Crown gall caused by Agrobacterium tumefaciens is a bacterial disease in rose nurseries around the world. Planting diseased seedlings, wounds by pruning or propagation, and insect damage can give this pathogen opportunities to invade plants and forms tumors (Agrios, 2005; Bliss et al., 1999). A small brown gall forms on the root, crown, and stem in the early disease progress, developed into a hard and large gall. Galls occurred on roots turned to dark brown or grey colors as symptoms gradually progress (Chen et al., 1999). When galls formed on crown or stem, they can damage and exert pressure on the vascular cambium as galls get bigger, which limits the flow of water and nutrients (Best et al., 2004). Crown galls can cause reductions in growth, yield, and quality of roses. In severe cases, they can result in death. In hydroponic cultures, pathogens can be rapidly spread by circulating with water and nutrient solution. About 80% of domestic cut roses are grown hydroponically (Kwon et al., 2014). Commercial rose cultivars that use cutting for production suffer from crown gall disease (Han et al., 2008).
The crown gall disease is one of the diseases hard to control during the cultivation of cut roses. Although some studies have been conducted using a non-pathogenic strain Agrobacterium radiobacter K84 to prevent crown gall, its control efficacy on rose roots is low (Park and Cha, 2001). There is a possibility of the emergence of strains that are resistant to a non-pathogenic strain. The non-pathogenic strain has no effect on already infected plants (Park and Cha, 2001; Xu et al., 2011). Selection of resistant cultivars has been performed to manage crown gall by A. tumefaciens (Kim et al., 2014; Zhao et al., 2005; Zhou et al., 1996, 2000). Rose breeding by intervarietal hybrids or interspecific hybrids has been actively carried out at home and abroad. However, studies on how to test the resistance of rose against crown gall to prevent this disease for the development of resistant cultivars or the production of healthy and high-quality seedlings are insufficient.
Although there is a method to test resistance by inoculating A. tumefaciens directly into individuals, it is not easy to assess resistance due to seasonal and environmental effects (Moore, 1976; Zhao et al., 2005; Zhou et al., 2000). According to Brown (1923), incidence rate of crown gall disease differed among seasons. Resistance test using an in vitro culture method with stem explants of rose has been reported (Zhou et al., 1996). This method minimizes the influence of the external environment that may affect the evaluation of resistance. It allows repeated experiments under the same environmental conditions in the laboratory.
Accordingly, this study was conducted to investigate the level of disease susceptibility of domestically bred roses to crown gall using an in vitro culture method with nodal explants.
Materials and Methods
Plant materials
Rose cultivars used in the study were collected from the National Institute of Horticultural and Herbal Science, Gyeonggi-do Agricultural Research & Extension Services, Jeollabuk-do Agricultural Research & Extension Services, Kainos nursery, and Jangsu nursery, Korea (Supplementary Table 1). Rose seedlings were planted in a mixture of sterilized soil and soil for roses (Rosenerde, Flora gard, Germany) in pots at the greenhouse in the University of Seoul. Resistance test was conducted for 58 cultivars developed in Korea and six foreign cultivars.
Isolation of pathogen
Galls were isolated from the root, crown, and stem of roses infected with crown gall disease, immersed in 2% sodium hypochlorite for 10 min and washed three times with sterile water for surface sterilization. The internal tissue of the sterile gall was cut with a sterile scalpel into less than 5 mm × 5 mm in size and placed in 5 ml of sterile water. After maceration with sterile water at room temperature for 30 min, the gall extract was diluted 10 times with sterile water and 0.1 ml of the diluted solution was streaked onto NASA medium (Clark, 1969) known as a selective medium for A. tumefaciens. Brick red colonies with white edge were checked after incubation at 28°C for 48 h in the dark and a single colony was isolated. All isolates were maintained on nutrient broth medium with 20% glycerol and placed at −70°C for long-term storage (Park et al., 2021).
Selection of pathogenic isolate
A single colony of A. tumefaciens grown for 2 days on nutrient agar medium was streaked onto three selective medium: D1 medium (Perry and Kado, 1982), MacConkey medium (Bopp et al., 1999), and NASA medium (Clark, 1969). Characteristic colonies that appeared in each medium were examined while they were incubated at 28°C for 48 h in the dark. Polymerase chain reaction (PCR) analysis showed the presence of agrocinopine, nopaline, and octopine gene fragments. These genes are known to be related to virulence of pathogenic strains (Chandrasekaran et al., 2019). Among isolates identified as A. tumefaciens based on characteristics of some selective medium, one pathogenic strain was selected for pathogenicity test.
Bacterial inoculum preparation and in vitro plant tissue culture
In order to investigate the susceptibility of Korean and foreign rose cultivars to A. tumefaciens, the selected pathogenic strain RC12 strain was streaked onto yeast extract beef medium. After incubation at 25°C for 48 h, bacterial suspension was adjusted to 108 cfu/ml and was prepared for inoculation (Han et al., 2006; Zhou et al., 2000). Rose stems were collected from 58 Korean cultivars and six foreign cultivars and sterilized with 75% ethanol for 2 min followed by treatment with 3% sodium hypochlorite containing Tween 20 (0.1%) for 10 min. Stems having an axillary bud were cut into 4 cm or more and nodal segments were cultured in vitro on MS medium (Murashige and Skoog, 1962). Rates of shoot formation, contamination, and mortality were examined during culture. Upper parts of nodal explants and axillary shoots, cultured for more than 3 weeks were cut with scissors and subsequently inoculated with 3 μl of the bacterial suspension using a micropipette. These inoculated nodal explants of rose were cultured for 5 weeks under 12 h photoperiod at around 25°C.
Resistance test and evaluation
Resistance tests were conducted for 58 Korean cultivars and six foreign cultivars (‘Brocante’, ‘Show Flow’, ‘Ocean Song’, ‘Coral Natural’, ‘Fuego’, ‘Hera’). Resistance to crown gall was evaluated based on tumor formation rate (incidence rate) and tumor formation period. Rose explants with or without galls were recorded for 5 weeks after inoculation. To determine whether galls were caused by callus or by pathogens, galls were isolated and gall extract was spread onto NASA medium. Plates were incubated at 28°C for 24 h in the dark and the presence of distinctive colonies was observed. Principal component analysis, one of multivariate data analyses, was used to investigate the relationship between tumor formation period and the incidence rate.
Results and Discussion
Isolation and characterization of A. tumefaciens on selective medium
From June 2016 to December 2017, A. tumefaciens strains were isolated from 12 rose cultivars (‘Yellow Eye’, ‘Fuego’, ‘Dominica’, ‘Pink Party’, ‘Stella’, ‘Vintage’, ‘White Soda’, ‘Red Eagle’, ‘Silver Shadow’, ‘White Beauty’, ‘Sharbet’, and ‘Punch Bowl’). After investigating each of the characteristics of each strain for a total of 125 strains isolated from June 2016 to August 2016 using three selective media, 100 strains showed specific colony morphologies. Colonies were observed at 24 h after incubation. Distinct colonies were formed at 48 h after incubation. A. tumefaciens presented cream or bright sky-blue colonies during early growth stages and then showed a yellowish-green color on D1 medium. Its colonies change color from pink to red on MacConkey medium. It showed red colonies with a white edge on NASA medium. On ATMM medium, it showed creamy color colonies (Supplementary Fig. 1). ATGN medium [K2HPO4 0.079 M, pH to 7.0 W/NaOH 0.044 M, (NH4)SO4 0.015 M, MgSO4·7H2O 0.6 mM, CaCl2-2H2O 0.06 mM, MnSO4·H2O 0.0071 mM, glucose 0.5%] contains a carbon source that is suitable for stable growth of A. tumefaciens (Morton and Fuqua, 2012).
A. tumefaciens forms creamy, sky-blue, and yellowish-green colonies on D1 medium (Kado and Heskett, 1970) and red colonies on MacConkey medium as they are positive for lactose (Moore et al., 1988). A. tumefaciens grown on NASA medium presents colonies with a white translucent edge and a dark red center, a characteristic of Gram-negative bacteria. NASA medium was used for the isolation of A. tumefaciens from galls formed after resistance test (Supplementary Fig. 1C). It was chosen because a distinct colony could be formed on this medium, but not on other media (Davoodi et al., 2013). As characteristics of colony formed on selective medium matched with colony morphologies reported previously (Atlas, 2010; Clark, 1969; Islam et al., 2010; Kado and Heskett, 1970), strains isolated from roses with crown gall disease were confirmed to be A. tumefaciens.
Selection for pathogenic isolate
In order to investigate pathogenic genes related to the use of carbon sources of 86 A. tumefaciens strains selected based on the formation of characteristic colony morphology through selective medium, PCR analysis was carried out by Konkuk University (Chandrasekaran et al., 2019). Forty-seven pathogenic strains were identified by the presence of genes related to the expression of opine, such as agrocinopine, nopaline, and octopine known to be needed for the growth of A. tumefaciens: 45 were positive for agrocinopine and 2 were positive for nopaline. A. tumefaciens can produce opines and agrocinopines and, use them as carbon and energy sources (Ellis and Murphy, 1981). In addition, not all A. tumefaciens strains isolated from individuals infected with crown gall were identical in pathogenicity (Islam et al., 2010; Xu et al., 2011). A. tumefaciens RC12 isolate was chosen as an inoculation strain because it was positive for agrocinopine by PCR analysis and identified as a pathogenic A. tumefaciens (Chandrasekaran et al., 2019).
In vitro culture of rose stem explants
In vitro culture of 64 rose cultivars for resistance test against crown gall showed average shoot formation rate of 82.6% and average mortality of 12% (Supplementary Table 2). ‘Beast’, ‘Ever Spring’, and ‘Henney Leon’ all produced shoots without dead explants. ‘11-305’, ‘12-376’, ‘G13-335’, ‘Red Square’, and ‘Redwing’ had high average shoot formation rates of over 80% without dead explants. Among cultivars with above-average shoot formation rate, some cultivars showed high mortality. They showed good early growth, but began to turn brown and wither after 3 or 4 weeks. When tissue culture is established using stem explants of woody plants, explants can release phenolic compounds composed of oxide polyphenols and tannins, thereby causing the stem to brown and wither (Thorpe et al., 1991), consist with observations of the present study. The mortality rate differed depending on cultivars. Among cultivars with above-average mortality, ‘G12-46’, ‘G13-194’, ‘Redbi’, ‘Brocante’, ‘Blondy’, ‘Show Flow’, ‘Swai’, ‘Sweet Skin’, ‘Silver Shadow’, and ‘Fascinar’ had below average shoot formation rate. In particular, ‘13-251’, ‘G12-46’, ‘Brocante’, ‘Swai’, and ‘Silver Shadow’ had mortality rates of 40% or more with growth of saprophytes after their death, showing a high contamination rate.
According to Horn (1992) and Khosh-Khui and Sink (1982), organogenesis of rose in vitro varies by cultivar, resulting in differences in culture efficiencies. Cultivars with high shoot formation are suitable for the resistance test. However, some rose cultivars could not be subjected to a resistance test using in vitro culture because these cultivars had high mortality rates.
Shokri et al. (2015) has reported that the medium is contaminated by fungi and bacteria in the case of rose tissue collected from nature, showing a contamination rate of 42.5%. In March 2018, a preliminary test was performed to determine the growth ability of roses showing about 40% contamination rate by fungi and bacteria as a result of in vitro culture on MS medium. To reduce the contamination, surface sterilization time of 75% ethanol was increased from 20-30 s to 2 min. The concentration of sodium hypochlorite was increased from 2% to 3%, and the sterilization time of sodium hypochlorite was increased for an additional 5 min. As a result of adjusting the sterilization method to have the lowest rate of contamination, the contamination rate on medium in this study was decreased to an average of 16%.
Callus tissue can be formed on stem explants in vitro. It is a common phenomenon in an in vitro culture (Han et al., 2006; Hsia and Korban, 1996; Khosh-Khui and Sink, 1982). In the present study, callus formation was observed in 32 cultivars. ‘Ice Wing’ and ‘Jinii’ showed callus formation rate of 80% or more. ‘S-Pink’, ‘Rock Fire’, ‘Bobos’, and ‘Yellow Sun’ showed callus formation rate more than 20%. However, callus was not observed in 32 cultivars including ‘Love Letter’. In the case of ‘Deep Purple’, ‘Rock Fire’, ‘Redbi’, ‘Red Pocket’, ‘Bobos’, ‘Show Girl’, ‘Pink Heart’, and ‘White Beauty’, repeatedly used in the first, second, and third tests, there was a difference in callus formation. Therefore, it is necessary to confirm whether it is a callus or a tumor when it comes to an in vitro inoculation method.
Resistance test of rose cultivars against crown gall
Results of test for resistance to crown gall for 64 cultivars revealed that 40 of them formed tumors with an average tumor formation rate of 11.2% (Supplementary Table 3). At 11 days after inoculation, a tumor was first observed on the inoculation area of stem explants. In susceptible cultivars with tumor formation, the size of tumor increased over time with tumor surface color changed from white to brown. Han et al. (2006) and Park and Cha (2000) have reported that after infection by A. tumefaciens, small tumors can develop with increasing sizes, and tumor surface color turning brown. Six cultivars (‘12-376’, ‘G12-46’, ‘G13-335’, ‘Love Letter’, ‘Redbi’, and ‘Feel Lip’) had tumor formation rate of 16% to 25%. The other six cultivars (‘Deep Silver’, ‘Bobos’, ‘Jinii’, ‘Pink Shine’, ‘Pink Pop’, and ‘White Beauty’) were relatively high susceptible as 30% of explants formed tumors (Fig. 1). ‘Bobos’ especially had relatively high susceptibility with tumor formation of 45.8%. A. tumefaciens was transmitted not only to the inoculated site but also to the non-inoculated wounds. Martí et al. (1999) have also reported that A. tumefaciens could be detected at non-inoculation sites. Twenty-four cultivars (22 Korean cultivars and 2 foreign cultivars) appeared to be resistant to crown gall as they did not form tumor. These cultivars were: ‘11-305’, ‘A-Pink’, ‘S-Pink’, ‘Green Star’, ‘Redliner’, ‘Red Square’, ‘Redwing’, ‘Red Fit’, ‘Rich’, ‘Missha’, ‘Brocante’, ‘Sugar Ball’, ‘Swai’, ‘Sweet Skin’, ‘Silver Shadow’, ‘Ever Spring’, ‘Yellow Eye’, ‘Yellow Pop’, ‘Coral Natural’, ‘Fascinar’, ‘Pink Beauty’, ‘Pink Yar’, ‘Pink Condor’, and ‘Pink Fire’.
When utilizing germplasm that is resistant to crown gall for breeding, resistance can be transmitted to the progeny (Nam et al., 1997; Reynders-Aloisi et al., 1998). ‘Violetta’ known as crown gall-resistant cultivar described by Lee et al. (2015) exhibited one tumor in only one explant of the total number of repetitions, which resulted in a tumor formation rate of 3.4%. ‘Ocean Song’, the parental line of ‘Violetta’, also developed one tumor with a tumor formation rate of 1.6%, indicating that both cultivars were relatively resistant probably due to inherited resistance.
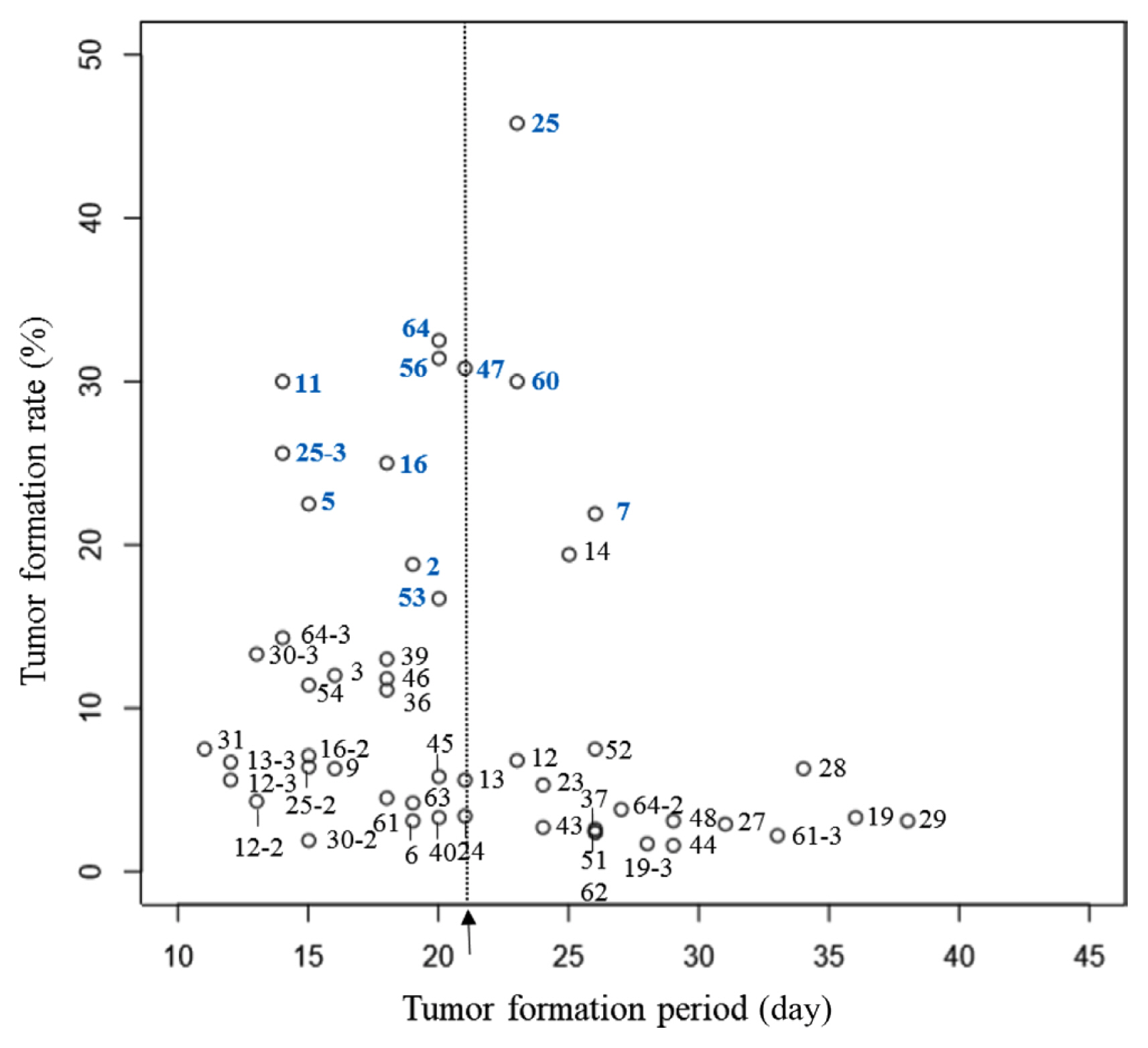
In the survey of tumor formation period, the average period was 21.1 days for the initial tumor formation and 28.6 days for the last tumor formation in 40 cultivars. ‘G12-46’, ‘Deep Silver’, ‘Deep Purple’, ‘Show Girl’, ‘Show Flow’, and ‘Pink Baby’ formed tumors in 15 days, while ‘12-376’, ‘G13-335’, ‘Wedding Cake’, and ‘Feel Lip’ formed additional tumors even at 40 days after inoculation, confirming that the period of tumor formation differed among cultivars. Tumor formation rate also differed depending on the cultivar. Six cultivars (‘Deep Silver’, ‘Bobos’, ‘Jinii’, ‘Pink Shine’, ‘Pink Pop’, and ‘White Beauty’) that had tumor formation rates of over 30% formed initial tumors within 23 days of inoculation. Twenty-three cultivars had tumor formation rates of less than 8%, 15 of which formed initial tumors after 20 days of inoculation (Supplementary Table 3). Six cultivars (‘Red Pocket’, ‘Blondy’, ‘Beast’, ‘Sharbet’, ‘Ocean Song’, and ‘Candy Party’) that had low tumor formation rates of around 5% formed initial tumors after 28 days of inoculation (Fig. 2). ‘Ocean Song’ used in breeding for resistance also belonged to this group of cultivars indicating that these six cultivars were relatively resistant to crown gall. Thirteen cultivars (‘12-376’, ‘13-251’, ‘G12-46’, ‘Deep Silver’, ‘Redbi’, ‘Bobos’, ‘Antique Curl’, ‘Wedding Cake’, ‘Jinii’, ‘Feel Lip’, ‘Pink Baby’, ‘Pink Shine’, and ‘White Beauty’) had higher than average tumor formation rates. In addition, their tumors formed faster than the average period. Eight cultivars (‘12-376’, ‘G12-46’, ‘G13-335’, ‘Love Letter’, ‘Antique Curl’, ‘Jinii’, ‘Feel Lip’, and ‘White Beauty’) showed above-average tumor formation rates with last tumors formed later than 28.6 days after inoculation, which was the average period for the last tumor formation (Fig. 3). Among six cultivars with tumor formation rate of 30% or more, ‘Deep Silver’, ‘Jinii’, and ‘White Beauty’ additionally formed tumors at 28 days after inoculation, and ‘G12-46’, ‘G13-335’, ‘Deep Silver’, ‘Jinii’, ‘Pink Shine’, and ‘Pink Pop’ with tumor formation rate of more than 20% additionally formed tumors at 26 days after the inoculation. Brown (1923) and Zhou et al. (2000) have also reported that the period of tumor formation varies by cultivar and that such difference could be related to resistance. According to Zhou et al. (2000), as for susceptible rootstocks cultured in vitro, over 40% of disease shoots formed tumors during the first week after inoculation.
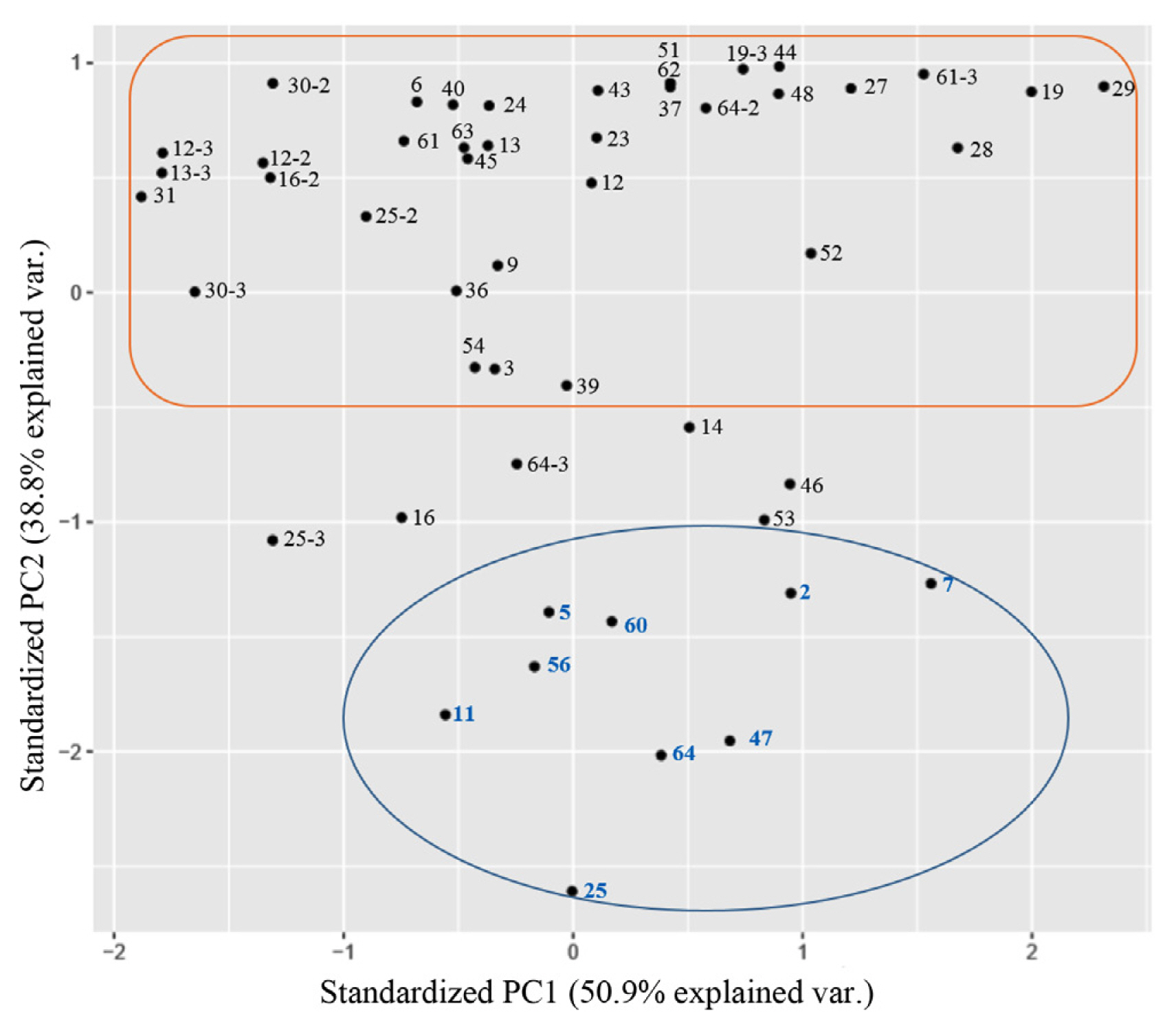
This study used a principal component analysis, one of multivariate data analyses, based on two scatter plots showing the period of tumor formation. Two components, PC1 and PC2, with cumulative sum of the eigenvalue of 90 were selected (Table 1). PC1 was the period of initial and last tumor formation, and PC2 was the tumor formation rate. Two groups were divided by principal component analysis (Fig. 4). The group at the bottom of the principal component analysis had nine cultivars (‘12-376’, ‘G12-46’, ‘G13-335’, ‘Deep Silver’, ‘Bobos’, ‘Jinii’, ‘Pink Shine’, ‘Pink Pop’, and ‘White Beauty’), all of which had an average tumor formation rate of 18% or more in scatter plots. Their initial tumors were observed within 26 days of inoculation. In particular, five cultivars (‘12-376’, ‘G12-46’, ‘Deep Silver’, ‘Pink Shine’, and ‘White Beauty’) of this group formed tumors earlier than the average period of the initial tumor formation. The group at the upper part of principal component analysis belonged to cultivars having below the average tumor formation rate with, additional tumor formed at 37 days after inoculation, indicating a high correlation between the period of initial tumor formation and tumor formation rate.
In 2019, eight Korean cultivars (‘Deep Purple’, ‘Rock Fire’, ‘Red Pocket’, ‘Bobos’, ‘Show Girl’, ‘Sugar Ball’, ‘Pink Heart’, and ‘White Beauty’) were re-collected from the National Institute of Horticultural and Herbal Science, and Gyeonggi-do Agricultural Research & Extension Services to test again. Among them, six cultivars (‘Deep Purple’, ‘Rock Fire’, ‘Red Pocket’, ‘Show Girl’, ‘Sugar Ball’, and ‘Pink Heart’) having below the average tumor formation rate did not form any tumors in the first test or formed tumors on one or two explants in the second test, showing a tumor formation of less than 5%, indicating a resistant response. No tumors appeared especially on ‘Sugar Ball’ in the first or the second tests. All six cultivars, except for ‘Bobos’ and ‘White Beauty’, showed a difference of less than 6% compared to the average tumor formation rate in the first test. ‘Bobos’ showed tumor formation rates of 45.8% and 6.4% in the first and second test, respectively. ‘White Beauty’ showed tumor formation rates of 32.5% and 3.8% in the first and second test, respectively. To further investigate the difference in tumor formation rate, roses grown at the greenhouse in the University of Seoul were cultured in vitro in a third test. ‘Bobos’ had a tumor formation rate of 25.6%, and ‘White Beauty’ had a tumor formation rate of 14.3%. The low tumor rate for susceptible cultivars might be due to growing conditions of rose samples in different environments. This difference has also been reported by Zhou et al. (2000). They reported that the incidence rate of crown gall was 90% for rose cultivars in further tests, although these cultivars were found to be resistant in a previous study. They assumed that such difference was caused by different growth environments of roses. Therefore, it is necessary to conduct resistance tests for many cultivars grown in different environments.
In conclusion, progressed in vitro inoculation method can be conducted under controlled conditions in the laboratory, which minimizes environmental influences and allows resistant breeding to reduce generation time. Value for resistance evaluation comprises both tumor formation rate and onset of crown gall disease. The data between the tumor formation rate and the period of initial tumor formation show that the incidence of crown gall and the first outbreak of crown gall disease had a high correlation. A total of 64 cultivars were tested for their resistance against crown gall in this study. This will provide information about resistance sources for breeding crown gall-resistant rose.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Supplement1
Supplement1 Print
Print