobacterium carotovorum subsp. brasiliensis isolated in Korea. Plant Pathol. J 30:117-124.



Lee, D. H., Lim, J.-A., Lee, J., Roh, E., Jung, K., Choi, M., Oh, C., Ryu, S., Yun, J. and Heu, S. 2013. Characterization of genes required for the pathogenicity of Pectobacterium carotovorum subsp. carotovorum Pcc21 in Chinese cabbage. Microbiology (Reading) 159(Pt 7):1487-1496.



Lee, S. M., Choi, Y. H., Kim, H., Kim, H. T. and Choi, G. J. 2020. Development of an efficient bioassay method for testing resistance to bacterial spot rot of Chinese cabbage. Res. Plant Dis 26:159-169.


Lee, Y., Kim, Y., Yeom, S., Kim, S., Park, S., Jeon, C. O. and Park, W. 2008. The role of disulfide bond isomerase A (DsbA) of Escherichia coli O157:H7 in biofilm formation and virulence. FEMS Microbiol. Lett 278:213-222.


Lee, Y., Oh, S. and Park, W. 2009. Inactivation of the Pseudomonas putida KT2440 dsbA gene promotes extracellular matrix production and biofilm formation. FEMS Microbiol. Lett 297:38-48.


Lee, Y., Seo, H., Yeom, J. and Park, W. 2011. Molecular characterization of the extracellular matrix in a Pseudomonas putida dsbA mutant: implications for acidic stress defense and plant growth promotion. Res. Microbiol 162:302-310.


Li, H. and Durbin, R. 2009. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25:1754-1760.




Liu, F., Hu, M., Zhang, Z., Xue, Y., Chen, S., Hu, A., Zhang, L.-H. and Zhou, J. 2022.
Dickeya manipulates multiple quorum sensing systems to control virulence and collective behaviors. Front. Plant Sci 13:838125.



Liu, Q., Chen, N., Chen, H. and Huang, Y. 2020. RNA-Seq analysis of differentially expressed genes of Staphylococcus epidermidis isolated from postoperative endophthalmitis and the healthy conjunctiva. Sci. Rep 10:14234.




López-Solanilla, E., García-Olmedo, F. and Rodríguez-Palenzuela, P. 1998. Inactivation of the sapA to sapF locus of Erwinia chrysanthemi reveals common features in plant and animal bacterial pathogenesis. Plant Cell 10:917-924.



Ma, B., Hibbing, M. E., Kim, H.-S., Reedy, R. M., Yedidia, I., Breuer, J., Breuer, J., Glasner, J. D., Perna, N. T., Kelman, A. and Charkowski, A. O. 2007. Host range and molecular phylogenies of the soft rot enterobacterial genera Pectobacterium and Dickeya
. Phytopathology 97:1150-1163.


Malko, A., Frantsuzov, P., Nikitin, M., Statsyuk, N., Dzhavakhiya, V. and Golikov, A. 2019. Potato pathogens in Russia’s regions: an instrumental survey with the use of real-time PCR/RT-PCR in matrix format. Pathogens 8:18.



Mansfield, J., Genin, S., Magori, S., Citovsky, V., Sriariyanum, M., Ronald, P., Dow, M., Verdier, V., Beer, S. V., Machado, M. A., Toth, I., Salmond, G. and Foster, G. D. 2012. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol 13:614-629.



Marković, S., Stanković, S., Jelušić, A., Iličić, R., Kosovac, A., Poštić, D. and Popović, T. 2021. Occurrence and identification of Pectobacterium carotovorum subsp. brasiliensis and Dickeya dianthicola causing blackleg in some potato fields in Serbia. Plant Dis 105:1080-1090.


Mattinen, L., Nissinen, R., Riipi, T., Kalkkinen, N. and Pirhonen, M. 2007. Host-extract induced changes in the secretome of the plant pathogenic bacterium Pectobacterium atrosepticum
. Proteomics 7:3527-3537.



Mattinen, L., Somervuo, P., Nykyri, J., Nissinen, R., Kouvonen, P., Corthals, G., Auvinen, P., Aittamaa, M., Valkonen, J. P. T. and Pirhonen, M. 2008. Microarray profiling of host-extract-induced genes and characterization of the type VI secretion cluster in the potato pathogen Pectobacterium atrosepticum
. Microbiology (Reading) 154(Pt 8):2387-2396.


Merlin, C., Gardiner, G., Durand, S. and Masters, M. 2002. The Escherichia coli metD locus encodes an ABC transporter which includes Abc (MetN), YaeE (MetI), and YaeC (MetQ). J. Bacteriol 184:5513-5517.




Mole, B. M., Baltrus, D. A., Dangl, J. L. and Grant, S. R. 2007. Global virulence regulation networks in phytopathogenic bacteria. Trends Microbiol 15:363-371.


Monson, R., Burr, T., Carlton, T., Liu, H., Hedley, P., Toth, I. and Salmond, G. P. C. 2013. Identification of genes in the VirR regulon of Pectobacterium atrosepticum and characterization of their roles in quorum sensing-dependent virulence. Environ. Microbiol 15:687-701.


Mulholland, V., Hinton, J. C., Sidebotham, J., Toth, I. K., Hyman, L. J., Perombelon, M. C., Reeves, P. J. and Salmond, G. P. C. 1993. A pleiotropic reduced virulence (Rvi−) mutant of Erwinia carotovora subspecies atroseptica is defective in flagella assembly proteins that are conserved in plant and animal bacterial pathogens. Mol. Microbiol 9:343-356.


Muturi, P., Yu, J., Li, J., Jiang, M., Maina, A. N., Kariuki, S., Mwaura, F. B. and Wei, H. 2018. Isolation and characterization of pectolytic bacterial pathogens infecting potatoes in Nakuru County, Kenya. J. Appl. Microbiol 124:1580-1588.



Park, T.-H., Choi, B.-S., Choi, A.-Y., Choi, I.-Y., Heu, S. and Park, B.-S. 2012. Genome sequence of Pectobacterium carotovorum subsp. carotovorum strain PCC21, a pathogen causing soft rot in Chinese cabbage. J. Bacteriol 194:6345-6346.




Pérombelon, M. C. M. 2002. Potato diseases caused by soft rot erwinias: an overview of pathogenesis. Plant Pathol 51:1-12.


Perombelon, M. C. M. and Kelman, A. 1980. Ecology of the soft rot Erwinias
. Annu. Rev. Phytopathol 18:361-387.

Plener, L., Boistard, P., González, A., Boucher, C. and Genin, S. 2012. Metabolic adaptation of Ralstonia solanacearum during plant infection: a methionine biosynthesis case study. PLoS ONE 7:e36877.



Portier, P., Pédron, J., Taghouti, G., Fischer-Le Saux, M., Caullireau, E., Bertrand, C., Laurent, A., Chawki, K., Oulgazi, S., Moumni, M., Andrivon, D., Dutrieux, C., Faure, D., Hélias, V. and Barny, M.-A. 2019. Elevation of Pectobacterium carotovorum subsp. odoriferum to species level as Pectobacterium odoriferum sp. nov., proposal of Pectobacterium brasiliense sp. nov. and Pectobacterium actinidiae sp. nov., emended description of Pectobacterium carotovorum and description of Pectobacterium versatile sp. nov., isolated from streams and symptoms on diverse plants. Int. J. Syst. Evol. Microbiol 69:3207-3216.


Rochex, A. and Lebeault, J.-M. 2007. Effects of nutrients on biofilm formation and detachment of a Pseudomonas putida strain isolated from a paper machine. Water Res 41:2885-2892.


Rossmann, S., Dees, M. W., Perminow, J., Meadow, R. and Brurberg, M. B. 2018. Soft rot Enterobacteriaceae are carried by a large range of insect species in potato fields. Appl. Environ. Microbiol 84:e00281-18.




Ryu, C.-M. 2015. Against friend and foe: type 6 effectors in plant-associated bacteria. J. Microbiol 53:201-208.



Shyntum, D. Y., Nkomo, N. P., Shingange, N. L., Gricia, A. R., Bellieny-Rabelo, D. and Moleleki, L. N. 2019. The impact of type VI secretion system, bacteriocins and antibiotics on bacterial competition of Pectobacterium carotovorum subsp. brasiliense and the regulation of carbapenem biosynthesis by iron and the ferric-uptake regulator. Front. Microbiol 10:2379.



Taté, R., Riccio, A., Caputo, E., Iaccarino, M. and Patriarca, E. J. 1999. The Rhizobium etli metZ gene is essential for methionine biosynthesis and nodulation of Phaseolus vulgaris
. Mol. Plant-Microbe Interact 12:24-34.


Torres, M., Uroz, S., Salto, R., Fauchery, L., Quesada, E. and Llamas, I. 2017. HqiA, a novel quorum-quenching enzyme which expands the AHL lactonase family. Sci. Rep 7:943.




Toth, I. K., Bell, K. S., Holeva, M. C. and Birch, P. R. 2003. Soft rot erwiniae: from genes to genomes. Mol. Plant Pathol 4:17-30.



Valecillos, A. M., Palenzuela, P. R. and López-Solanilla, E. 2006. The role of several multidrug resistance systems in Erwinia chrysanthemi pathogenesis. Mol. Plant-Microbe Interact 19:607-613.


van der Merwe, J. J., Coutinho, T. A., Korsten, L. and van der Waals, J. E. 2010.
Pectobacterium carotovorum subsp. brasiliensis causing blackleg on potatoes in South Africa. Eur. J. Plant Pathol 126:175-185.


Wang, C., Pu, T., Lou, W., Wang, Y., Gao, Z., Hu, B. and Fan, J. 2018. Hfq, a RNA chaperone, contributes to virulence by regulating plant cell wall-degrading enzyme production, type VI secretion system expression, bacterial competition, and suppressing host defense response in Pectobacterium carotovorum
. Mol. Plant-Microbe Interact 31:1166-1178.


- TOOLS
-
METRICS

- ORCID iDs
-
Eui-Hwan Chung

https://orcid.org/0000-0002-5048-8142Yunho Lee

https://orcid.org/0000-0003-0700-0399 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Supplement1
Supplement1 Print
Print



